ABSTRACT
Vaccination against measles, mumps, rubella, and varicella is recommended for all children in the US. Limitations manufacturing Oka/Merck strain varicella-zoster virus have hampered the availability of the combination vaccine (MMRV) against these 4 viruses, which drove the need to investigate an alternative manufacturing process. Healthy children 12-to-23 months of age at 71 US sites were randomized (1:1) to receive MMRV manufactured using an alternative process (MMRVAMP) or the currently licensed MMRV. Subjects received 2 0.5 mL doses 3 months apart. Sera were collected before and 6 weeks after Dose-1. Adverse experiences (AEs) were collected for 42 d after each dose and serious AEs and events of special interest for 180 d after Dose-2. Overall, 706 subjects were randomized to MMRVAMP and 706 to MMRV and 698 and 702 received at least 1 dose of study vaccine, respectively. The risk difference in response rates and geometric mean concentrations of antibody to measles, mumps, rubella, and varicella viruses 6 weeks after Dose-1 met non-inferiority criteria for MMRVAMP versus, MMRV. Response rates met acceptability criteria for each virus, and the seroconversion rate to varicella-zoster virus was 99.5% in both groups. Vaccine-related AEs were mostly mild-to-moderate in intensity and somewhat more common after MMRVAMP. Febrile seizures occurred at similar rates in both groups during the first 42 d after each vaccine dose. MMRVAMP is non-inferior to MMRV and represents an important advancement in maintaining an adequate supply of vaccines against these diseases.
Introduction
Vaccination against measles, mumps, rubella, and varicella is recommended for all children in the United States (US).Citation1 The routine schedule published by the Advisory Committee on Immunization Practices (ACIP) recommends measles, mumps, and rubella vaccine (MMR; M-M-R™ II, Merck & Co., Inc., Kenilworth, NJ) and varicella vaccine (VAR; Varivax™, Merck & Co., Inc., Kenilworth, NJ) at 12 to 15 months of age.Citation1-3 Both vaccines have been shown to be well tolerated, immunogenic, efficacious, and highly effective in reducing the incidence of measles, mumps, rubella, and varicella.Citation4-16 In 2014, the estimated coverage rates among US children 19 to 35 months of age were 91.5% for MMR and 91.0% for VAR.Citation17 Measles, mumps, rubella, and varicella vaccine (MMRV; ProQuad™, Merck & Co., Inc., Kenilworth, NJ) is considered an option for parents who prefer the combinationCitation2,3 (the American Academy of Pediatrics [AAP] has no preference for MMR plus VAR vs. MMRV for the first dose, as long as the parents are aware of the slightly increased risk of febrile seizures with MMRVCitation18). A second dose of each vaccine is recommended at 4 to 6 y of age, with MMRV being preferred by both ACIP and AAP.Citation2-4
In general, use of combination vaccines can improve coverage rates and timeliness by decreasing the number of injections that are due.Citation19,20 However, the production and availability of MMRV has been severely hampered by limitations in the supply of Oka/Merck strain varicella zoster virus (Oka/Merck-VZV) bulk materials in the manufacturing process. The Oka/Merck-VZV bulk material is not only used for MMRV production but also for VAR and the herpes zoster (shingles) vaccine (HZV; Zostavax™, Merck & Co., Inc., Kenilworth, NJ), both of which are recommended for universal use (in children and adults ≥60 years of age, respectively). Increased uptake of these vaccines would put great pressure on the availability of the VZV bulk vaccine for MMRV production.
An alternative manufacturing process (AMP) has been developed to increase the availability of Oka/Merck-VZV for use in varicella-containing vaccines. This study (NCT01536405) evaluated the safety, tolerability, and immunogenicity of a formulation of MMRV that contains Oka/Merck-VZV manufactured using the AMP (MMRVAMP) compared to the currently licensed MMRV.
Results
Subjects
Overall, 99.2% (1400/1412) of randomized subjects received Dose-1 of study vaccine; and among subjects who received dose 1, 90.4% (1266/1400) received Dose-2 (). Approximately 84.3% of subjects completed the study (received both doses, had all blood samples collected, and completed the 42-day safety data after each vaccination). The primary immunogenicity analyses were based on the Per-Protocol population. The Per-Protocol population was defined as subjects who received 1 dose of MMRVAMP or MMRV according to their vaccination group assignment, adhered to study instructions, and provided serum samples within the appropriate day ranges. The Per-Protocol population excluded subjects due to important deviations from the protocol that substantially affected the results of the primary immunogenicity endpoints. Therefore, the primary evaluation for each antigen was based on subjects in the Per-Protocol population who met the baseline antibody requirement for that antigen. The most commonly cited reason for exclusion was a missing blood sample after Dose-1. In general, the number of subjects who were excluded from the per-protocol analyses at each deviation category was comparable between groups for each antigen. All subjects who received a vaccine dose were included in the safety analysis.
Figure 1. Subject disposition. aNumber of subjects excluded from the per-protocol MMRVAMP postdose 1 immunogenicity analyses: Measles (69); Mumps (80); Rubella (90); and Varicella (112). bNumber of subjects excluded from the per-protocol MMRV postdose 1 immunogenicity analyses: Measles (81); Mumps (92); Rubella (109); and Varicella (114). cOne subject did not receive Dose 2, but was followed for 180 d postdose 1 and discontinued due to an AE (onset was Day 5 postdose 1) during the extended safety follow-up but before study completion.
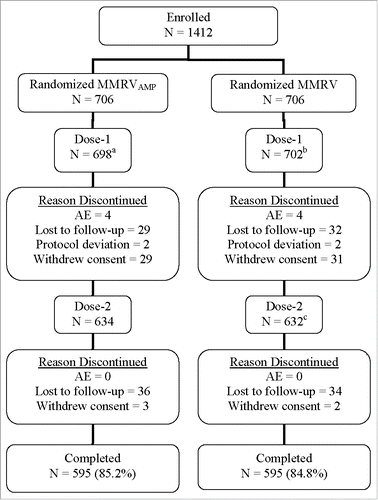
The number of subjects who discontinued was evenly distributed across the two groups. The most common reasons for discontinuation from the study were lost to follow-up (9.3%) and withdrawal of consent (4.7%). Four subjects in each group discontinued due to an AE.
Subjects across both groups were similar with respect to gender, age, and race (). The distribution of baseline serostatuses (>91% were initially seronegative for each virus) and unknown baseline serostatuses were comparable between the 2 groups. Approximately 30% of subjects received any prior medical therapy, the most common being acetaminophen (6.5% before Dose-1, 7.3% before Dose-2) and ibuprofen (4.7% before Dose-1, 7.0% before Dose-2). Use of acetaminophen (35.6% after Dose-1, 24.4% after Dose-2) and ibuprofen (25.9% after Dose-1, 19.7% after Dose-2) increased following administration of each respective dose. The percentages of subjects with any concomitant therapies were similar across both vaccination groups.
Table 1. Demographics.
Immunogenicity
A statistical analysis of the risk difference in the response rates and GMCs to measles, mumps, rubella, and VZV between groups in the Per-Protocol Population after Dose-1 can be found in . The results demonstrate non-inferiority of the response rate to each virus 6 weeks after Dose-1 in MMRVAMP as compared to MMRV. Additionally, the non-inferiority criterion regarding GMCs was met for measles, mumps, rubella, and VZV. The acceptability criteria regarding the response rate for each virus 6 weeks after Dose-1 was met at p < 0.001 for each virus.
Table 2. Summary of response rates and geometric mean concentrations (GMCs) after dose-1.
The VZV seroconversion rate was comparable between groups, with 99.5% of both groups (individually and in total) achieving seroconversion after Dose-1.
Safety
In subjects with safety follow-up, AEs were reported by 71.3% of subjects after Dose-1 and 65.6% after Dose-2 (). The number of subjects with vaccine-related AEs after Dose-1 was statistically significantly higher in the MMRVAMP group than in the MMRV group (risk difference [95% CI] of 6.3% [1.1%, 11.5%]).
Table 3. Adverse experience (AE) summary (days 1 to 42 following each vaccination).
Injection-site AEs ranged from 29.7% to 37.8% of participants, with the majority being mild to moderate in intensity across both doses. From Days 1 to 5 after Dose-1, the MMRVAMP group experienced statistically significantly (p = 0.001) more injection-site erythema events than the MMRV group (22.9% vs 15.8%; risk difference 7.0%, 95% CI [2.9%, 11.2%]). No injection-site AE after Dose-1 was associated with a hospitalization or discontinuation from the study.
The most frequently reported systemic AE was pyrexia, occurring in 23.5% of MMRVAMP subjects and 21.1% of MMRV subjects after Dose-1, and in 14.5% of MMRVAMP subjects and 17.6% of MMRV subjects after Dose-2. Subjects in the MMRVAMP group did experience statistically significantly more maculopapular rash events after Dose-1 than subjects in the MMRV group (1.6% and 0.4 %, respectively; risk difference 1.2%, 95% CI [0.1%, 2.5%]). Of note, subjects in the MMRVAMP group experienced marginally significantly more varicella-like rash events after Dose-1 than subjects in the MMRV group (4 events and 0 events, respectively; p = 0.045).
Two (0.3%) subjects in the MMRVAMP group and 4 (0.6%) subjects in the MMRV group discontinued from the study due to an AE with event onsets during Days 1 to 42 after Dose-1. One (0.1%) subject in the MMRVAMP group and 2 (0.3%) subjects in the MMRV group experienced SAEs that were judged by the investigators to be related to vaccination (). No subject died during the study.
Table 4. Serious adverse experience (SAE) listing.
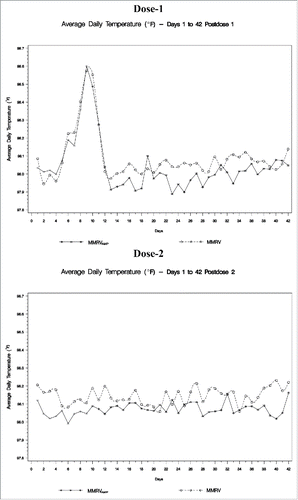
As shown in , the rate of fever (temperature ≥102.2°F [≥39.0°C] oral equivalent) in the MMRVAMP group from 1 to 5 d after Dose-1 was non-inferior to that in the MMRV group (6 events and 5 events, respectively; p < 0.001). The average daily temperatures between both vaccination groups were comparable, and neither group as a whole recorded an average daily temperature that exceeded 102.2°F (39.0°C) oral equivalent (). For both groups, the greatest frequency of subjects with a temperature of >100.4°F occurred 6 to 11 d after vaccination (≥19 subjects each day).
Table 5. Analysis of rates of fever by day range between vaccination groups - postdose 1 (all subjects as treated population).
One (1) febrile seizure occurred in the MMRVAMP group and 2 occurred in the MMRV group during the first 42 d after Dose-1; these events occurred during the expected time period post-vaccination and were considered vaccine related. One (1) febrile seizure that was not considered vaccine-related occurred in a MMRVAMP subject 28 d after Dose-2. In total, 12 febrile seizures (10 in the MMRVAMP group and 2 in the MMRV group) occurred in 9 subjects (7 in the MMRVAMP group and 2 in the MMRV group). Three subjects in the MMRVAMP group experienced 2 seizures each. All febrile seizure events resolved without clinical sequelae.
Seven (7) subjects in the MMRVAMP group and 5 subjects in the MMRV group experienced SAEs after Dose-1 of which 1 in the MMRVAMP group and 2 in the MMRV group were deemed by the investigator to be vaccine-related. Two (2) subjects in the MMRVAMP group and 2 subjects in the MMRV group experienced SAEs after Dose-2 of which none were deemed by the investigator to be vaccine-related.
Discussion
This study demonstrates that the immunogenicity of the investigational MMRVAMP is non-inferior to the currently licensed MMRV in healthy children 12 to 23 months of age, as evidenced by similar antibody response rates and GMCs to measles, mumps, rubella, and VZV 6 weeks after Dose-1. In addition, MMRVAMP induced acceptable antibody responses against all 4 viruses at this same time point. The vaccine was well tolerated and had an adverse event profile that was comparable to the licensed product. The rate of fever (temperature ≥102.2°F [≥39.0°C] oral equivalent) for MMRVAMP during the first 5 d after vaccination was similar to the rate of MMRV, and although the rate of injection-site adverse events (erythema, pain/tenderness, and swelling) was higher for MMRVAMP, these events were mostly mild in intensity, similar in size (generally ≤1 inch), and did not result in hospitalization or discontinuation from the study. It cannot be concluded from this study whether the increased potency of the VZV bulk material or the use of rHA instead of HSA or the combination of both may have caused the increased injection-site related AEs observed in the MMRVAMP group.
Febrile seizures occurred at similar rates in both groups during the 42 d following each vaccine dose (this includes the known high risk period for febrile seizures after Dose-1 of MMRV, which is approximately 5 to 12 d after vaccinationCitation21,22). There were more febrile seizures outside of the primary safety follow-up period in the MMRVAMP group compared to the MMRV group; none of these events, however, were assessed by the investigators to be related to the vaccine. Based on literature review, the incidence of febrile seizures in the second year of life is reported to be 1 to 2 per 1000 children per month in the general population (estimated prior to the introduction of many of the vaccines in the current pediatric schedule).Citation23-29 In addition, the number of febrile seizures observed in the MMRVAMP group is consistent with what would be expected for children in this age range based on estimates from incidence rates and times of follow-up. The observed imbalance between groups in the numbers of first occurrences of febrile seizures outside the primary safety period is not likely to be related to any true incidence difference between the groups.
Clinical AEs reported during the 42 d after Dose-2 were generally comparable between the 2 vaccination groups in terms of the incidence rates of adverse events overall, systemic AEs, and SAEs. During the extended safety follow-up period, the incidence rates of SAEs, medically attended AEs, and new or worsening chronic medical conditions that did not meet the definition of a SAE were similar.
The strength of this study is that it was adequately powered, well controlled, and over 85% of subjects completed the required follow-up. A limitation of this study was that the parental completion of a diary card for 42 d after each vaccination may have resulted in reporting fatigue over time. Another limitation of this study was that serum samples were not collected 6 weeks after Dose-2. This was done in order to facilitate enrollment. As a result it is not possible to compare the antibody response between the two groups after Dose-2.
The immunization programs in the US for measles, mumps, rubella, and varicella have been remarkably successful. Indigenous rubellaCitation30 and measlesCitation31 have been eliminated, although some recent outbreaks have occured.Citation32 Mumps and varicella are well controlled.Citation33 Consolidating and maintaining these successes depend on maintaining an adequate supply of vaccines, and MMRVAMP represents an important step in that direction. MMRVAMP measles, mumps, and rubella antibody responses are consistent with published results for MMRV vaccine and MMRVAMP VZV antibody responses are somewhat higher than previously reported. The safety profile of MMRVAMP is consistent with published results for MMRV vaccine, however injection-site reactions appear to be somewhat higher than previously reported.Citation34-38
Methods
Design
This was a randomized, double-blind (subject, investigator, sponsor, and laboratory) clinical trial conducted in 71 sites within the US from June 2012 to January 2014. The protocol was approved by the ethical review committee of each site and conducted in conformance with applicable local requirements.
Subjects
Healthy children 12 to 23 months of age with a negative history for measles, mumps, rubella, and varicella and without prior immunization against these diseases were eligible for the study. Exclusion criteria included receipt of any inactivated vaccine within 14 days, or any live vaccine within 30 days, prior to study entry; history of seizure disorder; febrile illness within 72 hours prior to study entry; or any congenital or acquired immune deficiency, neoplastic disease, or immunosuppression.
Subjects were allocated to a vaccination group using a randomized schedule generated by the study statistician. Subjects were randomized to receive either two 0.5 ml subcutaneous doses of MMRVAMP 3 months apart or two 0.5 ml subcutaneous doses of MMRV 3 months apart. The study was designed to have approximately 1400 subjects randomized in a 1:1 ratio to either one of the two groups. Based on approximately 700 subjects per group, and with an expected evaluability rate of 90%, the study provided 89.8% power across the primary immunogenicity hypotheses.
Vaccines
The supply of VZV bulk materials is the limiting factor for the production of MMRV. To obtain higher potency VZV bulk for use in MMRV, a modification was made to the Oka/Merck-VZV manufacturing process. The modification involves the introduction of an additional processing step to the upstream harvesting process, which provides higher potency bulk vaccine for use in MMRV. In addition, recombinant human albumin is used in the AMP. While the AMP provides higher potency bulk vaccine for use in the manufacture of MMRV this higher potency bulk vaccine is diluted in the manufacture of MMRV, such that the final product meets established quality specifications. Oka/Merck-VZV produced via this process and combined with MMR is referred to as MMRVAMP in this publication. Both MMRVAMP and MMRV are live, attenuated, lyophilized vaccines for the prevention of measles, mumps, rubella, and varicella in children 12 months through 12 y of age. They are indistinguishable in appearance. Both vaccines were packaged in single-dose glass vials with a multilingual booklet label and stored at 2° to 8°C.
Immunogenicity
Serum samples collected before and 6 weeks after Dose-1 were tested for concentrations of measles, mumps, rubella, and varicella. Antibody titers for measles, mumps, and rubella were evaluated by enzyme-linked immunosorbent assay (ELISA) methods, and antibody titers for varicella were evaluated by glycoprotein ELISA (gpELISA) methods.Citation39-41 Immunogenicity was evaluated by response rates at each time point and geometric mean concentrations (GMCs) of antibodies to each virus were evaluated 6 weeks after Dose-1. Serum samples were not collected 6 weeks after Dose-2 for antibody measurement.
The primary immunogenicity objectives were: (1) to demonstrate that MMRVAMP induces measles, mumps, rubella, and VZV antibody response rates that are non-inferior to those induced by MMRV 6 weeks after Dose-1; (2) to demonstrate that GMCs of measles, mumps, rubella, and VZV antibodies in subjects who received MMRVAMP are non-inferior 6 weeks after Dose-1 to those in subjects who received MMRV; and (3) to demonstrate that MMRVAMP induces acceptable measles, mumps, rubella, and VZV antibody response rates 6 weeks after Dose-1. Response rates were defined as follows:
Measles: percent of subjects with measles antibody concentration 255 mIU/mL 6 weeks after Dose-1 among subjects whose baseline concentration was (<255 mIU/mL)
Mumps: percent of subjects with mumps antibody concentration 10 mumps antibody units/mL 6 weeks after Dose-1 among subjects whose baseline concentration was <10 mumps antibody units/mL
Rubella: percent of subjects with rubella antibody concentration 10 IU/mL 6 weeks after Dose-1 among subjects whose baseline concentration was <10 IU/mL
VZV: percent of subjects with VZV antibody concentration 5 gpELISA units/mL 6 weeks after Dose-1 among subjects whose baseline concentration was <1.25 gpELISA units/mL
Non-inferiority for antibody response rate was defined as follows. For measles, mumps, and rubella, the lower bound of the 2-sided 95% confidence interval (CI) on the risk difference had to be >−5%. For VZV, the lower bound of the 2-sided 95% CI on the risk difference had to be >−10%.
Non-inferiority for GMCs was defined as follows. For each antigen, the lower bound of the 2-sided 95% CI for each GMC ratio (MMRVAMP/MMRV) had to be >0.67.
Acceptability of antibody response rates was defined as follows. For measles, mumps, and rubella, the lower bound of the 2-sided 95% CI for each antibody response rate had to be >90.0%. For VZV, the lower bound of the 2-sided 95% CI had to be >76.0%.
The seroconversion rate for VZV, defined as the percentage of subjects with baseline VZV concentration <1.25 gpELISA units/mL who have a concentration of ≥1.25 gpELISA units/mL after Dose-1, was an exploratory endpoint.
Safety
The primary safety objective was to demonstrate that the rate of fever (temperature ≥102.2·F [≥39.0·C] oral equivalent) Days 1 to 5 following Dose-1 of MMRVAMP is non-inferior to that following Dose-1 of MMRV. A secondary safety objective was to assess the overall safety and tolerability of MMRVAMP when administered to children 12 to 23 months of age.
Subjects were followed for 42 d following each dose. Parents/guardians recorded daily axillary temperatures and any injection-site or systemic adverse experiences (AEs) using a vaccination report card (VRC). All subjects were followed for serious AEs (SAEs) and febrile seizures (an event of special clinical interest which required it to be reported to the sponsor within 24 hours of the investigator being made aware of the event) from the time of enrollment through 180 d after Dose-2. At that time, a scripted questionnaire was used during a phone call to determine if any SAEs, medically-attended AEs, and new or worsening chronic medical conditions (not meeting the definition of SAE) had occurred since the 42-day safety follow-up period after Dose-2. Subjects were also instructed to call the study site immediately for an event that could potentially be an SAE at any time from the signing of the consent form to 180 d after Dose-2 of study vaccine.
Sponsor's role
This study was funded by Merck & Co., Inc. (sponsor). Although the sponsor formally reviewed a penultimate draft, the opinions expressed are those of the authorship and may not necessarily reflect those of the sponsor. All co-authors approved the final version of the manuscript.
Abbreviations
| AAP | = | American Academy of Pediatrics |
| ACIP | = | Advisory Committee on Immunization Practices |
| AE | = | Adverse experience |
| AMP | = | Alternative manufacturing process |
| CI | = | Confidence interval |
| ELISA | = | Enzyme-linked immunosorbant assay |
| GMC | = | Geometric mean concentration |
| gpELISA | = | Glycoprotein enzyme-linked immunosorbant assay |
| MMR | = | Measles: mumps: and rubella vaccine |
| MMRV | = | Measles: mumps: rubella: and varicella vaccine |
| Oka/Merck-VZV | = | Oka/Merck strain varicella-zoster virus |
| SAE | = | Serious adverse experience |
| US | = | United States |
| VAR | = | Varicella vaccine |
| VRC | = | Vaccination report card |
| VZV | = | Varicella-zoster virus |
Disclosure of potential conflicts of interest
Gary Marshall has been an investigator on clinical trials funded by Glaxo-SmithKline, Merck, Novartis, Pfizer, and Sanofi Pasteur. He also has received honoraria from these companies for service on advisory boards.
Gary Marshall, Shelly Senders, Julie Shepard, and Jerry Twiggs were investigators for the sponsor supported by research grants.
Julie Gardner, Darcy Hille, Jonathan Hartzel, Jon Stek, and Frans Helmond are employees of the sponsors and may hold stock and/or stock options from the sponsors.
Rowan Valenzuela is an employee of Covance which was contracted by the sponsor to conduct this study.
Author contributions
Gary Marshall, Shirley Senders, Julie Shepard, and Jerry Twiggs: enrollment of subjects and/or data collection, analysis and interpretation of data, and preparation of manuscript.
Darcy Hille, Rowan Valenzuela, Jon Stek, and Frans Helmond: analysis and interpretation of data, and preparation of manuscript.
Julie Gardner and Jonathan Hartzel: study concept and design, analysis and interpretation of data, and preparation of manuscript.
Acknowledgments
The authors would like to thank all the subjects who participated in this study and their parents or legal guardian; K. Beck (Merck & Co., Inc.) for her contributions to clinical trial operations; The Protocol 027 Study group: A. Acevedo, G. Adams, J. Alvey, W. Anderson, T. Benton, H. Bernstein, J. Calcagno, D. Cardona, K. Coverston, W. Daly, D. DeSantis, W. Douglas, R. Dracker, C. Duffy, M. Fernando, N. Forbush, E.R. Franklin, D. Freeman, A. Gabrielsen, A.G. Garscadden, U. Goswami, M.W. Halenkamp, B. Harvey, J. Hedrick, W. Hitchcock, J. Hoekstra, M. Husseman, A.J. Infante, A. Ituriaga, C. Jordan, R. Khaira, S. Khamis, R. Kratz, B. Lantry, T. Latiolas, M. Leonardi, M. Levinson, W. Lorentz, M.F. Drusano, E. Malacaman, S.R. Manson, C. Marchant, T.A. J. McAreavey, McKnight, K. McLelland, D. Mitchell, R. Mussleman, C.G. Nassim, B.W. Nauert, A. Naz, S. Owens, W. Parker, A. Pruitt, M.M. Rey, K. Rouse, R. Rupp, V. Sanchez-Bal, Z.G. Sanders, M. Schane, G. Schlichter, S. Shapiro, R. Sheikh, L.C. Sigg, P. Silas, C.K. Stratford, R. Strzinek, T. Sullivan, M. Tipton, M. Varman, L. Weiner, J. White, R. Yogev, M. Yudovich, A.M. Zomcik.
Funding
Funding for this research was provided by Merck & Co., Inc.
References
- Centers for Disease Control and Prevention. Recommended immunization schedules for persons aged 0 through 18 years—United States, 2015. http://www.cdc.gov/vaccines/schedules/downloads/child/0–18yrs-schedule.pdf (accessed January 25, 2016).
- Centers for Disease Control and Prevention (CDC). Use of combination measles, mumps, rubella, and varicella, vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2010; 59:1-15; PMID:20075837
- Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep 2011; 60(RR-2):1-61.
- U.S. Food an Drug Administration. M-M-R ®II (Measles, Mumps, and Rubella Virus Vaccine Live) [package insert]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM123789.pdf
- U.S. Food an Drug Administration. Varivax (Varicella Virus Vaccine Live) [package insert]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/UCM142812.pdf
- Bottiger M, Christenson B, Romanus V, Taranger J, Strandell A. Swedish experience of two dose vaccination programme aiming at eliminating measles, mumps, and rubella. BMJ 1987; 295:1264-7; PMID:3120971; http://dx.doi.org/10.1136/bmj.295.6608.1264
- Centers for Disease Control and Prevention. Summary of notifiable diseases, United States, 1998. MMWR Morb and Mortal Wkly Rep 1999; 47:1-93.
- Centers for Disease Control and Prevention. Measles. In: W Atkinson, C Wolfe, S Humiston, R Nelson, eds. Epidemiology and prevention of vaccine-preventable diseases. 6 ed. Atlanta (GA): Centers for Disease Control and Prevention, 2000:115-33.
- Nguyen HQ, Jumaan AO, Seward JF. Decline in mortality due to varicella after implementation of varicella vaccination in the United States. N Engl J Med 2005; 352:450-8; PMID:15689583; http://dx.doi.org/10.1056/NEJMoa042271
- Centers for Disease Control and Prevention. Varicella-related deaths, United States, January 2003 – June 2004. MMWR Morb and Mortal Wkly Rep 2005; 54:272-4.
- Seward JF, Watson BM, Peterson CL, Mascola L, Pelosi JW, Zhang JX, Maupin TJ, Goldman GS, Tabony LJ, Brodovicz KG, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA 2002; 287:606-11; PMID:11829699; http://dx.doi.org/10.1001/jama.287.5.606
- Maupin T, Civen R, Jumaan A, Xiao H, Seward J, Mascola L. Varicella outbreaks in an active surveillance site; Antelope Valley, CA, 1995–2003. Abstract presented at the 38th Annual National Immunization Conference, Nashville, TN. May 11–14, 2004.
- Clements DA, Zaref JI, Bland CL, Walter EB, Coplan PM. Partial uptake of varicella vaccine and the epidemiological effect on varicella disease in 11 day-care centers in North Carolina. Arch Pediatr Adolesc Med 2001; 155:455-61; PMID:11296072; http://dx.doi.org/10.1001/archpedi.155.4.455
- Vázquez M, LaRussa P, Gershon A, Steinberg SP, Freudigman K, Shaprio ED. The effectiveness of the varicella vaccine in clinical practice. N Engl J Med 2001; 344:955-60; http://dx.doi.org/10.1056/NEJM200103293441302
- Vázquez M, LaRussa P, Gershon A, Niccolai LM, Muehlenbein CE, Steinberg SP, Shapiro ED. Effectiveness over time of varicella vaccine. JAMA 2004; 291:851-5; http://dx.doi.org/10.1001/jama.291.7.851
- Clements D, Moreira S, Coplan P, Bland C, Walter E. Postlicensure study of varicella vaccine effectiveness in a day-care setting. Pediatr Infect Dis J 1999; 18:1047-50; PMID:10608622; http://dx.doi.org/10.1097/00006454-199912000-00004
- Hill HH, Elam-Evans LD, Yankey D, Singleton JA, Kolasa M, Centers for Disease Control and Prevention (CDC). National, state, and selected local area vaccination coverage among children aged 19–35 months - United States, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:889-96; PMID:26313470; http://dx.doi.org/10.15585/mmwr.mm6433a1
- American Academy of Pediatrics Committee on Infectious Diseases. Policy statement—Prevention of varicella: update of recommendations for use of quadrivalent and monovalent varicella vaccines in children. Pediatrics 2011; 128:630-2; PMID:21873692; http://dx.doi.org/10.1542/peds.2011-1968
- Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006; 25:507-12; PMID:16732148; http://dx.doi.org/10.1097/01.inf.0000222413.47344.23
- Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007; 26:496-500; PMID:17529866; http://dx.doi.org/10.1097/INF.0b013e31805d7f17
- Jacobsen SJ, Ackerson BK, Sy LS, Tran TN, Jones TL, Yao JF, Xie F, Cheetham TC, Saddier P. Observational safety study of febrile convulsion following first dose MMRV vaccination in a managed care setting. Vaccine 2009; 27:4656-61; PMID:19520201; http://dx.doi.org/10.1016/j.vaccine.2009.05.056
- Klein NP, Fireman B, Yih WK, Lewis E, Kulldorff M, Ray P, Baxter R, Hambidge S, Nordin J, Naleway A, et al. Measles-mumps-rubella-varicella combination vaccine and the risk of febrile seizures. Pediatrics 2010; 126:e1-8; PMID:20587679; http://dx.doi.org/10.1542/peds.2010-0665
- Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia 1994; 35:S1-S6; PMID:8275976; http://dx.doi.org/10.1111/j.1528-1157.1994.tb05932.x
- Pavlovic MV, Jarebinski MS, Pekmezovic TD, Marjanovic BD, Levic ZM. Febrile convulsions in a Serbian region: a 10-year epidemiological study. Eur J Neurol 1999; 6:39-42; PMID:10209348; http://dx.doi.org/10.1046/j.1468-1331.1999.610039.x
- Forsgren L, Sidenvall R, Blomquist HKS, Heijbel J. A prospective incidence study of febrile convulsions. Acta Paediat Scand 1990; 79:550-7; PMID:2386045; http://dx.doi.org/10.1111/j.1651-2227.1990.tb11510.x
- Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia 1975; 16:1-66; PMID:804401; http://dx.doi.org/10.1111/j.1528-1157.1975.tb04721.x
- Verburgh ME, Bruijnzeels MA, van der Wouden JC, van Suijlekom-Smit LWA, van der Velden J, Hoes AW, Offringa M. Incidence of febrile seizures in The Netherlands. Neuroepidemiology 1992; 11:169-72; PMID:1291879; http://dx.doi.org/10.1159/000110928
- Vestergaard M, Hviid A, Madsen K, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA 2004; 292(3):351-7; PMID:15265850; http://dx.doi.org/10.1001/jama.292.3.351
- van den Berg BJ, Yerushalmy J. Studies on convulsive disorders in young children. I. Incidence of febrile and nonfebrile convulsions by age andother factors. Pediatr Res 1969; 3:298-304; PMID:5807059.
- Reef SE, Cochi SL. The evidence for the elimination of rubella and congenital rubella syndrome in the United States: a public health achievement. Clin Infect Dis 2006; 43:S123-5; PMID:16998770; http://dx.doi.org/10.1086/505943
- Katz SL, Hinman AR. Summary and conclusions: measles elimination meeting, 16–17 March 2000. J Infect Dis 2004; 189:S43-7; PMID:15106088; http://dx.doi.org/10.1086/377696
- Clemmons NS, Gastanaduy PA, Fiebelkorn AP, Redd SB, Wallace GS. Measles—United States, January 4-April 2, 2015. MMWR Morb Mortal Wkly Rep 2015; 64:373-6; PMID:25879894.
- Adams DA, Jajosky RA, Ajani U, Kriseman J, Sharp P, Onwen DH, Schley AW, Anderson WJ, Grigoryan A, Aranas AE, et al. Summary of notifiable diseases—United States, 2012. MMWR Morb Mortal Wkly Rep 2014; 61:1-121; PMID:25233134.
- Shinefield H, Black S, Digilio L, Reisinger K, Blatter M, Gress JO, Brown ML, Eves KA, Klopfer SO, Schödel F, et al. Evaluation of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J 2005; 24:665-9; PMID:16094217; http://dx.doi.org/10.1097/01.inf.0000172902.25009.a1
- Shinefield H, Black S, Williams W, Marchant C, Reisinger K, Stewart T, Meissner HC, Guerrero J, Klopfer SO, Xu J, et al. Dose-response of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children. Pediatr Infect Dis J 2005; 24:670-5; PMID:16094218; http://dx.doi.org/10.1097/01.inf.0000172901.29621.e9
- Lieberman JM, Williams WR, Miller JM, Black S, Shinefield H, Henderson F, Marchant CD, Werzberger A, Halperin S, Hartzel J, et al. The safety and immunogenicity of a quadrivalent measles, mumps, rubella and varicella vaccine in healthy children: a study of manufacturing consistency and persistence of antibody. Pediatr Infect Dis J 2006; 25:615-22; PMID:16804432; http://dx.doi.org/10.1097/01.inf.0000220209.35074.0b
- Shinefield H, Black S, Thear M, Coury D, Reisinger K, Rothstein E, Xu J, Hartzel J, Evans B, Digilio L, et al. Safety and immunogenicity of a measles, mumps, rubella and varicella vaccine given with combined Haemophilus influenzae type b conjugate/hepatitis B vaccines and combined diphtheria-tetanus-acellular pertussis vaccines. Pediatr Infect Dis J 2006; 25:287-92; PMID:16567978; http://dx.doi.org/10.1097/01.inf.0000207857.10947.1f
- Bernstein HH, Eves K, Campbell K, Black SB, Twiggs JD, Reisinger KS, Conti RM, Flodmark CE, Rombo L, Klopfer S, et al. Comparison of the safety and immunogenicity of a refrigerator-stable versus a frozen formulation of ProQuad (measles, mumps, rubella, and varicella virus vaccine live). Pediatrics 2007; 119:e1299-305; PMID:17502347; http://dx.doi.org/10.1542/peds.2006-2283
- Keller PM, Lonengan K, Neff BJ, Morton DA, Ellis RW. Purification of individual varicella-zoster virus (VZV) glycoprotein gpI, gpII, and gpIII and their use in ELISA for detection of VZV glycoprotein-specific antibodies. J Virol Methods 1986; 14:177-88; PMID:3021804; http://dx.doi.org/10.1016/0166-0934(86)90048-0
- Wasmuth EH, Miller WJ. Sensitive enzyme-linked immunosorbent assay for antibody to varicella-zoster virus using purified VZV glycoprotein antigen. J Med Virol 1990; 32:189-93; PMID:2177782; http://dx.doi.org/10.1002/jmv.1890320310
- Provost PJ, Krah DL, Kuter BJ, Morton DH, Schofield TL, Wasmuth EH, White CJ, Miller WJ, Ellis RW. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine 1991; 9:111-6; PMID:1647574; http://dx.doi.org/10.1016/0264-410X(91)90266-9