ABSTRACT
We previously reported an increased frequency of antibodies to hypocretin (HCRT) receptor 2 in sera obtained from narcoleptic patients who received the European AS03-adjuvanted vaccine Pandemrix (GlaxoSmithKline Biologicals, s.a.) for the global influenza A H1N1 pandemic in 2009 [A(H1N1)pdm09]. These antibodies cross-reacted with a particular fragment of influenza nucleoprotein (NP) – one of the proteins naturally contained in the virus used to make seasonal influenza vaccine and pandemic influenza vaccines. The purpose of this commentary is to provide additional insights and interpretations of the findings and share additional data not presented in the original paper to help the reader appreciate the key messages of that publication. First, a brief background to narcolepsy and vaccine-induced narcolepsy will be provided. Then, additional insights and clarification will be provided on the following topics: 1) the critical difference identified in the adjuvanted A(H1N1)pdm09 vaccines, 2) the contributing factor likely for the discordant association of narcolepsy between the AS03-adjuvanted pandemic vaccines Pandemrix and Arepanrix (GlaxoSmithKline Biologicals, s.a.), 3) the significance of detecting HCRT receptor 2 (HCRTr2) antibodies in some Finnish control subjects, 4) the approach used for the detection of HCRTr2 antibodies in vaccine-associated narcolepsy, and 5) the plausibility of the proposed mechanism involving HCRTr2 modulation in vaccine-associated narcolepsy.
Introduction
Our recent publicationCitation1 identified an increased frequency of antibodies to hypocretin (HCRT) receptor 2 in sera obtained from narcoleptic patients who received the European AS03-adjuvanted vaccine Pandemrix (GlaxoSmithKline Biologicals, s.a., Dresden, Germany) for the global influenza A H1N1 pandemic in 2009 [A(H1N1)pdm09]. These antibodies cross-reacted with a particular fragment of influenza nucleoprotein (NP) – one of the proteins naturally contained in the virus used to make seasonal influenza vaccine and pandemic influenza vaccines. Mass spectrometry analysis of seasonal influenza vaccines detected the lowest amounts of nucleoprotein in the subunit vaccine Agrippal (Novartis Vaccines and Diagnostics S.r.l., Rosia, Italy) and the MF-59 adjuvanted subunit vaccine Chiromas (Novartis Vaccines and Diagnostics S.r.l., Rosia, Italy). Mass spectrometry analysis of adjuvanted pandemic influenza vaccines used during A(H1N1)pdm09 demonstrated greater quantities of NP in Pandemrix compared to the Canadian AS03-adjuvanted vaccine Arepanrix (GlaxoSmithKline Biologicals, Quebec, Canada), in agreement with two other studies.Citation2,3 Only trace quantities of NP were detected in the adjuvanted pandemic influenza vaccine Focetria (Novartis Vaccines & Diagnostics, S.r.l., Rosia, Italy), and this was similar to the trace amounts detected in the seasonal influenza vaccines Agrippal and Chiromas that are manufactured using similar subunit vaccine purification steps as Focetria. An analysis of the immune response in non-influenza-exposed subjects immunized with Focetria in 2009 demonstrated that the trace amounts of NP in Focetria would not elicit durable NP antibody responses necessary for subsequent cross-reactivity to hypocretin receptor 2 (HCRTr2).
The purpose of this commentary is to provide additional insights and interpretations of the findings and share additional data not presented in the original paper to help the reader appreciate the key messages of that publication.Citation1 First, a brief background to narcolepsy and vaccine-induced narcolepsy will be provided to provide context for the subsequent discussions.
Narcolepsy
Narcolepsy is a chronic disorder presenting with excessive daytime sleepiness and, its variant with cataplexy, referred to as type 1 narcolepsy (T1N), is tightly associated with HLA-DQB1*0602.Citation4 Investigations in patients with narcolepsy-cataplexy have revealed that the neuropeptide HCRT,Citation5 also known by the term orexin,Citation6 is deficient in the cerebrospinal fluid in the majority of these patients. In the few postmortem brain studies conducted, investigations have identified a loss of hypothalamic cells.Citation7,8 To date, the exact mechanism culminating in the loss of HCRT-producing cell bodies of the perifornical lateral hypothalamus in narcoleptic patients remains unclear.
Dogs with hereditary narcolepsy have a mutation in the HCRTr2 gene.Citation9 Murine models recapitulating the human narcolepsy phenotype have been generated by loss of function of the HCRTr2Citation10 or the preprohypocretin geneCitation11 and also with genetic ablation of HCRT neurons.Citation12 These findings related to the HCRTr2 in canine and murine narcolepsy may be important clues to understanding human narcolepsy.
In humans, another idiopathic sleep disorder termed excessive daytime sleepiness has been linked by single-stranded conformational polymorphism analysis to an HCRTr2 coding variant resulting in a Pro11Thr change that impaired HCRT ligand binding-related signaling.Citation13 These findings suggest that alterations in HCRT ligand or its receptors can disrupt neurotransmission and may play key roles in sleep-related disorders, including narcolepsy.
Vaccine-induced narcolepsy
More than 1300 cases of vaccine-associated narcolepsy have been spontaneously reported to the European Medicines Agency (EMA) Eudravigilance databaseCitation14 as of January 2015 associated with an AS03-adjuvanted influenza vaccine manufactured in Europe that was distributed to more than 30.5 million people in European Union/European Economic Area (EU/EEA countries) during A(H1N1)pdm09.Citation15 In 2012, observational studies in Finland and the combined counties of Sweden reported an association between narcolepsy and vaccination with a European A(H1N1)pdm09 vaccine adjuvanted with AS03, an oil-in-water based emulsion.Citation16
Larger epidemiologic studies in Ireland in 2012, England in 2013, Norway in 2013, and France in 2013 confirmed the association of narcolepsy in children and adolescents receiving the European AS03-adjuvanted pandemic influenza vaccine.Citation16 A retrospective study from England in 2014 examining 75 children developing narcolepsy between 2008 and 2011 demonstrated disease development after seasonal influenza vaccine (without adjuvant), after European AS03-adjuvanted pandemic influenza vaccination, and also with influenza illness.Citation17 A recently published case-coverage study from England has also demonstrated a significantly increased risk of narcolepsy in adults following administration of the European AS03-adjuvanted pandemic influenza vaccine but the risk was lower than that seen in children using a similar study design.Citation18
To date, no increased-risk has been reported for the MF59-adjuvanted A(H1N1)pdm09 vaccine, for which an estimated 6.5 million doses were distributed in EU/EEA and 25 million doses were used in Europe and Latin America.Citation19 In Canada, an estimated 12 million doses were administered of another AS03-adjuvanted pandemic vaccine, Arepanrix,Citation20 and a lower association with narcolepsyCitation21 was observed with it compared to the European AS03-adjuvanted A(H1N1)pdm09 vaccine Pandemrix, used in Europe. These observations support the concept that vaccine-associated narcolepsy is not due solely to the characteristics of the adjuvant. The following sections will now provide additional clarifications/insights related to the published study and assumes that the reader has reviewed that study.Citation1
Could differences in the adjuvanted A(H1N1)pdm09 vaccines explain the discordant development of vaccine-associated narcolepsy?
Indeed, in the original publication,Citation1 we identified two differences in the MF59-adjuvanted pandemic vaccine and AS03-adjuvanted pandemic vaccines: 1) a mutation in the nucleoprotein mimic sequence contained in the respective vaccine reassortant strains X-181 and X-179a and 2) the total amount of nucleoprotein in the final vaccine products.
In order to identify the mimic sequence, we used a bioinformatics approach comparing vaccine influenza proteins to all members of the HCRT family (including HCRT receptors) because preclinical models of narcolepsyCitation9,10,22 and human sleep disorders,Citation13 have identified a role for the HCRT receptors in sleep dysregulation. These seminal studies by Lin/Mignot and colleagues,Citation9 Willie and colleagues,Citation10 Hungs/Mignot and colleagues,Citation22 and Thompson and colleaguesCitation13 helped us to avoid the bias of focusing only on HCRT. We thus identified a sequence from influenza nucleoprotein, “YDKEEIRRIWR,” that was similar (e-value = 0.0061) to a sequence from the first extracellular domain (N-terminus) of HCRTr2, “YDDEEFLRYLWR.” Nine out of 11 amino acids were identical or structurally similar resulting in a 75–82% resemblance. Cross-reactivity was subsequently confirmed with two different platforms, enzyme-linked immunosorbent assay (ELISA) and microscopy, using peptide competition assays (based on the isoleucine and methionine variants of NP, a scramble peptide of the NP variants, and a recombinant peptide to the first external domain of human HCRTr2) (please see Fig. 3 in ref. 1). Of interest was that the NP mimic contained in the wildtype influenza virus and in the X-179a vaccine reassortant strain contained an isoleucine at position 116 and this was different from the X-181 vaccine reassortant that contained a methionine substitution at position 116.
However, vaccine strains can mutate when growing in eggs, and both NP peptide variants (containing either isoleucine or methionine at position 116) could potentially be present in all influenza vaccines regardless of the initial vaccine reassortant strain used. This was illustrated in the original reportCitation23 by the identification of both NP peptide populations in the NP sequence trace from the high-yielding donor strain X-157 (please see Fig. S13 in ref. 1) used to generate the vaccine reassortants X-179a and X-181 used for the A(H1N1)pdm09 vaccines. The sequence trace illustrates that the NP peptide populations containing either isoleucine or methionine residues actually reflect a single nucleotide mutation, adenine to guanine, in the underlined wobble position (isoleucine = ATA while methionine = ATG, respectively). These observations would also explain why other influenza reassortant lineages generated from X-157 alternate in their content of either the isoleucine variant or the methionine variant of NP (Figs. S11 and S12Citation1).
Therefore, the difference in the MF59-adjuvanted A(H1N1)pdm09 vaccine and the European AS03-adjuvanted A(H1N1)pdm09 vaccine explaining the discordant development of vaccine-associated narcolepsy may be the total amount of nucleoprotein. Our observations with mass spectrometry at Stanford demonstrated that the lowest amount of NP was found in the subunit-purified vaccine when compared to other split-virion and subunit-purified vaccines (please see Table 2 in ref. 1). The similar blocking of serum antibody-binding to HCRTr2 expressed on the cell membrane by the isoleucine and methionine variants of the NP peptide suggested that this one residue difference in these variants may not be contributing to any difference in antibody cross-reactivity in vaccine-associated narcolepsy. However, these NP peptide variants differentially bound (Table S3Citation1) to the major histocompatibility complex (MHC) allele product tightly associated with narcolepsy development (HLA-DQB1*0602). This differential binding could contribute to differences in T helper activity in antibody generation.Citation24 Thus, the abovementioned differences in total amount of influenza nucleoprotein contained in AS03-adjuvanted A(H1N1)pdm09 vaccines compared to the MF59-adjuvanted A(H1N1)pdm09 vaccine would translate into differences in total amount of NP peptides. Therefore, the AS03-adjuvanted A(H1N1)pdm09 vaccines would have sufficient amounts of NP peptides available for MHC-binding and subsequent immune response generation in the setting of adjuvant.
In Canada, why was the AS03-adjuvanted pandemic vaccine20 associated with a lower risk of narcolepsy?
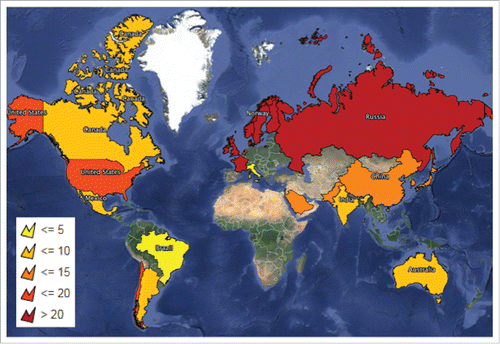
We believe that the low NP content in the vaccine explains the lack of disease development with the MF59-adjuvanted A(H1N1)pdm09 vaccine while the low projected population coverage for HLA DQB1*06:02 in Canada (5.60 % based on linkage disequilibrium for DR-DQ) explains the lower incidence of narcolepsy in Canada with the Canadian AS03-adjuvanted A(H1N1)pdm09 vaccine compared to the European AS03-adjuvanted A(H1N1)pdm09 vaccine. is a world heat map indicating the projected population coverage for HLA-DQB1*06:02 in the various countries. It is worth noting that the countries reporting vaccine-associated narcolepsy with the European AS03-adjuvanted A(H1N1)pdm09 vaccine also have a greater percentage of the population carrying the narcolepsy risk-allele, HLA-DQB1*06:02, compared to countries not reporting A(H1N1)pdm09 vaccine-associated narcolepsy. Canada does not have a high percentage of the population carrying the narcolepsy risk-allele and would explain why the risk of narcolepsy was lower with the Canadian AS03-adjuvanted A(H1N1)pdm09 vaccine.
Figure 1. World heat map shading in light yellow the countries with the lowest projected population coverage for HLA-DQB1*0602 (less than or equal to 5%) and progressively getting darker with dark red indicating the countries with the highest projected population coverage for HLA-DQB1*0602 (greater than 20%). Map produced using Mapline (https://mapline.com/).
Why were some Finnish control subjects' sera from 2004/2005 positive for HCRTr2 antibodies?
Indeed we detected the presence of autoantibodies from some of the Finnish children not known to have narcolepsy in 2004/2005 (whose HLA haplotype was unknown) in the one serum sample timepoint that was available for testing. Since follow-up sera were not available on these subjects whose records were anonymous (for protection of privacy), the detection of HCRTr2 antibodies might reflect, the first steps to disease developmentCitation17 in some individuals likely to be carrying the narcolepsy susceptibility allele. This likelihood is high given that the projected coverage for the HLA-DQB1*0602 allele in Finland is 31.3% of the population.Citation25 Therefore, the detection of HCRTr2 antibodies in these normal-appearing Finnish subjects does not diminish their potential importance in pathogenesis for narcolepsy.
Why were antibodies to HCRTr2 detected with higher frequency in vaccine-associated narcolepsy compared to older studies on primary narcolepsy?
Tanaka and colleagues detected autoantibodies in 5% of narcoleptic patients (HCRTr2>HCRT) compared to 3% of controls when analyzing sera obtained decades after disease onset using a radioligand-based assay in 2006.Citation26 In addition to the long interval from time of disease onset for the sera tested, Tanaka and colleagues suggested that their cell-free system might have prevented the correct conformational presentation of the receptors necessary for antibody detection. Indeed, G-protein coupled receptors (GPCRs) like HCRTr2 are integral membrane proteins and are innately unstable when purified out of a cell's stabilizing membrane, and this limitation precludes them from a wide range of structural and biophysical techniques that are used for soluble proteins.Citation27,28 For example, in order to obtain crystal structures of agonists of GPCRs, the GPCR must be stabilized, and this has been achieved through a variety of techniques.Citation29 Our approach therefore selected an assay system that would not only detect binding but would also permit the fluidity of GPCRs as they are anchored in a cell membrane.
We chose to do our analysis on sera from vaccine-associated narcolepsy using a cell line in order to preserve the correct conformation and fluidity of the receptor in the cell membrane. This difference in assay technique may explain the higher detection of HCRTr2 antibodies in our investigationsCitation1 using an in-cell ELISA assay. Furthermore, instead of using an arbitrarily assigned cutoff for positivity, we crosschecked with two more independent methods. First, positive signals detected by ELISA were corroborated with immunofluorescence microscopy for punctate staining typical for GPCRs.Citation30 Second, we additionally performed co-localization studies using a commercial antibody to the first extracellular domain of human HCRTr2. As mentioned in our publication,Citation1 the use of cell-based assays has similarly enabled the detection of acetylcholine receptor antibodies in 60% of patients with myasthenia gravis that were previously considered negative by radioimmunoprecipitation assay.Citation31 Following-up from the earlier studies by Tanaka and colleagues, our results emphasize the potential importance of the conformation of the HCRT receptor for serum antibody detection.
Is the proposed mechanism of HCRTr2 modulation in vaccine-associated narcolepsy plausible?
Despite the >99 % association of HLA-DQB1*06:02 with narcolepsy-cataplexy associated with low cerebrospinal fluid (CSF) hypocretin-1, narcolepsy with low CSF hypocretin-1 can also occur in subjects not positive for HLA-DQB1*06:02.Citation32 Furthermore, a recent case report describes a patient with isolated cataplexy in the context of two non-diagnostic multiple sleep latency tests and normal CSF-hypocretin-1 levels (>217 pg/mL).Citation33 This individual gradually developed excessive daytime sleepiness and low CSF-hypocretin-1 (<110 pg/mL).Citation33 These findings might be considered in context with data in the previously mentioned canine models of narcolepsy with HCRTr2 dysregulation (but normal CSF hypocretin-122 and in context with a report of polymorphic HCRTr2 coding variant being associated with impaired HCRT ligand binding and aberrant signaling in patients with “excessive daytime sleepiness.”Citation13
All these anomalies suggest that sleep dysregulation in narcolepsy may be multifactorial and not always due to isolated deficiency of HCRT. As recently demonstrated in a non-autoimmune mouse model of narcolepsy-like disease triggered by intranasal challenge with H1N1 influenza virus,Citation34 other mechanisms could be involved in primary narcolepsy including cellular dysfunction resulting from viral proteins such as NP that accumulates in dendritic spines to reduce excitatory synaptic activity.Citation35
Therefore, our previous reportCitation1 suggest that vaccine-associated narcolepsy may be reflecting a spectrum of immune-mediated disease development starting with modulation of HCRTr2 signaling and then culminating in loss of HCRT-producing neurons. Indeed, modulation of HCRTr2 signaling has been demonstrated in four seminal studies of the HCRT ligand and receptor as described below.
Lin and colleaguesCitation9 in 1999 indicated that they determined that canine narcolepsy is caused by disruption of the HCRT (orexin) receptor 2 gene, that these changes most likely disrupt proper membrane localization and/or cause loss of function of this strongly evolutionarily conserved protein, and that an autoimmune process directed against the HCRTr2 or more complex neuroimmune interactions may be involved in the pathophysiology of human narcolepsy. Nishino and colleaguesCitation7 in 2000 identified two subjects with an unquestionable diagnosis of narcolepsy-cataplexy that had similar (255 pg/mL) and elevated (638 pg/mL) levels of HCRT compared to controls, and suggested the possibility of receptor/effector-mediated deficiency (as opposed to a defect in hypocretin production).
Thannickal and colleaguesCitation36 reported in 2003 that the correlation between percentage loss of axons and percentage increase in gliosis and the [mRNA] message density for HCRTr2 suggests that this receptor or antigens linked to it may be associated with processes that intensify the pathological process in narcolepsy. Specifically, they suggested that the loss of HCRT function in narcolepsy results from a cytotoxic or immunologically-mediated attack focused on HCRTr2 or an antigen anatomically linked to HCRTr2. These investigators indicated that the striking similarity of the symptoms and pharmacology of narcolepsy in HCRTr2 mutant animals to those in HCRT-deficient humansCitation37 suggests that normal HCRTr2 operation is necessary for adequate regulation of HCRT release at terminal sites.
Finally, Wu and colleaguesCitation38 in 2011 concluded that, HCRTr2 is a vital element in a feedback loop integrating HCRT, acetylcholine, and norepinephrine function. In the absence of functional HCRTr2 [in canine narcolepsy], HCRT levels were not affected by monoaminergic and cholinergic drugs [prazosin, physostigmine, methamphetamine, labetalol, and phenylephrine], despite the strong modulation of cataplexy by these drugs. Conversely, strong transient reductions of HCRT levels by these drugs did not produce episodes of cataplexy in normal dogs suggesting that HCRTr2 mutation caused drug-induced cataplexy by virtue of its long-term effect on the functioning of other brain systems, rather than by increasing the magnitude of phasic changes in HCRT level.
The crucial HCRT receptors for this feedback suggested by Wu and colleaguesCitation38 may be on HCRT neurons, and a recent in vitro studyCitation39 demonstrated that such autoreceptors exists and are excitatory. Alternatively, or in addition, crucial receptors may be on non-HCRT cells, which produce an excitatory feedback excitation of HCRT cells.Citation40 The absence of this feedback could alter the morphology of neurons in this circuit resulting in sleep disturbances characteristic of narcolepsy.
Conclusion
The complexity of this disease process is why our original investigationCitation1 assembled an international team of 20 experts spanning vaccine discovery and development, informatics, formulations, in vitro cell biology, biologics (antibody) profiling, quantitative sciences, assay development, epidemiology, neurosciences, immunology, and mass spectrometry. The diversity of expertise provided a system of crosschecks for the previously published scientific literature and evolved into an integrated approach. This helped us to avoid the pitfall of “re-inventing the wheel” for the various assays and analyses used in our studies and enabled us to build on the important scientific clues revealed by earlier investigations (e.g., Tanaka and colleaguesCitation26 and the potential importance of the HCRT receptor conformation). More lessons are sure to be learned from ongoing studies in narcolepsy and vaccine-associated narcolepsy.
Abbreviations
| HCRT | = | hypocretin |
| A(H1N1)pdm09 | = | global influenza A H1N1 pandemic in 2009 |
| NP | = | influenza nucleoprotein |
| HCRTr2 | = | hypocretin receptor 2 |
| T1N | = | type 1 narcolepsy |
| EMA | = | European Medicine Agency |
| EU/EAA | = | European Union/European Economic Area |
| ELISA | = | enzyme-linked immunosorbent assay |
| MHC | = | major histocompatibility complex |
| GPCRs | = | G-protein coupled receptors |
| CSF | = | cerebrospinal fluid |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Ahmed SS, Volkmuth W, Duca J, Corti L, Pallaoro M, Pezzicoli A, Karle A, Rigat F, Rappuoli R, Narasimhan V, et al. Antibodies to influenza nucleoprotein cross-react with human hypocretin receptor 2. Sci Transl Med 2015; 7:294ra105; PMID:26136476
- Jacob L, Leib R, Ollila HM, Bonvalet M, Adams CM, Mignot E. Comparison of Pandemrix and Arepanrix, two pH1N1 AS03-adjuvanted vaccines differentially associated with narcolepsy development. Brain Behav Immun 2015; 47:44-57; PMID:25452148
- Vaarala O, Vuorela A, Partinen M, Baumann M, Freitag TL, Meri S, Saavalainen P, Jauhiainen M, Soliymani R, Kirjavainen T, et al. Antigenic Differences between AS03 Adjuvanted Influenza A (H1N1) Pandemic Vaccines: Implications for Pandemrix-Associated Narcolepsy Risk. PLoS One 2014; 9:e114361; PMID:25501681; http://dx.doi.org/10.1371/journal.pone.0114361
- van der Heide A, Verduijn W, Haasnoot GW, Drabbels JJ, Lammers GJ, Claas FH. HLA dosage effect in narcolepsy with cataplexy. Immunogenetics 2015; 67:1-6; PMID:25277311; http://dx.doi.org/10.1007/s00251-014-0808-z
- de LL, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 1998; 95:322-7; PMID:9419374; http://dx.doi.org/10.1073/pnas.95.1.322
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998; 92:573-85; PMID:9491897; http://dx.doi.org/10.1016/S0092-8674(00)80949-6
- Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet 2000; 355:39-40; PMID:10615891; http://dx.doi.org/10.1016/S0140-6736(99)05582-8
- Peyron C, Faraco J, Rogers W, Ripley B, Overeem S, Charnay Y, Nevsimalova S, Aldrich M, Reynolds D, Albin R, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 2000; 6:991-7; PMID:10973318; http://dx.doi.org/10.1038/79690
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, de Jong PJ, Nishino S, Mignot E. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 1999; 98:365-76; PMID:10458611; http://dx.doi.org/10.1016/S0092-8674(00)81965-0
- Willie JT, Chemelli RM, Sinton CM, Tokita S, Williams SC, Kisanuki YY, Marcus JN, Lee C, Elmquist JK, Kohlmeier KA, et al. Distinct narcolepsy syndromes in Orexin receptor-2 and Orexin null mice: molecular genetic dissection of Non-REM and REM sleep regulatory processes. Neuron 2003; 38:715-30; PMID:12797957; http://dx.doi.org/10.1016/S0896-6273(03)00330-1
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, et al. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 1999; 98:437-51; PMID:10481909; http://dx.doi.org/10.1016/S0092-8674(00)81973-X
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001; 30:345-54; PMID:11394998; http://dx.doi.org/10.1016/S0896-6273(01)00293-8
- Thompson MD, Comings DE, Abu-Ghazalah R, Jereseh Y, Lin L, Wade J, Sakurai T, Tokita S, Yoshida T, Tanaka H, et al. Variants of the orexin2/hcrt2 receptor gene identified in patients with excessive daytime sleepiness and patients with Tourette's syndrome comorbidity. Am J Med Genet B Neuropsychiatr Genet 2004; 129B:69-75; PMID:15274044; http://dx.doi.org/10.1002/ajmg.b.30047
- EudraVigilance management system (DBMS). European Medicines Agency, 2015
- Johansen K. The roles of influenza virus antigens and the AS03 adjuvant in the 2009 pandemic vaccine associated with narcolepsy needs further investigation. Dev Med Child Neurol 2014; 56:1041-2; PMID:25052448; http://dx.doi.org/10.1111/dmcn.12543
- Ahmed SS, Schur PH, MacDonald NE, Steinman L. Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants. J Autoimmun 2014; 50:1-11; PMID:24559657; http://dx.doi.org/10.1016/j.jaut.2014.01.033
- Winstone AM, Stellitano L, Verity C, Andrews N, Miller E, Stowe J, Shneerson J. Clinical features of narcolepsy in children vaccinated with AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine in England. Dev Med Child Neurol 2014; 56:1117-23; PMID:25041214; http://dx.doi.org/10.1111/dmcn.12522
- Stowe J, Miller E, Andrews N, Kosky C, Leschziner G, Shneerson JM, Hall A, Eriksson S, Reading P, Dennis G, et al. Risk of Narcolepsy after AS03 Adjuvanted Pandemic A/H1N1 2009 Influenza Vaccine in Adults: A Case-Coverage Study in England. Sleep 2016; [Epub ahead of print] PMID:26856903
- Tsai TF. MF59 adjuvanted seasonal and pandemic influenza vaccines. Yakugaku Zasshi 2011; 131:1733-41; PMID:22129867; http://dx.doi.org/10.1248/yakushi.131.1733
- European Center for Disease P, Control. Enhanced Monitoring of Vaccine Safety for 2009 Pandemic Vaccines. 2013.
- Montplaisir J, Petit D, Quinn MJ, Ouakki M, Deceuninck G, Desautels A, Mignot E, De WP. Risk of narcolepsy associated with inactivated adjuvanted (AS03) A/H1N1 (2009) pandemic influenza vaccine in Quebec. PLoS One 2014; 9:e108489; PMID:25264897; http://dx.doi.org/10.1371/journal.pone.0108489
- Hungs M, Fan J, Lin L, Lin X, Maki RA, Mignot E. Identification and functional analysis of mutations in the hypocretin (orexin) genes of narcoleptic canines. Genome Res 2001; 11:531-9; PMID:11282968; http://dx.doi.org/10.1101/gr.GR-1610R
- Ahmed SS, Plotkin SA, Black S, Coffman RL. Assessing the safety of adjuvanted vaccines. Sci Transl Med 2011; 3:93rv2; PMID:21795590
- Hampe CS. B Cell in Autoimmune Diseases. Scientifica (Cairo) 2012; 2012:1-18; http://dx.doi.org/10.6064/2012/215308
- Bui HH, Sidney J, Dinh K, Southwood S, Newman MJ, Sette A. Predicting population coverage of T-cell epitope-based diagnostics and vaccines. BMC Bioinformatics 2006; 7:153; PMID:16545123; http://dx.doi.org/10.1186/1471-2105-7-153
- Tanaka S, Honda Y, Inoue Y, Honda M. Detection of autoantibodies against hypocretin, hcrtrl, and hcrtr2 in narcolepsy: anti-Hcrt system antibody in narcolepsy. Sleep 2006; 29:633-8; PMID:16774153
- Robertson N, Jazayeri A, Errey J, Baig A, Hurrell E, Zhukov A, Langmead CJ, Weir M, Marshall FH. The properties of thermostabilised G protein-coupled receptors (StaRs) and their use in drug discovery. Neuropharmacology 2011; 60:36-44; PMID:20624408; http://dx.doi.org/10.1016/j.neuropharm.2010.07.001
- de Hoog HP, Lin JieRong EM, Banerjee S, Decaillot FM, Nallani M. Conformational antibody binding to a native, cell-free expressed GPCR in block copolymer membranes. PLoS One 2014; 9:e110847; PMID:25329156; http://dx.doi.org/10.1371/journal.pone.0110847
- Ring AM, Manglik A, Kruse AC, Enos MD, Weis WI, Garcia KC, Kobilka BK. Adrenaline-activated structure of beta2-adrenoceptor stabilized by an engineered nanobody. Nature 2013; 502:575-9; PMID:24056936; http://dx.doi.org/10.1038/nature12572
- Hislop JN, von Zastrow M. Analysis of GPCR localization and trafficking. Methods Mol Biol 2011; 746:425-40; PMID:21607873; http://dx.doi.org/10.1007/978-1-61779-126-0_25
- Vincent A, Waters P, Leite MI, Jacobson L, Koneczny I, Cossins J, Beeson D. Antibodies identified by cell-based assays in myasthenia gravis and associated diseases. Ann NY Acad Sci 2012; 1274:92-8; PMID:23252902; http://dx.doi.org/10.1111/j.1749-6632.2012.06789.x
- Han F, Lin L, Schormair B, Pizza F, Plazzi G, Ollila HM, Nevsimalova S, Jennum P, Knudsen S, Winkelmann J, et al. HLA DQB1*06:02 negative narcolepsy with hypocretin/orexin deficiency. Sleep 2014; 37:1601-8; PMID:25197808
- Drakatos P, Leschziner G. Cataplexy with Normal Sleep Studies and Normal CSF Hypocretin - an Explanation? J Clin Sleep Med 2015; 12(3):449-50
- Tesoriero C, Codita A, Zhang MD, Cherninsky A, Karlsson H, Grassi-Zucconi G, Bertini G, Harkany T, Ljungberg K, Liljestrom P, et al. H1N1 influenza virus induces narcolepsy-like sleep disruption and targets sleep-wake regulatory neurons in mice. Proc Natl Acad Sci U S A 2016; 113:E368-77; PMID:26668381; http://dx.doi.org/10.1073/pnas.1521463112
- Brask J, Chauhan A, Hill RH, Ljunggren HG, Kristensson K. Effects on synaptic activity in cultured hippocampal neurons by influenza A viral proteins. J Neurovirol 2005; 11:395-402; PMID:16162482; http://dx.doi.org/10.1080/13550280500186916
- Thannickal TC, Siegel JM, Nienhuis R, Moore RY. Pattern of hypocretin (orexin) soma and axon loss, and gliosis, in human narcolepsy. Brain Pathol 2003; 13:340-51; PMID:12946023; http://dx.doi.org/10.1111/j.1750-3639.2003.tb00033.x
- Thannickal TC, Moore RY, Nienhuis R, Ramanathan L, Gulyani S, Aldrich M, Cornford M, Siegel JM. Reduced number of hypocretin neurons in human narcolepsy. Neuron 2000; 27:469-74; PMID:11055430; http://dx.doi.org/10.1016/S0896-6273(00)00058-1
- Wu MF, Nienhuis R, Maidment N, Lam HA, Siegel JM. Role of the hypocretin (orexin) receptor 2 (Hcrt-r2) in the regulation of hypocretin level and cataplexy. J Neurosci 2011; 31:6305-10; PMID:21525270; http://dx.doi.org/10.1523/JNEUROSCI.0365-11.2011
- Yamanaka A, Tabuchi S, Tsunematsu T, Fukazawa Y, Tominaga M. Orexin directly excites orexin neurons through orexin 2 receptor. J Neurosci 2010; 30:12642-52; PMID:20861370; http://dx.doi.org/10.1523/JNEUROSCI.2120-10.2010
- Valko PO, Gavrilov YV, Yamamoto M, Reddy H, Haybaeck J, Mignot E, Baumann CR, Scammell TE. Increase of histaminergic tuberomammillary neurons in narcolepsy. Ann Neurol 2013; 74:794-804; PMID:24006291; http://dx.doi.org/10.1002/ana.24019
