ABSTRACT
Background: Although the killed whole-cell and live attenuated plague vaccine have been licensed, they are rarely used today because of toxicities, limited evidence of efficacy against plague, poor immune persistence required booster immunization every year, and limited commercial availability. This study was a randomized phase 2a clinical trial aimed to evaluating the immunogenicity and safety of a novel subunit plague vaccine. Methods: 240 healthy adults aged 18–55 y were enrolled and randomly assigned at a ratio of 1:1 to receive 2 doses of 15 or 30 mcg vaccine at a 28-day interval between doses. Blood samples were collected at day 0, 28 and 56. Adverse events were collected during the first 28 d after each vaccination. Serious Adverse Event was observed throughout the study period. Results: 239 participants received the first dose at day 0 and 238 received the second dose at day 28. Antibodies to envelope antigen faction 1 (F1) and recombinant virulence antigen (rV) were increased at day 28, and boosted significantly at day 56. For anti-F1 antibodies, geometric mean titer (GMT) and geometric mean fold increase (GMFI) were significantly higher in 30 mcg group than in the 15 mcg group(each P1< 0.05 at day 28 and each P1< 0.001 at day 56), with similar seroconversion rate of antibodies between 15 and 30 mcg group at both of the 2 time points. For anti-rV antibodies, seroconversion rate at day 28 in 30 mcg group was higher than that in 15 mcg group. However, GMT and GMFI of anti-rV antibodies were increased to approximately the same levels in the 2 groups. Similar booster immune response was also noticed in both groups at day 56. The injections were well tolerated, with mainly mild or moderate local and systemic adverse reactions (lower than grad 3). The proportion of pain at injection site was higher in 30 mcg group. None of SAEs were reported during 56 d. Conclusion: The plague vaccine comprised of F1 and rV antigens showed good safety and immunogenicity in adults aged 18–55 y old. The data show that the 30 mcg formulation is generally more immunogenic than the 15 mcg formulation, and represents the preferred formulation for further clinical development. It will be important to evaluate the long-term efficacy for appropriate formulations of the plague subunit vaccine.
Introduction
Plague is a potentially fatal infectious disease in humans caused by the gram-negative bacterium Yersinia pestis, transmitted naturally from rodent reservoirs to humans via fleas. Infections of Yersinia pestis bacterium mainly lead to bubonic, pneumonic and septicemic plague. Pneumonic plague is typically diagnosed in humans with high mortality.Citation1,2 Due to the potential person-to-person transmission of pneumonic plague, the disease was considered to be the highest risk threat for potential bioterrorism agents. Yersinia pestis has a long history as an agent of biowarfare, and poses a serious threat to international security.Citation3-6 In human history, there were 3 major pandemics of plague all over the world, about 200 million people died from the disease. The trend of plague epidemic has increased in recent years. Some areas in Africa, Asia, and the Americas still have the outbreak of plague.Citation7-13 A safe and effective vaccine is urgently needed.
Although the killed whole-cell and live attenuated plague vaccines have been licensed, these cause significant adverse reactions, including fever, headache, malaise, lymphadenopathy, erythema and induration at the injection site. They are rarely used today because of toxicities, limited evidence for efficacy to prevent plague, short immune persistence with booster immunization every year and limited commercial availability.Citation13,14 Based on the researches in the last 20 years, it has focused on recombinant subunit vaccines which were formed with envelope antigen faction 1 (F1) and virulence antigen (V) as the main composition provide greater protection than vaccines comprised of either subunit alone.Citation15-17 The F1 protein is a capsule-like structure around the surface of the Yersinia with the function of enabling it to escape phagocytosis. The V protein is the virulence factor and protective antigen of the bacterium, and contributes to the type-3 secretion system. With the function of V protein, Yersinia can survives and breeds in phagocytes, and be released finally.Citation18-20
This study was aimed to assess the safety and immunogenicity of the new type of plague subunit vaccine which was comprised of natural F1 antigen and recombined V antigen (F1+rV).
Results
Baseline characteristics of participants and compliance with follow-up for safety observation
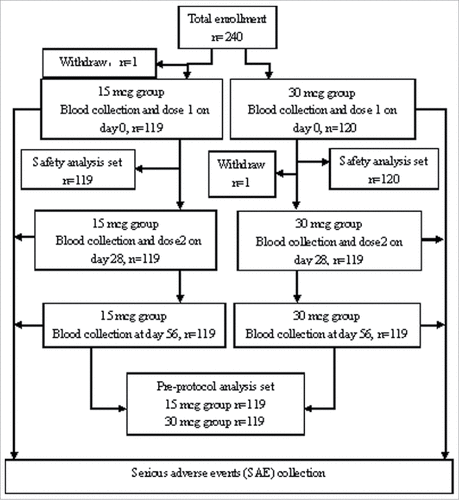
240 participants aged 18–55 y were enrolled and randomly assigned to receive 15 or 30 mcg plague vaccine, with 120 participants in each group (). One participant did not receive the first dose in 15 mcg group and another participant did not receive the second dose in 30 mcg vaccine group, they were excluded from the final analyses. Completion rates of vaccination and blood samples collection were 99.71% for all visits in each group. The demographic characteristics (age, sex and BMI) of each group were showed in . There were no significant differences between the 2 groups (all P > 0.05), and the distributions of gender in each group were equally comparable.
Table 1. Baseline characteristics of participants and compliance with follow-up for safety observation.
Immunogenicity
One part of assessment immunogenicity of plague vaccine was based on the antibodies to F1 antigen. The levels of antibodies to F1 were similar in 15 and 30 mcg vaccine groups with similar GMTs were approximately 1.00 at baseline. After one dose of plague vaccine at day 28, compared with GMT of 13.88 and GMFI of 13.48 in 15 mcg group, they were increased to 22.10 in 30 mcg vaccine group. The GMT and GMFI in the 15 mcg group were marginally lower than that in the 30 mcg group (P1 = 0.0338 and P1 = 0.0231). Seroconversion rates of F1 antibody were approximately 80% in each group (78.99% in 15 mcg group and 84.03% in 30 mcg group). After two doses of vaccination, the GMT and GMFI were 47.55 and 46.19 in 15 mcg group at day 56, and they were significantly increased to 90.53 in 30 mcg group (P1 = 0.0004 and P1 = 0.0002). Seroconversion rates were increased to 95% (). Except for 8 participants in 15 mcg group and 4 in 30 mcg group, all other participants in each group who received 2 doses of vaccine were seroconverted for specific F1 antibody. For the same dose comparison, GMT, GMFI and seroconversion rate of participants in 15 mcg group at day 56 were significant higher than those in 30 mcg group at day 28.
Table 2. Immunogenicity of F1 antibody at baseline and after vaccination.
The distributions of anti-F1 antibodies titers were shown in . The accumulation curve of anti-F1 antibodies showed that more than 50% participants in 15 and 30 mcg group produced specific antibody, with titers more than 16 at day 28, and increased to 64 at day 56. The titers of antibodies in 30 mcg group seemed higher than that in 15 mcg group at day 28 and 56 (). Compare with titers of antibodies in 30 mcg group at day 28, the titer was higher in 15 mcg group at day 56, and GMT, GMFI, seroconversion rate were significantly higher (, P2 < 0.001, P2 < 0.001 and P2 = 0.0246).
Another part of assessment immunogenicity of plague vaccine was based on the antibodies to rV. The level of antibodies to rV in 15 mcg group was similar with that in 30 mcg group at baseline (day 0). It was highly significantly increased at day 28 and 56. GMTs at day 28 were 395.81 and 551.71 in 15 and 30 mcg groups with 35.74 and 51.75 fold increases compared with those at baseline, and then they were approximately 2500 with over 220 fold increases at day 56. The seroconversion rates were increased from 54.62% to 99.16% in 15 mcg group and from 71.43% to 99.16% in 30 mcg group at day 28 and 56 (). Only one participant in each 15 and 30 mcg group who received 2 doses was not seroconverted for anti-rV antibodies. For comparison of the same vaccination dose, GMT, GMFI and seroconversion rate of antibodies to rV in 15 mcg group at day 56 were also significantly higher than those in 30 mcg group at day 28.
Table 3. Immunogenicity of rV IgG antibody at baseline and after vaccination.
The distributions of anti-rV antibodies titers were shown in . The findings were quite similar to that of anti-F1 antibodies. The accumulation curve of anti-rV antibodies showed that more than 50% participants in 15 and 30 mcg group produced antibody titers than 256 at day 28, and increased to 1024 at day 56. The titers in 30 mcg group seemed higher than those in 15 mcg group at day 28 and 56 (). Compared with titers in 30 mcg group at day 28, the anti-rV antibodies titer was also higher in 15 mcg group at day 56, and GMT, GMFI, seroconversion rate were significantly higher as well (, each P2 < 0.001).
Safety
The administration of 2 injections of plague vaccine was well tolerated at 2 dose levels. The frequencies of local, systemic and unsolicited adverse reactions/events were similar in 15 and 30 mcg groups. The adverse reactions mainly were erythema/redness, pain, induration, swelling, pruritus in injection site, and general symptoms as fever, muscle pain, fatigue, and headache. Most of the adverse events were mild or moderate (lower than grade 3). For the solicited local adverse reaction, pain at the injection site was the most common local adverse reaction in each group, with significantly different rates (P = 0.0219, ). For solicited systemic adverse reaction, fever was the most common adverse reaction in each group, with similar rates of 5.88% and 8.33%. The frequencies of other reaction/events were all less than 5%.
Table 4. Adverse reaction/events in the participants.
One participant in 15 mcg group reported redness in injection site with a diameter over 30 mm (grade 3), and recovered within 3 d without treatment. Another unsolicited adverse event in 30 mcg group was papules on the face and neck with a diameter over 30 mm (grade 3) during day 8–28 after the first dose. It was not related to the vaccination. None of serious adverse events (SAEs) was reported during 56 d.
Discussion
This is the first report of a phase 2a clinical trial for safety and immunogenicity of a recombinant subunit plague vaccine (F1 + rV) in a cohort including healthy adults aged 18–55. Although many studies in mice has been demonstrated that vaccine could provide strong protection, none of the previous licensed vaccines were reported to have been evaluated in a randomly clinical trial, and only one clinical study of recombinant subunit plague vaccine (rF1 + rV) was published.Citation21-23 One phase 2a clinical trial of DynPort Vaccine Company LLC in United States, was performed with 80 mcg and 160 mcg rF1V, and another one of phase 2b was performed with 80 mcg rF1V, but the results were not published. In 2005, Williamson et al found that 5–40 mcg antigens of rF1rV were immunogenic with a peak in titers after the booster dose at day 21.Citation22
Lower doses of 15 and 30 mcg antigens of F1rV were assessed in our study, we found that the 2 formulations of the new vaccine showed good safety profiles and robust immunogenicity in China. In our study we found that GMTs of antibody to V antigen were approximate 11.00 in 2 study groups at baseline. This suggested that participants had prior exposure to antigens sharing significant homology with Y. pestis antigens, even though this study was conducted in an area non-endemic for plague. The genus Yersinia includes 3 human pathogenic species: Yersinia pestis, Yersinia pseudotuberculosis and Yersinia enterocolitica. The pathogenicity of these 3 species is dependent on the presence of a 70-kb conserved virulence plasmid with polynucleotide sequence homology ranging from 55 to 90%.Citation24 Y. pestis is ordinarily transmitted by the bite of a flea, while the enteropathogenic Y. pseudotuberculosis and Y. enterocolitica are transmitted by an oral-fecal route. Yersiniosis which was related with Yersinia enterocolitica, have been frequently reported from many countries in the world.Citation25 Thus, it is accepted that antibodies to V antigen may exist prior to vaccination.Citation26
The measurements of specific antibodies to F1 and rV of participants in 15 and 30 mcg group revealed that the plague vaccine comprised of F1+ rV antigens was immunogenic, with different responses in each group and visit time. For anti-F1 antibody, the titers increased after the first dose and to greater levels after booster immunization. Compared to baseline, they were increased about 50 and 90 fold in 15 and 30 mcg groups after 2 doses vaccination. Although seroconversion rates were almost similar, the titers of antibodies as GMT and GMFI in 30 mcg group were approximately 2-fold of those in 15 mcg group at the same point. For anti-rV antibody, responses were stronger than those to F1, and titers were higher over 200 fold at day 56 when comparing to baseline. The increasing trends were almost similar in 2 doses groups, with higher seroconversion rates in 30 mcg group at day 28 and finally to equal levels at day 56. To the same immune procedure, 30 mcg F1 antigens displayed better immunogenicity, and 2 doses of rV antigen had similar immune response. Consideration of the same dose vaccination, compared levels of antibodies in 15 mcg group at day 56 with those in 30 mcg group at day 28, the results clearly showed that 2 doses vaccination had greater immunogenicity than that in one dose vaccination.
The vaccine was well tolerated. The outcomes of safety observation were generally similar in 15 and 30 mcg groups. The adverse reactions were mild and did not affect the accomplishment of 2 doses vaccination with finally completion rates approximately 100%. Although one case of related grade 3 local adverse reaction in 15 mcg group was reported, there was no significant difference to 30 mcg group.
Limitations of the study included: (1) control group was not include in the study arms. (2) The lack of long time immunologic observation makes it impossible to assess immunity durability. In fact, the clinical trial site was in Yancheng City of Jiangsu Province, which was not a high prevalence area of plague in China, specific antibodies to plague were at very low level, and baseline levels of antibodies were good control for vaccination. We had conducted a long time follow-up for all the participants about 12 months, and the data was still in the collection. Immunity durability will be researched in the future study.
Although plague is primarily a disease of rodents in nature, humans are occasionally infected through a secondary host, for example domestic pet (cat or dog), or a wild rabbit, rarely, through another infected person.Citation27 The future availability of this vaccine, to provide more comprehensive protection in humans, will bring the goal of disease prevention or eradication.
In conclusion, the desirable safety and immunogenicity of a novel subunit plague vaccine (F1 + rV) were demonstrated in healthy adults aged 18–55 y. Based on our data, 2 doses were more immunogenic than one dose in both groups. Thirty mcg dose of F1 antigen was more immunogenic than 15 mcg dose at day 56, while 15 and 30 mcg doses of rV antigens had comparably immunogenic at the same point.. A 30 mcg dose formulation, containing 30 mcg F1 and 30 mcg rV, is proposed for further efficacy trials. It will be important to assess the long-term efficacy for appropriate formulations of the subunit plague vaccine.
Participants and methods
Study design and participants
The phase 2a random clinical trial was conducted from October to December, 2014, in Yancheng City of Jiangsu Province in China. It was designed by the Jiangsu Provincial Center for Disease Control and Prevention (JSCDC) and Lanzhou Institute of Biological Products Co., Ltd (sponsor of the study and manufacture of the vaccine). The study was approved by the institutional ethics committee of JSCDC and national regulatory agencies. The trial was undertaken by JSCDC in accordance with Good Clinical Practice (GCP) and Good Clinical Laboratory Practice (GCLP) in China. (ClinicalTrials.gov: NCT02596308).
Healthy adults aged 18–55 y old were enrolled and provided the written informed consent prior to the initiation of study procedures. Adult was excluded according to the inclusion and exclusion criteria (detailed inclusion and exclusion criteria are described in the Appendix Text S1). The participants were randomly assigned in a 1:1 ratio to receive 2 doses of tested 15 or 30 mcg plague vaccine at a 28-day interval between doses. 5.0 ml blood sample was obtained from each participant at day 0, 28 and 56, respectively. Serum were collected and independently tested for immunogenicity by the National Institute for Food and Drug Control (NIFDC, Beijing, China). Safety observation was proceeded post each dose, including observations of solicited injection-site and systemic adverse reactions (AE) within 7 days, and unsolicited events within 28 d post-each dose. Serious adverse events (SAEs) were collected throughout the trial ().
Vaccine
The tested vaccine was formulated by mixing natural F1 protein (F1) and recombinant V protein (rV). F1, which has been widely used as a plague vaccine antigen was extracted and purified from a live attenuated Y. pestis strain EV76. This strain was named as EV by following the first 2 letters of a patient's name who died of bubonic plague in Madagascar in 1926.Citation28 rV antigen was expressed in E. coli. The dose ratio of F1 and rV was maintained at 1:1. The two vaccine formulations were: 15 mcg (containing 15 mcg F1 and 15 mcg rV) and 30 mcg (containing 30 mcg F1 and 30 mcg rV). All components suspended in 1.0 ml of buffered saline and aluminum adjuvant. All the investigational vaccines were repackaged under Good Manufacturing Practice (GMP) conditions in identical appearance with different vaccine numbers.
Randomization and masking
The participants were randomly assigned in a 1:1 ratio to receive tested 15 mcg and 30 mcg plague vaccine according to a randomization list (with a block size of 10) that was generated by an independent statistician who did not participated in any other part of the trial. The two vaccine formulations had identical packaging with blindly labeled sequential codes. All the participants and investigators were masked to the treatment allocation.
Immunogenicity assessments
Serum was promptly centrifuged and stored at −20°C until testing. IgG antibodies to F1 and rV were detected by indirect and double antigen sandwich ELISA methods. For F1 antibodies, the serum of day 28 and 56 were 10-fold serially diluted and then measured with ELISA kit. Seroconvesion for F1 antibody defined as antibodies after vaccination was 4 folds increased compared to preimmunization. For antibodies to rV, the serum of day 0 were 10-fold serially diluted and of day 28 and 56 were 80-fold diluted, then measured with plate coated with rV antigen. The kits and plates were developed by Lanzhou Institute of Biological Products Co., Ltd. Antibody titers were also measured using a threshold of 1:80, seroconvesion was defined as an rV antibody titer of at least 1:320 in initially seronegative participants (rV antibody <1:80) and as a 4-fold increase in titer from pre- to postvaccination.
Safety assessments
Participants were monitored 30 minutes after vaccination. Solicited injection site reaction (pain, symptoms of skin and mucosa, induration, erythema, swelling, rash and pruritus) and systemic adverse reaction (fever, allergic reaction, headache, drowsiness, emesis, diarrhea, muscle pain and cough) were recorded until day 28 after each dose in diary card by participants. Solicited reactions occurred within 7 d post each dose were defined as injection-site and systemic adverse reactions with the symptom had been listed in this study. Unsolicited events defined as the other adverse reactions with the symptom never listed in this study exclude solicited reactions within 28 d post each dose. Adverse reactions were graded as 1, 2, 3, 4 according to the guidelines of vaccine clinical trials classification standard in China (detailed classification standards are described in the Appendix Text S2 and S3). Data on SAE were collected throughout the trial. Whether AE and SAEs were attributable to vaccination or not was judged by the investigators.
Statistical analysis
Participants, who received at least one dose of vaccine and safety follow-up, were included in the analysis set of safety. Immunogenicity analysis was conducted in the per-protocol set (PPS), which consisted of the participants who received 2 doses vaccination and donated blood samples at day 0, 28, 56 during the protocol-specified time frames.
Statistical comparisons were made using 2-sided tests with an α value of 0.05. GMT and GMFI were summarized with 95% confidence intervals (CIs) and compared by the Student's t test or t’ test, respectively. Comparisons of seroconversion rate, baseline characteristics and adverse reactions/events rate were conducted by Chi-square test or Fisher's exact test. The statistical analyses were performed by an independent statistician using SAS (version 9.1, SAS Institute Inc., Cary, NC, USA).
Abbreviations
| Y. pestis | = | Yersinia pestis |
| F1 | = | envelope antigen faction 1 |
| rV | = | recombinant virulence antigen |
| AE | = | adverse event |
| AR | = | adverse reaction |
| GMT | = | geometric mean titer |
| GMFI | = | geometric mean fold increase |
| SAE | = | serious adverse event |
| CI | = | confidence interval |
| IEC | = | independent ethics committee |
| CFDA | = | Chinese Food and Drug Administration |
| GCP | = | Good Clinical Practice |
| GCLP | = | Good Clinical Laboratory Practice |
| JSCDC | = | Jiangsu Provincial Center for Disease Control and Prevention |
Authors' contributions
Y-M Hu, K Chu, L Jiao, Y-L Chang and B-X Wang contributed to conception and design of the study; Y-M Hu, K Chu and W-G Chen coordinated the clinical aspects of the study; J-J Xu, Z-H Yuan, Z-Y Li, W-G Chen, F-Y Meng contributed to collecting data and managing participants; Li Luo analyzed the data; Y-M Hu, K Chu, J-L Hu, J-X Li and B-X Wang wrote the paper
All authors read and approved the final manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplementary files
Download MS Word (58.5 KB)Acknowledgments
The authors would like to thank the volunteers who participated in this study, and also thank all the investigators of Jiangsu Provincial Center for Diseases Control and Prevention and Yancheng Center for Diseases Control and Prevention, who were responsible for collecting data and managing participants.
Funding
This work was supported by Lanzhou Institute of Biological Products Co.,Ltd.
References
- Prentice MB, Rahalison L. Plague. Lancet 2007; 369:1196-207; PMID:17416264; http://dx.doi.org/10.1016/S0140-6736(07)60566-2
- Cobbs CG, Chansolme DH. Plague. Dermatologic clinics 2004; 22:303-12; PMID:15207311; http://dx.doi.org/10.1016/j.det.2004.03.007
- pestisQuenee LE, Cornelius CA, Ciletti NA, Elli D, Schneewind O. Yersinia pestis caf1 variants and the limits of plague vaccine protection. Infect Immun 2008; 76:2025-36; PMID:18347051; http://dx.doi.org/10.1128/IAI.00105-08
- Inglesby TV, Dennis DT, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Koerner JF, et al. Plague as a biological weapon: medical and public health management. Working Group on Civilian Biodefense. Jama 2000; 283:2281-90; PMID:10807389; http://dx.doi.org/10.1001/jama.283.17.2281
- Zilinskas RA. The anti-plague system and the Soviet biological warfare program. Crit Rev Microbiol 2006; 32:47-64; PMID:16610337; http://dx.doi.org/10.1080/10408410500496896
- Greenfield RA, Drevets DA, Machado LJ, Voskuhl GW, Cornea P, Bronze MS. Bacterial pathogens as biological weapons and agents of bioterrorism. Am J Med Sci 2002; 323:299-315; PMID:12074485; http://dx.doi.org/10.1097/00000441-200206000-00003
- Anisimov AP, Lindler LE, Pier GB. Intraspecific diversity of Yersinia pestis. Clin Microbiol Rev 2004; 17:434-64; PMID:15084509; http://dx.doi.org/10.1128/CMR.17.2.434-464.2004
- Stenseth NC, Atshabar BB, Begon M, Belmain SR, Bertherat E, Carniel E, Gage KL, Leirs H, Rahalison L. Plague: past, present, and future. PLoS Med 2008; 5:e3; PMID:18198939; http://dx.doi.org/10.1371/journal.pmed.0050003
- Human plague: review of regional morbidity and mortality, 2004-2009. Wkly Epidemiol Rec 2009; 85:40-5; PMID:20151494
- Bevins SN, Baroch JA, Nolte DL, Zhang M, He H. Yersinia pestis: examining wildlife plague surveillance in China and the USA. Integrative zoology 2012; 7:99-109; PMID:22405453; http://dx.doi.org/10.1111/j.1749-4877.2011.00277.x
- Ratsitorahina M, Chanteau S, Rahalison L, Ratsifasoamanana L, Boisier P. Epidemiological and diagnostic aspects of the outbreak of pneumonic plague in Madagascar. Lancet 2000; 355:111-3; PMID:10675169; http://dx.doi.org/10.1016/S0140-6736(99)05163-6
- Jones RT, Borchert J, Eisen R, MacMillan K, Boegler K, Gage KL. Flea-Associated Bacterial Communities across an Environmental Transect in a Plague-Endemic Region of Uganda. PloS one 2015; 10:e0141057; PMID:26485147; http://dx.doi.org/10.1371/journal.pone.0141057
- Butler T. Plague gives surprises in the first decade of the 21st century in the United States and worldwide. Am J Trop Med Hyg 2013; 89:788-93; PMID:24043686; http://dx.doi.org/10.4269/ajtmh.13-0191
- Feodorova VA, Corbel MJ. Prospects for new plague vaccines. Exp Rev Vaccin 2009; 8:1721-38; PMID:19943765; http://dx.doi.org/10.1586/erv.09.129
- Anderson GW, Jr., Heath DG, Bolt CR, Welkos SL, Friedlander AM. Short- and long-term efficacy of single-dose subunit vaccines against Yersinia pestis in mice. Am J Trop Med Hyg 1998; 58:793-9; PMID:9660466
- Heath DG, Anderson GW, Jr., Mauro JM, Welkos SL, Andrews GP, Adamovicz J, et al. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 1998; 16:1131-7; PMID:9682370; http://dx.doi.org/10.1016/S0264-410X(98)80110-2
- Williamson ED, Eley SM, Griffin KF, Green M, Russell P, Leary SE, Oyston PC, Easterbrook T, Reddin KM, Robinson A. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol Med Microbiol 1995; 12:223-30; PMID:8745007; http://dx.doi.org/10.1111/j.1574-695X.1995.tb00196.x
- Williams RC, Jr., Gewurz H, Quie PG. Effects of fraction I from Yersinia pestis on phagocytosis in vitro. J Infect Dis 1972; 126:235-41; PMID:4559742; http://dx.doi.org/10.1093/infdis/126.3.235
- Straley SC, Skrzypek E, Plano GV, Bliska JB. Yops of Yersinia spp. pathogenic for humans. Infect Immun 1993; 61:3105-10; PMID:8335339
- Anisimov AP, Dentovskaya SV, Panfertsev EA, Svetoch TE, Kopylov P, Segelke BW, Zemla A, Telepnev MV, Motin VL. Amino acid and structural variability of Yersinia pestis LcrV protein. Infect Genet Evolution 2010; 10:137-45; PMID:19835996; http://dx.doi.org/10.1016/j.meegid.2009.10.003
- Jefferson T, Demicheli V, Pratt M. Vaccines for preventing plague. Cochrane Database Syst Rev 2000; 2:CD000976; PMID:10796565
- Williamson ED, Flick-Smith HC, Lebutt C, Rowland CA, Jones SM, Waters EL, Gwyther RJ, Miller J, Packer PJ, Irving M. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect Immun 2005; 73:3598-608; PMID:15908389; http://dx.doi.org/10.1128/IAI.73.6.3598-3608.2005
- Williamson ED, Oyston PC. Protecting against plague: towards a next-generation vaccine. Clin Exp Immunol 2013; 172:1-8; PMID:23480179; http://dx.doi.org/10.1111/cei.12044
- Roggenkamp A, Geiger AM, Leitritz L, Kessler A, Heesemann J. Passive immunity to infection with Yersinia spp. mediated by anti-recombinant V antigen is dependent on polymorphism of V antigen. Infect Immun 1997; 65:446-51; PMID:9009295
- Ranjbar R, Afshar D. Development of a loop-mediated isothermal amplification assay for rapid detection of Yersinia enterocolitica via targeting a conserved locus. Iran J Microbiol 2015; 7:185-90; PMID:26697156
- Tomaso H, Mooseder G, Al Dahouk S, Bartling C, Scholz HC, Strauss R, Treu TM, Neubauer H. Seroprevalence of anti-Yersinia antibodies in healthy Austrians. Eur J Epidemiol 2006; 21:77-81; PMID:16450210; http://dx.doi.org/10.1007/s10654-005-5047-z
- Runfola JK, House J, Miller L, Colton L, Hite D, Hawley A, Mead P, Schriefer M, Petersen J, Casaceli C, et al. Outbreak of Human Pneumonic Plague with Dog-to-Human and Possible Human-to-Human Transmission–Colorado, June-July 2014. MMWR Morb Mortal Wkly Rep 2015; 64:429-34; PMID:25928467
- Cui Y, Yang X, Xiao X, Anisimov AP, Li D, Yan Y, Zhou D, Rajerison M, Carniel E, Achtman M, et al. Genetic variations of live attenuated plague vaccine strains (Yersinia pestis EV76 lineage) during laboratory passages in different countries. Infect Genet Evolution 2014; 26:172-9; PMID:24905600; http://dx.doi.org/10.1016/j.meegid.2014.05.023