?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background: Inactivated vaccines for hepatitis A virus (HAV) infection are widely used in China. Mass vaccination programs drive the need for data on long-term persistence of vaccine-induced protection. Methods: A prospective, randomized, open-label clinical trial was conducted to compare geometric mean concentrations (GMCs) and seroconversion rates (SRs) of anti-HAV antibody elicited by the inactivated vaccines Healive and Havrix for 5 y post immunization, in which 400 healthy children were randomly assigned in a 3:1 ratio to receive 2 doses of Healive or Havrix at 0 and 6 month. Anti-HAV antibody concentration was detected by microparticle enzyme immunoassay (MEIA) during the study. Furthermore, an attempt was made to predict persistence of protective immunogenicity by using a suitable statistical model. Results: The GMCs were significantly higher after vaccination with Healive than after Havrix as comparator vaccine at 1, 6, 7, 18, 30, 42, 54 and 66 month (P < 0.01) with the peak point at 7 month (3427.2 mIU/ml for Healive and 1441.9 mIU/ml for Comparator). Similarly significant differences of SRs were found between the 2 groups at 1 and 6 month (P < 0.01). Afterwards, the SRs of both groups reached 100% at 7 month and did not decline until 66 month(99.1% for Healive and 97.5% for Comparator). A linear mixed model with a change point at 18 month(Model 3) was found to be suitable to predict persistence of protective immunogenicity induced by vaccines. It was estimated that the duration of protection for Healive was at least 20 y with a lower limit of GMC 95% confidence interval (CI) no less than 20 mIU/mL. Conclusions: Compared with Havrix, the new preservative-free inactivated hepatitis A vaccine (Healive) in 2 doses showed better persistence of antibody concentrations for 5 y after full-course immunization among children and the persistence of protective immunogenicity was estimated for at least 20 y.
Introduction
Hepatitis A which can cause asymptomatic-to-severe illness is a significant endemic and epidemic disease of global importance.Citation1-3 The prevalence of hepatitis A infection varies according to hygienic conditions, being very high in countries where the majority of infections are clinically undetected.Citation4-7 In May, 2013, an outbreak of symptomatic hepatitis A virus (HAV) infections occurred in the USA.Citation8 In Japan, 342 cases of HAV infection had been reported in 2014, which was considered as a nationwide HAV outbreak.Citation9 In China, hepatitis is also a huge public health problem and infection with HAV remains the leading cause of acute viral hepatitis, especially for children and young adults.Citation10
China has long experience using inactivated HAV vaccines.Citation11,12 The review reported by Cui et al.Citation12 demonstrated that inactivated HAV vaccines manufactured in China were immunogenic, effective, and safe. Liu et al.Citation2 conducted a study in the healthy young adults at colleges in Nanchang City, China and found that 1 or 2 doses of inactivated hepatitis A vaccine Healive® (Sinovac Biotech, Beijing, China) induced high rates of seroconversion (anti-HAV IgG concentration ≥20 mIU/mL) persisting for at least 36 months. However, mass vaccination programs drive the need for data on long-term persistence of vaccine-induced protection.Citation13
In 2006, a double-blind, randomized and controlled clinical trial was conducted in healthy volunteers aged 1-8 y from Changzhou city, Jiangsu province of China to compare the immunogenicity of anti-HAV antibodies among 3 consecutive production lots of Healive® and Havrix® (GlaxoSmithKline Biologicals) as comparator vaccine. Total 400 subjects were randomized into 4 groups with 100 subjects per group, receiving one of the 3 lots of Healive or the comparator vaccine. The vaccination was 2-dose regimen at 0-6 months. The three lots of Healive showed statistically indistinguishable clinical performance with 100% seroconversion rates (SRs) and 3237-3814 mIU/ml geometric mean concentrations (GMCs) 1 month after the second dose.Citation14
The aim of the present analysis is to compare the immunogenicity between Healive and Comparator further for 5 y and predict persistence of protective immunogenicity induced by both vaccines with a suitable statistical model.
Results
Participants included in analysis
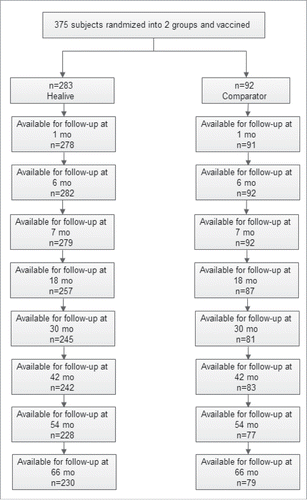
Of 400 children, 375 participants who completed their full-course immunization were invited to participate in the follow-up phase with 283 in Healive and 92 in Comparator ().
Demographic characteristics of both groups at baseline in the full analysis set (FAS) are presented in . There were no statistically significant differences in gender ratio, age, height and weight between 2 groups.
Table 1. Demographic characteristics of participants.
Observed immunogenicity of Anti-HAV antibodies over time
GMCs and SRs of anti-HAV antibodies in the 2 groups at each timepoint from 1 to 66 month are shown in . At 1 month after the first dose, GMCs of Healive and Comparator were 29.1 and 20.3 mIU/ml respectively and increased slowly in the following 5 months. At 7 month, i.e. 1 month after dose-2, GMCs of both groups reached the peak point (3427.2 mIU/ml for Healive and 1441.9 mIU/ml for Comparator). Later, there were a sharp decline with 572.0 and 386.0 mIU/ml at 18 month for Healive and Comparator respectively and a relatively slower rate of decline from 30 to 66 month (ranged from 468.7 to 257.1 mIU/ml for Healive and 300.1 to 168.1 mIU/ml for Comparator). The GMCs were significantly higher in Healive than in Comparator at each timepoint from 1 to 66 month (P < 0.01). Similarly significant differences of SRs were found between the 2 groups at 1 month (75.2% for Healive and 50.6% for Comparator) and 6 month (97.5% for Healive and 87.0% for Comparator) (P < 0.01) . Afterwards, the SRs of both groups reached 100% at 7 month and did not decline until 66 month(99.1% for Healive and 97.5% for Comparator).
Table 2. Geometric mean concentrations and seroconversion rates over time in healthy children.
Model results
278 participants in Healive and 92 participants in Comparator were included in the model research with 5 in Healive excluded for reasons of sample contamination and data missing (see model selection section for details).
The predicted GMCs and SRs from 7 to 306 month after the first dose using Model 3 are shown in . GMCs (95% CI) were predicted to 33.0 (24.3-45.0) mIU/mL at 246 month and 16.1 (11.0-23.6) mIU/mL at 306 month for Healive. During this period, 260 month was found to be the farthest timepoint with protective immunogenicity, where the GMC (95% CI) was estimated to 27.9 (20.2-38.7) mIU/mL with a lower limit no less than 20 mIU/mL. (Not listed in , the same below). In a similar way, the farthest timepoint with protective immunogenicity for Comparator was 209 month where the GMC (95% CI) was estimated to 33.2(20.0-55.2) mIU/mL. With regard to SRs, in order to guarantee a lower limit no less than 80%, it was estimated that the SR (95% CI) was 84.9% (80.1%-88.9%) at 157 month for Healive and 89.1% (80.9%-94.7%) at 116 month for Comparator.
Table 3. Observed and Predicted Geometric Mean Concentrations and Seroconversion Rates.
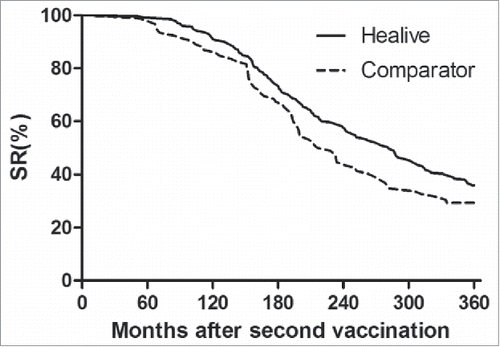
The predicted SRs using Model 3 for both groups are shown in and the SRs of Healive were higher than Comparator at all the timepoints until 360 month after the second dose.
Discussion
This study was designed to compare GMCs and SRs of anti-HAV antibody elicited by the inactivated vaccines Healive and Havrix as comparator vaccine for 5 y post immunization, and then a suitable statistical model was used to predict the persistence of protective immunogenicity for the 2 vaccines.
The GMCs were significantly higher in Healive than in Comparator at each timepoint from 1 to 66 month (P < 0.01). The SRs of both groups reached 100% at 7 month and did not decline until 66 month. There were no significant differences of SRs between the 2 groups at each timepoint except 1 and 6 month. Since each company has different antigen dissociation technique and dissociation rate, which is a patent technology and not open to the public, the dose unit of U(Healive) and El.U(Havrix) is different and the antigen content could not be compared directly. However, it does not matter to make comparison of the immunogenicity above and protective immunogenicity persistence between the 2 licensed products which is what is most pertinent to the use of the products in the field and population.
Because the advantage of linear mixed models fitting to longitudinal data was recognized by several studies,Citation2,15-19 4 previously used linear mixed models were applied to fit our follow-up data and Model 3 was confirmed to be a suitable one. Similar to many clinical trials showed,Citation2,20,Citation21 the GMCs declined rapidly in the first 12 months after 2 doses and slowly later, therefore, the timepoint of 18 month after the first dose was used as the chang point in Model 3. Apart from anti-HAV serostatus at 1 month, we also considered the inclusion of other covariates (age and gender), however, it had no statistically significant differences and did not improve goodness of fit.
A series of previous trials had explored the protective immunogenicity persistence of Havrix based on statistical models. Van Damme et al.Citation22 assumed a constant basic exponential decline and predicted the persistence of protective immunogenicity for at least 20 y among healthy adults with a 0-1-6 month vaccination schedule. Hammitt et al.Citation23 used a log-linear model of decline in GMC of antibody, and predicted that children would retain protective immunogenicity levels of anti-HAV for more than 22 y with a 0-1-6 month vaccination schedule or for more than 27 y with a 0-1-12 month vaccination schedule. Based on 2 of the longest follow-up studies (participants were vaccinated according to a 0-12 mo and a 0-6 mo schedule, respectively) in adults,Citation24,25 Niel et al.Citation16 reported a duration of protective immunogenicity at least 25 y with a linear mixed model, which was confirmed appropriate to describe the profile of HAV antibody decline phase and it was also applied in our persistence estimation.
In our study, the persistence of protective immunogenicity for Havrix was less than 20 y which was shorter compared with the studies mentioned above. ELISA method (Enzyme-Linked Immunosorbent Assay) to measure anti-HAV antibody concentration was adopted and anti-HAV antibody concentration no lower than 20 mIU/mL was considered the indicator of seroprotection for all the 4 studies including ours. Considering there are wide statistical bounds around the data in the published and current study, all results could lie within the range of variation of following such cohorts and modeling long-term changes based on a few years of data.
There were some limitations to our study as follows. The period of observations was limited to describe the whole profile of anti-HAV antibody elicited by the vaccines and a longer follow-up study need to be conducted to further adjust and verify the present results. In addition, results from single site might have resulted in selection bias.
In conclusion, compared with Havrix, the new preservative-free inactivated hepatitis A vaccine Healive with 0-6 month vaccination schedule showed better immunogenic for 5 y after full-course immunization among children and the persistence of protective immunogenicity was estimated for at least 20 y.
Materials and methods
Clinical trial methodology
The three consecutive production lots of Healive were combined as one group with the consistency of immunogenicity according to regulatory acceptance criteriaCitation14 taken into consideration. Therefore, the 400 healthy children were assigned in a 3:1 ratio to receive 2 doses of Healive (0.5 mL/dose) contained 250 U antigen and 0.25 mg alum without preservative or the comparator vaccine Havrix (0.5 mL/dose) contained 720 ELISA units (El.U) antigen and 0.25 mg alum with 2-phenoxyethanol as preservative.Volunteers who completed their full-course immunization were monitored annually for 5 y. Blood samples were collected at 0, 1, 6, 7, 18, 30, 42, 54 and 66 month with screening blood samples collected before immunization at 0 and 6 month. Anti-HAV antibody concentrations were assessed by microparticle enzyme immunoassay (MEIA) during the study. Both seroconversion and protective immunogenicity were defined as the ability of hepatitis A vaccines to elicit an immune response of at least 20 mIU/mL.Citation12,26
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Study protocols were approved by the Ethics Review Committee of the Changzhou Center for Disease Control and Prevention. Written informed consents were obtained from all participants prior to the performance of the study. The trial was registered at Clinicaltrials.gov (NCT00534885).
Statistical methods
GMCs and SRs of anti-HAV antibody were calculated based on the actual observed data without missing data imputation. Logarithmic transformation (log10) had been made before the calculation of GMC and 95% CI. Student’s t-test or Mann–Whitney U test (for non-normally distributed data) was used to compare GMCs when relevant, and Chi-square test or Fisher’s exact test (when data were sparse) was used to compare SRs. The significance level was 0.05 (2-sided). All data were handled and analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
Predicative models for persistence of protective Immunogenicity
In order to identify a suitable model for our data set, 4 linear mixed model structures used previously Citation15-17,Citation20,27,Citation28 to analyze antibody persistence following vaccination were investigated.
Model 1 is most widely used Citation15,17,Citation20,27,Citation28 to predict the persistence of protective immunogenicity induced by HAV. It is a linear mixed effects model involving linear antibody decay containing fixed and random effects for both slope and intercept parameters:
Where Yij is log10 antibody concentration for subject i observed at time tj, a and ai are the population-level (fixed effect) and individual level (random effect) intercepts and b and bi are the population-level and individual-level slope corresponding to the rate of linear antibody decay. εij is the residual error between model prediction and the observed value. The model was fitted to the data from 7 month (timepoint of antibody concentration peak) to 66 month, that is, the model was only fitted to the antibody concentrations decline phase.
Model 2Citation17 is a segmented linear mixed effects model which contains fixed and random effects for all slope and intercept parameters except for the indicator δij (contains only fixed effects). This model was fitted to antibody concentrations from 1 to 66 month:
Where δij = 1 for time up to the antibody concentrations peak and δij = 0 for time post the peak.
Model 3Citation16,29 is a linear trend model with one change point during the antibody concentrations decline phase:
Where δj = 1 for time post the change point and δj = 0 for time from the antibody concentrations peak up to the change point.
Model 4Citation16 is a simple model including only different exponent parameters of time:
Same as Model 1 and 3, Model 4 are only fitted to data during the antibody concentration decline phase.
Models were constructed and fitted using SAS 9.3 MIXED procedure with unconstrained variance–covariance matrices. AIC Citation30 and BIC Citation31 were calculated to choose the suitable model to predict persistence of protective immunogenicity for Healive and Comparator, where smaller AIC and BIC values indicate better model fitting.
Model selection
Because of one sample contamination at 42 month, the observation (4C283) in Healive was excluded in the data set to build statistical models. In addition, 4 observations (4C397, 4C140, 4C115, 4C164) in Healive whose antibody concentrations were missing at 7 month were also excluded. Missing values were imputed with the last consecutive measurements for data from 1 to 7 month and the next consecutive measurements for data from 7 to 66 month, considering that the GMCs of both groups reached the highest level at 7 month.
Parameter estimates and fit statistics for each modeling approach are shown in . Serostatus at 1 month was retained in each model with statistically significant coefficient (P < 0.01). According to the result showed in , a much smaller average concentration decline rate after 18 month compared to the decline rate from 7 to 18 month were found. Therefore, the use of Model 1 that assumed the constant basic logarithmic decline rate would have resulted in a shorter duration of protection. Similar to Model 1, Model 2 with concentration increasing phase added also assumed the constant basic logarithmic decline rate from 7 month onward. Both Models above had considerably higher AIC or BIC than other 2 models. Considering the different antibody concentration decline rates, Model 3 with one change point (18 month) during the antibody concentrations decline performed relative smaller AIC and BIC. Compared to Model 3, Model 4 had an improvement in AIC and BIC, however, the positive coefficients of implied an infinite duration of protection, as antibody concentrations were ultimately predicted to increase over time and it was unreasonable.
Table 4. Parameter estimates and fit statistics for each modeling approach.
On the basis of modeling results, Model 3 was chosen as a suitable model. When observed and predicted values were compared using Model 3, R2 ranged from 79.04% to 98.37% for Healive and 76.60% to 96.70% for Comparator at each timepoint from 7 to 66 month (data not shown). These values reflected a high level of agreement between the observed and predicted GMCs over time.
Disclosure of potential conflicts of interest
We declare that we have no conflict of interest exist in the submission of this manuscript, and manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.
Funding
This work was partially supported by Research Grants 81273176, 81302509 and 81473069 from the National Natural Science Foundation of China.
References
- Ryszkowska A, Gladysz A, Inglot M, Molin I. [Prevalence of anti-HAV antibodies in selected groups of children]. Przegl Epidemiol 2000; 54(3-4):375-83; PMID:11349601
- Liu XE, Chen HY, Liao Z, Zhou Y, Wen H, Peng S, Liu Y, Li R, Li J, Zhuang H. Comparison of Immunogenicity Between Inactivated and Live Attenuated Hepatitis A Vaccines Among Young Adults: A 3-Year Follow-up Study. J Infect Dis 2015; 212(8):1232-6; PMID:25969561; http://dx.doi.org/10.1093/infdis/jiv213
- McIntyre N. Clinical presentation of acute viral hepatitis. Br Med Bull 1990; 46(2):533-47; PMID:2116216
- Zago-Gomes MP, Stantolin GC, Perazzio S, Aikawa KH, Gonçalves CS, Pereira FE. Prevalence of anti-hepatits A antibodies in children of different socioeconomic conditions in Vila Velha, ES. Rev Soc Bras Med Trop 2005; 38(4):285-9; PMID:16082472; http://dx.doi.org/10.1590/S0037-86822005000400001
- Forbes A, Williams R. Increasing age–an important adverse prognostic factor in hepatitis A virus infection. J R Coll Physicians Lond 1988; 22(4):237-9; PMID:3230539
- Forbes A, Williams R. Changing epidemiology and clinical aspects of hepatitis A. Br Med Bull 1990; 46(2):303-18; PMID:2198989
- Shapiro CN, Margolis HS. Worldwide epidemiology of hepatitis A virus infection. J Hepatol 1993; 18 Suppl 2:S11-4; PMID:8182265; http://dx.doi.org/10.1016/S0168-8278(05)80371-X
- Collier MG, Khudyakov YE, Selvage D, Adams-Cameron M, Epson E, Cronquist A, Jervis RH, Lamba K, Kimura AC, Sowadsky R, et al. Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: an epidemiological case study. Lancet Infect Dis 2014; 14(10):976-81; PMID:25195178; http://dx.doi.org/10.1016/S1473-3099(14)70883-7
- Ishii K, Kiyohara T, Yoshizaki S, Kawabata K, Kanayama A, Yahata Y, Takahashi T, Kinoshita H, Saitou T, Sunagawa T, et al. Epidemiological and genetic analysis of a 2014 outbreak of hepatitis A in Japan. Vaccine 2015; 33(45):6029-36.
- Cao J, Bi S, Meng Q, Shen L, Zheng H, Zhang Y. Genotyping of acute hepatitis a virus isolates from China, 2003-2008. J Med Virol 2011; 83(7):1134-41; PMID:21520140; http://dx.doi.org/10.1002/jmv.22086
- Wang XY, Xu ZY, Ma JC, von Seidlein L, Zhang Y, Hao ZY, Han OP, Zhang YL, Tian MY, Ouyang PY, et al. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: results from 8-year follow-up. Vaccine 2007; 25(3):446-9; PMID:16949710; http://dx.doi.org/10.1016/j.vaccine.2006.08.004
- Cui F, Liang X, Wang F, Zheng H, Hutin YJ, Yang W. Development, production, and postmarketing surveillance of hepatitis A vaccines in China. J Epidemiol 2014; 24(3):169-77; PMID:24681843; http://dx.doi.org/10.2188/jea.JE20130022
- Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol 2011; 83(11):1885-91; PMID:21915861; http://dx.doi.org/10.1002/jmv.22200
- Jiang WP, Chen JT, Wang X, Wang YL, Liu Y, Chen WY, Xu WG, Qiu YZ, Yin WD. Immunogenicity and safety of three consecutive lots of a new preservative-free inactivated hepatitis A vaccine (Healive): a double-blind, randomized and controlled trial. Vaccine 2008; 26(18):2297-301; PMID:18395305; http://dx.doi.org/10.1016/j.vaccine.2007.11.008
- Bovier PA, Bock J, Ebengo TF, Frösner G, Glaus J, Herzog C, Loutan L. Predicted 30-year protection after vaccination with an aluminum-free virosomal hepatitis A vaccine. J Med Virol 2010; 82(10):1629-34; PMID:20827757; http://dx.doi.org/10.1002/jmv.21883
- Hens N, Habteab GA, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine 2014; 32(13):1507-13; PMID:24508042; http://dx.doi.org/10.1016/j.vaccine.2013.10.088
- Lopez EL, Contrini MM, Mistchenko A, Kieffer A, Baggaley RF, Di Tanna GL, Desai K, Rasuli A, Armoni J. Modeling the Long-term Persistence of Hepatitis A Antibody After a Two-Dose Vaccination Schedule in Argentinean Children. Pediatr Infect Dis J 2015; 34(4):417-25; PMID:25764099; http://dx.doi.org/10.1097/INF.0000000000000605
- Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. Oxford Statistical Science Series. 2009: Oxford University Press.
- Fitzmaurice GM, L.N.W.J., Applied Longitudinal Analysis. II ed. 2011: Hoboken, New Jersey: John Wiley & Sons, Inc.
- Desai K, Coudeville L, Bailleux F. Modelling the long-term persistence of neutralizing antibody in adults after one dose of live attenuated Japanese encephalitis chimeric virus vaccine. Vaccine 2012; 30(15):2510-5; PMID:22342547; http://dx.doi.org/10.1016/j.vaccine.2012.02.005
- Overbosch D, Peyron F, Picot N, Varichon JP, Dumas R, Chambonneau L, Weber F. Combined typhoid fever and hepatitis A vaccine: comparison of immunogenicity and safety to concomitant monovalent vaccine over 3 years. J Travel Med 2005; 12(6):319-26; PMID:16343383; http://dx.doi.org/10.2310/7060.2005.12604
- Van Damme P, Thoelen S, Cramm M, De Groote K, Safary A, Meheus A. Inactivated hepatitis A vaccine: reactogenicity, immunogenicity, and long-term antibody persistence. J Med Virol 1994; 44(4):446-51; PMID:7897379; http://dx.doi.org/10.1002/jmv.1890440425
- Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, Bell BP, McMahon BJ. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis 2008; 198(12):1776-82; PMID:18976095; http://dx.doi.org/10.1086/593335
- Van Herck K, Jacquet JM, Van Damme P. Antibody persistence and immune memory in healthy adults following vaccination with a two-dose inactivated hepatitis A vaccine: long-term follow-up at 15 years. J Med Virol 2011; 83(11):1885-91; PMID:21915861; http://dx.doi.org/10.1002/jmv.22200
- Van Herck K, Crasta PD, Messier M, Hardt K, Van Damme P. Seventeen-year antibody persistence in adults primed with two doses of an inactivated hepatitis A vaccine. Hum Vaccin Immunother 2012; 8(3):323-7; PMID:22327499; http://dx.doi.org/10.4161/hv.18617
- Andre F, Van Damme P, Safary A, Banatvala J. Inactivated hepatitis A vaccine: immunogenicity, efficacy, safety and review of official recommendations for use. Expert Rev Vaccines 2002; 1(1):9-23; PMID:12908508; http://dx.doi.org/10.1586/14760584.1.1.9
- Bailleux F, Coudeville L, Kolenc-Saban A, Bevilacqua J, Barreto L, André P. Predicted long-term persistence of pertussis antibodies in adolescents after an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine, based on mathematical modeling and 5-year observed data. Vaccine 2008; 26(31):3903-8; PMID:18555563; http://dx.doi.org/10.1016/j.vaccine.2008.04.089
- Bovier PA, Bock J, Loutan L, Farinelli T, Glueck R, Herzog C. Long-term immunogenicity of an inactivated virosome hepatitis A vaccine. J Med Virol 2002; 68(4):489-93; PMID:12376955; http://dx.doi.org/10.1002/jmv.10244
- Wiedermann G, Kundi M, Ambrosch F, Safary A, D'Hondt E, Delem A. Inactivated hepatitis A vaccine: long-term antibody persistence. Vaccine 1997; 15(6-7):612-5; PMID:9178459; http://dx.doi.org/10.1016/S0264-410X(96)00242-3
- Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control 1974; 19:716-723; http://dx.doi.org/10.1109/TAC.1974.1100705
- GE S. Estimating the dimension of a model. Ann Statist 1978; 6:461-464; http://dx.doi.org/10.1214/aos/1176344136