ABSTRACT
Dendritic cells (DCs) play an important role in the induction of antitumor immunity. Therefore, they are used as anti-cancer vaccines in clinical studies in various types of cancer. DC vaccines are generally well tolerated and able to induce antigen-specific T cell responses in melanoma patients. After DC vaccinations, functional tumor-specific T cells are more frequently detected in stage III melanoma patients, as compared to patients with advanced melanoma, indicating that the tumor load influences immunological responses. Furthermore, long-lasting clinical responses were rarely seen in metastatic melanoma patients after DC vaccination. Since more potent treatment options are available, e.g. immune checkpoint inhibitors and targeted therapy, DC vaccination as monotherapy may not be preferred in the treatment of advanced melanoma. However, encouraging results of DC vaccines combined with ipilimumab have been reported in advanced melanoma patients with an objective response rate of 38%. DC vaccines show promising clinical results in stage III patients, although clinical efficacy still needs to be proven in a phase 3 trial. The clinical and immunological results of DC vaccination in stage III melanoma patients might be further improved by using naturally circulating DCs (myeloid DCs and plasmacytoid DCs) and neoantigens to load DCs.
Introduction
Dendritic cells (DCs) were first discovered by Steinman and Cohn in 1973.Citation1 DCs are the most potent antigen-presenting cells of the immune system. Under steady state conditions, immature DCs sample peripheral tissues in search for pathogens or tissue injury, but when encountering danger signals, they quickly differentiate into activated (mature) DCs and migrate to lymphoid organs to induce an adaptive immune response. In lymphoid tissues, mature DCs initiate immune responses by presenting captured antigens to naïve T cells, in the form of peptide-major histocompatibility complex (MHC) molecule complexes. These T cells will proliferate and differentiate into effector cells that are able to kill target cells in an antigen-dependent manner.Citation2
Under ideal circumstances, tumor growth would be controlled by an in vivo cancer-immunity cycle, in which DCs take up tumor material and induce tumor-specific T cells that infiltrate the tumor bed and kill their target cells by recognition of specific antigens, thereby releasing new tumor antigens that can be picked up by DCs again. However, in cancer patients, this cycle is hampered: tumor antigens may not be detected by DCs, DCs and T cells may treat antigens as self rather than foreign, T cells may not properly infiltrate tumors, or factors in the tumor microenvironment might suppress effector cells.Citation3 Therapeutic vaccination, like DC vaccination, can be used to overcome some of these problems and thus accelerate and expand the production of tumor-specific T cells. The first clinical study of a DC vaccine was reported in 1996 by Hsu and colleaguesCitation4 in patients with B-cell lymphomas. Since then, multiple studies with DC vaccination have been reported in various tumor types, e.g., melanoma, prostate cancer, and glioma.Citation2 Here, we will focus on DC vaccination in melanoma patients.
Dendritic cell vaccines
The goal of DC vaccination is to induce tumor-specific T cell responses by injecting activated DCs loaded with tumor antigens.Citation5 Over the past years, different sources of DCs, maturation factors, and ways of tumor antigen loading have been used in clinical trials in melanoma patients.Citation6 Until recently, most DCs for immunotherapy were in vitro differentiated from precursors like monocytes, by culturing them in the presence of interleukin (IL) 4 and granulocyte-macrophage colony-stimulating factor (GM-CSF).Citation7 Additionally, these immature monocyte-derived DCs (moDCs) need to be matured, as mature DCs induce more potent anti-tumor immune responses than immature DCs in melanoma patients.Citation8,9 Different methods have been used to mature DCs, including cytokine cocktails consisting of monocyte-conditioned medium, tumor necrosis factor-α, prostaglandin E2, IL1-β, and IL-6; prophylactic vaccines used as TLR ligands; and electroporation with mRNA encoding CD40L, CD70, and constitutively active TLR4.Citation10-12 Finally, the mature DCs must be loaded with relevant tumor-antigens, for which several methods have been applied, including short peptides, long peptides, tumor cell lysates, and mRNA transfection.Citation13
Recently, naturally circulating DCs have been used to vaccinate advanced melanoma patients. Different subsets of naturally circulating DCs can be distinguished in the human peripheral blood by the expression of surface molecules: BDCA2+ plasmacytoid DCs (pDCs) and 2 subsets of myeloid DCs (mDCs): CD1c+ (also known as BDCA1+) mDCs and CD141+ mDCs, which differ in function and localization. Activated pDCs secrete large amounts of interferon-α (IFN-α) in response to viral products and they can induce the maturation of B cells into plasma cells, while mDCs are specialized in immunity against bacteria and fungi.Citation2,6,14 Until now, pDCs and CD1c+ mDCs have been used in clinical trials with advanced melanoma patients.Citation15,16 GM-CSF was used to activate CD1c+ mDCs and Frühsommer-meningoencephalitis vaccine to activate pDCs. Both mDCs as well as pDCs can be loaded with melanoma-associated peptides.Citation15,16 The most important advantages of using naturally circulating DCs over moDCs are a highly standardized rapid isolation procedure with antibody-coated magnetic beads (CliniMACS Prodigy), resulting in clinically applicable purified DCs, and the absence of an extensive culture period (overnight versus 8–9 d in moDCs), which may have a positive effect on the immunological capacity.Citation15,16 This makes naturally circulating DCs more suitable for large scale multicenter application.
Immune monitoring of DC vaccination
Immunologic monitoring is of great importance in clinical trials to determine the efficacy of DC vaccination. Enzyme-linked immunosorbent spot assays and tetramer analyses of tumor-specific T cell responses in peripheral blood are commonly used, but a low prevalence of tumor-specific T cells in peripheral blood makes these procedures less suitable for routine immunomonitoring. Besides peripheral blood samples, we evaluate skin-test infiltrating lymphocyte cultures from delayed-type hypersensitivity (DTH) biopsies taken within 2 weeks after each cycle of DC vaccinations.Citation17,18 These biopsies are analyzed for the presence of antigen-specific CD8+ T cells by tetrameric MHC-peptide complexes and for the occurrence of functional T cell responses, by measuring specific production of cytokines in response to different target cells. Since this assay takes multiple parameters of T cells into account, including their capacity to migrate into the skin and to produce cytokines upon antigen encounter, it is probably more suitable to monitor T cell responses.
Safety
DC vaccination has proven to be a safe treatment in melanoma patients. Most common adverse events are grade 1–2 flu-like symptoms and local injection site reactions. Flu-like symptoms usually last up to 48 hours and consist mostly of fever, fatigue and chills. Injection site reactions, are usually small and self-limiting within 2–7 d.Citation19 DC vaccination is rarely associated with severe immune-related toxicity, which is in sharp contrast to immune checkpoint inhibitors and cytokines, that frequently show grade 3–4 immune-related adverse events.Citation20 This can be explained by nonspecific activation of the immune system by these immunotherapeutic agents as compared with an antigen-specific activation by DC vaccination. Therapy-related grade 3 adverse events, including hepatitis and pneumonitis, occurred only in patients treated in protocols using prophylactic vaccines as TLR ligands to mature DCs. These adverse events were attributed to the BCG vaccine used in this DC maturation cocktail.Citation11 No grade 4 or 5 therapy-related adverse events were observed in our DC vaccination trials. Thus, DC vaccinations are generally well tolerated in melanoma patients.
Position of DC vaccination in the treatment of advanced melanoma
Most clinical studies in advanced melanoma patients were performed with moDCs.Citation21-25 Although antigen-specific immune responses were found, long-lasting clinical responses were limited in advanced melanoma patients. A recent meta-analysis showed objective response rates of 8.5% in 1205 advanced melanoma patients treated with DC vaccination.Citation26 The only phase 3 trial comparing moDCs monotherapy with dacarbazine in advanced melanoma patients was stopped prematurely due to low response rates (< 10%) in both treatment arms.Citation25 In retrospect, this trial was probably performed too early, since DC vaccination was still in development, leading to a variable quality of the DC vaccines and suboptimal maturation of DCs.
We have conducted 2 small proof of principle clinical studies exploiting naturally circulating DCs in advanced melanoma patients, the first study using pDCs and the second study using CD1c+ mDCs. Tumor-specific T cell responses were found after vaccination with both DC populations. Objective responses were found in a limited number of patients treated with either subset. However, the study with pDCs did show an improved overall survival (OS) for patients treated with pDCs as compared with matched historical controls (median OS 22.0 months vs. 7.6 months), and the objective responses (14%) and prolonged progression-free survival in the mDC trial were seen in patients with functional antigen-specific T cells in blood and DTH.Citation15,16
In the last years, multiple new therapies have been approved for the treatment of advanced melanoma. The clinical outcome after DC vaccination needs to be compared with the results of these approved therapeutic options, including immune checkpoint inhibitors and targeted therapies. Immune checkpoint molecules that down-regulate pathways of T cell activation, like cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed death-1 (PD-1), can be blocked with monoclonal antibodies (mAbs; ).Citation27 A pooled analysis of phase 2–3 trials with anti-CTLA-4 mAb ipilimumab in advanced melanoma patients showed a median OS of 9.5 months and a plateau at 21% in the survival curve around 3 y after start of ipilimumab.Citation28 Trials with anti-PD-1 mAbs nivolumab and pembrolizumab in advanced melanoma patients showed approximately 2-times higher response rates than ipilimumab and significantly longer progression-free survival.Citation29,30 However, long-term survival rates of these anti-PD-1 mAbs are still pending. The combination of ipilimumab and nivolumab resulted even in an objective response rate of 58%, however, at the expense of significant toxicity.Citation30 Besides the immune checkpoint inhibitors, BRAF inhibitors (vemurafenib, dabrafenib) have shown significant improvement of OS in patients with an activating BRAF mutation ().Citation31,32 However, responses are short-lived in many patients due to various resistant mechanisms.Citation33 Recently, it became apparent that adding a MEK inhibitor (cobimetinib, trametinib) to a BRAF inhibitor was associated with a further improvement of survival as compared to BRAF inhibition alone (), with overall response rates of 64–68% and median progression-free survival of 9.3–11.4 months.Citation34-36
Figure 1. Immune therapy and targeted therapy of melanoma. aBy injecting activated dendritic cells (DCs) loaded with tumor antigens, DC vaccination aims to induce tumor associated antigen-specific T cells. Antigen presentation by DCs and co-stimulation signals (B7-CD28) result in T cell activation and proliferation. bTo keep an immune response in control, CTLA-4 is then up regulated on the surface of T cells, which binds stronger to B7 than CD28 and causes an inhibitory signal. Blocking CTLA-4 with monoclonal antibodies (ipilimumab) enhances T cell activation.Citation49 cBinding of PD-1 on the T cells to PD-L1 on the tumor results in downregulation of effector functions of T cells, which inhibits the killing of tumor cells. Blockade of this ligation by anti-PD-1 antibodies (nivolumab, pembrolizumab) makes it possible for T cells to maintain their antitumor functions, which allow them to kill tumor cells.Citation50 dBRAF is a kinase that is part of the RAS-BRAF-MEK-ERK mitogen-activated protein kinase (MAPK) pathway of cell proliferation. The tumors of approximately 40–60% of advanced melanoma patients harbor activating BRAF V600 mutations. The mutated kinase is constitutively active, which results in unregulated cell proliferation. This process can be blocked by selective BRAF inhibitors (vemurafenib, dabrafenib).Citation33 eSingle-agent BRAF inhibition results commonly in progressive disease due to acquired resistance, which is commonly caused by genetic escape mechanisms resulting in MAPK pathway independant signaling. Upfront inhibition of both MEK (cobimetinib, trametinib) and the mutated BRAF kinases might counteract this form of resistance.Citation34 (+) indicates a stimulatory effect; (−) indicates an inhibitory effect. Abbrevations: CTLA-4, cytotoxic T-lymphocyte-associated antigen 4, MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, programmed death ligand-1; TCR, T cell receptor.
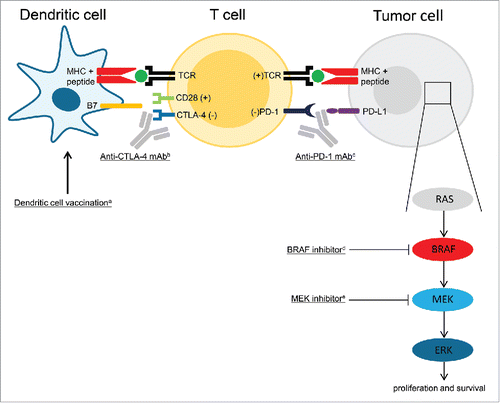
Altogether, these results suggest that DC vaccination monotherapy is not preferred in the treatment of advanced melanoma patients, despite the capability of inducing tumor-specific T cells. This may be explained by several immunosuppressive counter mechanisms against these T cells induced by the tumor (microenvironment), e.g. release of immunosuppressive cytokines, induction of regulatory T cells (Tregs) and myeloid derived suppressor cells, and expression of immune checkpoint molecules, like CTLA-4 and PD-1.Citation27,37 Therefore, the clinical efficacy of DC vaccination in advanced melanoma patients might be improved by combining it with other therapies that neutralize these immunosuppressive counter mechanisms. The mild toxicity profile of DC vaccination makes it an ideal candidate for combination-treatment. Unfortunately, combination of DC vaccination with the anti-CD25 mAb daclizumab did not show an enhancement of the efficacy of the DC vaccine, despite inducing a depletion of Tregs in the peripheral blood. This might be explained by simultaneous depletion of CD25+ effector T cells by daclizumab.Citation38 Combining DC vaccination with immune checkpoint inhibitors blocking CTLA-4 or PD-1 might be more effective. A phase 2 trial of moDCs in combination with ipilimumab conducted by Wilgenhof and colleagues showed tolerability and an objective response rate of 38% in 39 heavily pre-treated advanced melanoma patients, which supports further investigation of this combination.Citation39 Clinical studies with the combination of DC vaccination and anti-PD-1 mAbs in melanoma patients have not been described yet, but preclinical data support a potential synergistic effect.Citation40
Dendritic cell vaccination in stage III melanoma
Stage III melanoma patients have a high risk of recurrent disease, even after a radical lymph node dissection (RLND) with curative intent.Citation41 Therefore, adjuvant treatments that will improve survival rates are warranted. Stage III melanoma patients may have a more potent immune system than patients with advanced disease due to a lower tumor burden.Citation37 Therefore, DC vaccination might be more effective in stage III melanoma patients than in advanced melanoma patients. A retrospective analysis of 78 stage III patients treated with moDCs showed functional tumor-specific T cells in DTH skin-test biopsies of 71% of patients, which is substantially higher than in patients with distant metastasis (23%).Citation17,19 Furthermore, OS was significantly higher in this population, when compared to 209 matched controls who underwent RLND without adjuvant DC vaccination (median OS 63.6 months versus 31.0 months; p = 0.018).Citation19 However, these promising results have to be confirmed in a prospective randomized phase 3 clinical trial and should be compared to other (potential) adjuvant treatments, like IFN-α, ipilimumab and anti-PD-1 mAbs. IFN-α is an unattractive adjuvant treatment in stage III melanoma patients, since it only minimally improves survival and comes with substantial toxicity.Citation42 Ipilimumab has recently been approved by the Food and Drug Administration based on a significant improvement of recurrence-free survival compared to placebo, but OS data are still awaited.Citation43 However, adjuvant ipilimumab induced significant grade 3–4 adverse events and 49% of patients did not complete the treatment schedule due to drug-related adverse events.Citation43 Furthermore, the dosage of 10 mg/kg used in this trial could be debated, because a dosage of 3 mg/kg is commonly used in advanced melanoma patients. Clinical trials with adjuvant anti-PD-1 mAbs are currently ongoing (NCT02388906, NCT02362594). The mild toxicity profile of DC vaccination gives this therapy an advantage over immune checkpoint inhibitors when a phase 3 trial shows comparable clinical results, despite the fact that manufacturing a cellular product is more labor-intensive.
Future perspectives of DC vaccination in melanoma patients
Until now, all trials in stage III melanoma patients have been performed with moDCs, but currently we are conducting a trial (NCT02574377) in which immunogenicity of combined adjuvant mDC and pDC vaccination vs. adjuvant mDC or pDC vaccination alone is tested in stage III melanoma patients. Naturally circulating DCs have complementary functions and they can activate each other. Co-culture of mDCs and pDCs during activation augments the expression of co-stimulatory molecules and secretion of proinflammatory cytokines, providing the rationale for combining mDCs and pDCs in a DC vaccine.Citation44 In a murine tumor model, immunization with a mixture of activated pDC and mDC resulted in increased levels of antigen-specific CD8+ T cells and an enhanced antitumor response compared with immunization with either DC subset alone.Citation45
Besides the source of DCs, the antigens used in DC vaccination might influence the clinical effectiveness. Melanoma differentiation antigens, e.g., tyrosinase and gp100, are frequently used, as they are commonly expressed on melanoma cells and DCs with these antigens have shown to induce antigen-specific T cell responses.Citation22 Furthermore, cancer-testis antigens (e.g. MAGE-A3) have been used in melanoma patients to load DCs.Citation23 However, melanoma is the tumor with the highest prevalence of somatic mutations, resulting in the formation of many neoantigens, which have proven to play an important role in antitumor immunity.Citation46 These results formed the basis for a proof of concept study with a DC vaccine loaded with patient-specific neoantigens, which resulted in an enhanced CD8+ T cell response against some of these tumor neoantigens.Citation47 Although these results are promising, the biggest challenge of such a personalized vaccination strategy will be the identification of the optimal immunogenic neoantigens.Citation48 To induce a broad immune response, it might be best to combine melanoma differentiation antigens, cancer-testis antigens, and neoantigens in DC vaccines.
Finally, combinations of DC vaccination and immune checkpoint inhibitors deserve further investigation in melanoma patients, since the first results of combination-treatment look promising.
Conclusions
In conclusion, DC vaccination is safe and adjuvant DC vaccination monotherapy shows promising results in stage III melanoma patients after RLND, while in advanced melanoma patients DC vaccination might be more suited as combination-treatment with immune checkpoint inhibitors. However, clinical effectiveness of DC vaccination monotherapy in stage III melanoma still has to be proven in a prospective randomized phase 3 clinical trial. Furthermore, the efficacy of DC vaccines might be further improved by using naturally circulating DCs as a source for vaccination and neoantigens to load the DCs.
Abbreviations
| CTLA-4 | = | cytotoxic T-lymphocyte-associated antigen 4 |
| DC(s) | = | dendritic cells |
| DTH | = | delayed-type hypersensitivity |
| GM-CSF | = | granulocyte-macrophage colony-stimulating factor |
| IL | = | interleukin |
| IFN-α | = | interferon-α |
| OS | = | overall survival |
| mAb | = | monoclonal antibody |
| mDCs | = | myeloid dendritic cells |
| MHC | = | major histocompatibility complex |
| moDCs | = | monocyte-derived dendritic cells |
| PD-1 | = | programmed death-1 |
| PD-L1 | = | programmed death ligand-1 |
| pDCs | = | plasmacytoid dendritic cells |
| RLND | = | radical lymph node dissection |
| TCR | = | T cell receptor |
| Tregs | = | regulatory T cells |
Disclosure of potential conflicts of interest
WRG received speakers fees from Astellas, Bayer, Bavarian Nordic, Bristol-Myers Squibb, Janssen-Cilag and ESMO; WRG participated in advisory boards of Amgen, Astellas, Bayer, Bristol-Myers Squibb, Dendreon, Janssen-Cilag, Morphosys, Sanofi and Transgene; WRG participated in ad hoc consultancy for Psioxus Therapeutics, Sotio and Transgene; WRG is founder of Carcinos (global oncology education: immunotherapy of cancer).
References
- Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med 1973; 137:1142-62; PMID:4573839; http://dx.doi.org/10.1084/jem.137.5.1142
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012; 12:265-77; PMID:22437871; http://dx.doi.org/10.1038/nrc3258
- Chen DS, Mellman I. Oncology Meets Immunology: The Cancer-Immunity Cycle. Immunity 2013; 39:1-10; PMID:23890059; http://dx.doi.org/10.1016/j.immuni.2013.07.012
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996; 2:52-8; PMID:8564842; http://dx.doi.org/10.1038/nm0196-52
- Figdor CG, de Vries IJ, Lesterhuis WJ, Melief CJ. Dendritic cell immunotherapy: mapping the way. Nat Med 2004; 10:475-80; PMID:15122249; http://dx.doi.org/10.1038/nm1039
- Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin Cancer Res 2016; 22:1897-906; PMID:27084743; http://dx.doi.org/10.1158/1078-0432.CCR-15-1399
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179:1109-18; PMID:8145033; http://dx.doi.org/10.1084/jem.179.4.1109
- de Vries IJ, Lesterhuis WJ, Scharenborg NM, Engelen LP, Ruiter DJ, Gerritsen MJ, Croockewit S, Britten CM, Torensma R, Adema GJ, et al. Maturation of dendritic cells is a prerequisite for inducing immune responses in advanced melanoma patients. Clin Cancer Res 2003; 9:5091-100; PMID:14613986
- Jonuleit H, Giesecke-Tuettenberg A, Tuting T, Thurner-Schuler B, Stuge TB, Paragnik L, Kandemir A, Lee PP, Schuler G, Knop J, et al. A comparison of two types of dendritic cell as adjuvants for the induction of melanoma-specific T-cell responses in humans following intranodal injection. Int J Cancer 2001; 93:243-51; PMID:11410873; http://dx.doi.org/10.1002/ijc.1323
- de Vries IJ, Adema GJ, Punt CJ, Figdor CG. Phenotypical and functional characterization of clinical-grade dendritic cells. Methods Mol Med 2005; 109:113-26; PMID:15585917
- Bol KF, Aarntzen EH, Pots JM, Olde Nordkamp MA, van de Rakt MW, Scharenborg NM, de Boer AJ, van Oorschot TG, Croockewit SA, Blokx WA, et al. Prophylactic vaccines are potent activators of monocyte-derived dendritic cells and drive effective anti-tumor responses in melanoma patients at the cost of toxicity. Cancer Immunol Immunother 2016; 65:327-39; PMID:26861670; http://dx.doi.org/10.1007/s00262-016-1796-7
- Bonehill A, Van Nuffel AM, Corthals J, Tuyaerts S, Heirman C, Francois V, Colau D, van der Bruggen P, Neyns B, Thielemans K. et al. Single-step antigen loading and activation of dendritic cells by mRNA electroporation for the purpose of therapeutic vaccination in melanoma patients. Clin Cancer Res 2009; 15:3366-75; PMID:19417017; http://dx.doi.org/10.1158/1078-0432.CCR-08-2982
- Zhou YL, Bosch ML, Salgaller ML. Current methods for loading dendritic cells with tumor antigen for the induction of antitumor immunity. J Immunother 2002; 25:289-303; PMID:12142552; http://dx.doi.org/10.1097/00002371-200207000-00001
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science 1999; 284:1835-7; PMID:10364556
- Tel J, Aarntzen EH, Baba T, Schreibelt G, Schulte BM, Benitez-Ribas D, Boerman OC, Croockewit S, Oyen WJ, van Rossum M, et al. Natural human plasmacytoid dendritic cells induce antigen-specific T-cell responses in melanoma patients. Cancer Res 2013; 73:1063-75; PMID:23345163; http://dx.doi.org/10.1158/0008-5472.CAN-12-2583
- Schreibelt G, Bol KF, Westdorp H, Wimmers F, Aarntzen EH, Duiveman-de Boer T, van de Rakt MW, Scharenborg NM, de Boer AJ, Pots JM, et al. Effective clinical responses in metastatic melanoma patients after vaccination with primary myeloid dendritic cells. Clin Cancer Res 2015; 22:2155-66; PMID:26712687; http://dx.doi.org/10.1158/1078-0432.CCR-15-2205
- Aarntzen EH, Bol K, Schreibelt G, Jacobs JF, Lesterhuis WJ, Van Rossum MM, Adema GJ, Figdor CG, Punt CJ, De Vries IJ. Skin-test infiltrating lymphocytes early predict clinical outcome of dendritic cell-based vaccination in metastatic melanoma. Cancer Res 2012; 72:6102-10; PMID:23010076; http://dx.doi.org/10.1158/0008-5472.CAN-12-2479
- de Vries IJ, Lesterhuis WJ, Barentsz JO, Verdijk P, van Krieken JH, Boerman OC, Oyen WJ, Bonenkamp JJ, Boezeman JB, Adema GJ, et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nat Biotechnol 2005; 23:1407-13; PMID:16258544; http://dx.doi.org/10.1038/nbt1154
- Bol KF, Aarntzen EHJG, in 't Hout FEM, Schreibelt G, Creemers JHA, Lesterhuis WJ, Gerritsen WR, Grunhagen DJ, Verhoef C, Punt CJ et al. Favorable overall survival in stage III melanoma patients after adjuvant dendritic cell vaccination. Oncoimmunology 2016; 5:e1057673; PMID:26942068; http://dx.doi.org/10.1080/2162402X.2015.1057673
- Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK, Kershaw MH. Autoimmunity associated with immunotherapy of cancer. Blood 2011; 118:499-509; PMID:21531979; http://dx.doi.org/10.1182/blood-2011-01-325266
- Aarntzen EH, De Vries IJ, Lesterhuis WJ, Schuurhuis D, Jacobs JF, Bol K, Schreibelt G, Mus R, De Wilt JH, Haanen JB, et al. Targeting CD4(+) T-helper cells improves the induction of antitumor responses in dendritic cell-based vaccination. Cancer Res 2013; 73:19-29; PMID:23087058; http://dx.doi.org/10.1158/0008-5472.CAN-12-1127
- Aarntzen EH, Schreibelt G, Bol K, Lesterhuis WJ, Croockewit AJ, de Wilt JH, van Rossum MM, Blokx WA, Jacobs JF, Duiveman-de Boer T, et al. Vaccination with mRNA-electroporated dendritic cells induces robust tumor antigen-specific CD4+ and CD8+ T cells responses in stage III and IV melanoma patients. Clin Cancer Res 2012; 18:5460-70; PMID:22896657; http://dx.doi.org/10.1158/1078-0432.CCR-11-3368
- Wilgenhof S, Van Nuffel AM, Corthals J, Heirman C, Tuyaerts S, Benteyn D, De Coninck A, Van Riet I, Verfaillie G, Vandeloo J, et al. Therapeutic vaccination with an autologous mRNA electroporated dendritic cell vaccine in patients with advanced melanoma. J Immunother 2011; 34:448-56; PMID:21577140; http://dx.doi.org/10.1097/CJI.0b013e31821dcb31
- de Vries IJ, Bernsen MR, Lesterhuis WJ, Scharenborg NM, Strijk SP, Gerritsen MJ, Ruiter DJ, Figdor CG, Punt CJ, Adema GJ. Immunomonitoring tumor-specific T cells in delayed-type hypersensitivity skin biopsies after dendritic cell vaccination correlates with clinical outcome. J Clin Oncol 2005; 23:5779-87; PMID:16110035; http://dx.doi.org/10.1200/JCO.2005.06.478
- Schadendorf D, Ugurel S, Schuler-Thurner B, Nestle FO, Enk A, Brocker EB, Grabbe S, Rittgen W, Edler L, Sucker A, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol 2006; 17:563-70; PMID:16418308; http://dx.doi.org/10.1093/annonc/mdj138
- Anguille S, Smits EL, Lion E, van Tendeloo VF, Berneman ZN. Clinical use of dendritic cells for cancer therapy. Lancet Oncol 2014; 15:e257-67; PMID:24872109; http://dx.doi.org/10.1016/S1470-2045(13)70585-0
- Weber J. Immune checkpoint proteins: a new therapeutic paradigm for cancer–preclinical background: CTLA-4 and PD-1 blockade. Semin Oncol 2010; 37:430-9; PMID:21074057; http://dx.doi.org/10.1053/j.seminoncol.2010.09.005
- Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol 2015; 33:1889-94; PMID:25667295; http://dx.doi.org/10.1200/JCO.2014.56.2736
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015; 372:2521-32; PMID:25891173; http://dx.doi.org/10.1056/NEJMoa1503093
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015; 373:23-34; PMID:26027431; http://dx.doi.org/10.1056/NEJMoa1504030
- McArthur GA, Chapman PB, Robert C, Larkin J, Haanen JB, Dummer R, Ribas A, Hogg D, Hamid O, Ascierto PA, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014; 15:323-32; PMID:24508103; http://dx.doi.org/10.1016/S1470-2045(14)70012-9
- Ascierto PA, Minor D, Ribas A, Lebbe C, O'Hagan A, Arya N, Guckert M, Schadendorf D, Kefford RF, Grob JJ, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013; 31:3205-+; PMID:23918947; http://dx.doi.org/10.1200/JCO.2013.49.8691
- Alcala AM, Flaherty KT. BRAF inhibitors for the treatment of metastatic melanoma: clinical trials and mechanisms of resistance. Clin Cancer Res 2012; 18:33-9; PMID:22215904; http://dx.doi.org/10.1158/1078-0432.CCR-11-0997
- Larkin J, Ascierto PA, Dreno B, Atkinson V, Liszkay G, Maio M, Mandalà M, Demidov L, Stroyakovskiy D, Thomas L, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014; 371:1867-76; PMID:25265494; http://dx.doi.org/10.1056/NEJMoa1408868
- Long GV, Stroyakovskiy D, Gogas H, Levchenko E, de Braud F, Larkin J, Garbe C, Jouary T, Hauschild A, Grob JJ, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014; 371:1877-88; PMID:25265492; http://dx.doi.org/10.1056/NEJMoa1406037
- Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, Lichinitser M, Dummer R, Grange F, Mortier L, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015; 372:30-9; PMID:25399551; http://dx.doi.org/10.1056/NEJMoa1412690
- Gajewski TF, Meng Y, Blank C, Brown I, Kacha A, Kline J, Harlin H. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev 2006; 213:131-45; PMID:16972901; http://dx.doi.org/10.1111/j.1600-065X.2006.00442.x
- Jacobs JF, Punt CJ, Lesterhuis WJ, Sutmuller RP, Brouwer HM, Scharenborg NM, Klasen IS, Hilbrands LB, Figdor CG, de Vries IJ, et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: a phase I/II study in metastatic melanoma patients. Clin Cancer Res 2010; 16:5067-78; PMID:20736326; http://dx.doi.org/10.1158/1078-0432.CCR-10-1757
- Wilgenhof S, Corthals J, Heirman C, van Baren N, Lucas S, Kvistborg P, Thielemans K, Neyns B. Phase II study of autologous monocyte-derived mRNA electroporated dendritic cells (TriMixDC-MEL) plus ipilimumab in patients with pretreated advanced Melanoma. J Clin Oncol 2016; 34:1330-8; PMID:26926680; http://dx.doi.org/10.1200/JCO.2015.63.4121
- Ge Y, Xi H, Ju S, Zhang X. Blockade of PD-1/PD-L1 immune checkpoint during DC vaccination induces potent protective immunity against breast cancer in hu-SCID mice. Cancer Lett 2013; 336:253-9; PMID:23523609; http://dx.doi.org/10.1016/j.canlet.2013.03.010
- van Akkooi AC, Bouwhuis MG, van Geel AN, Hoedemaker R, Verhoef C, Grunhagen DJ, Schmitz PI, Eggermont AM, de Wilt JH. Morbidity and prognosis after therapeutic lymph node dissections for malignant melanoma. Eur J Surg Oncol 2007; 33:102-8; PMID:17161577; http://dx.doi.org/10.1016/j.ejso.2006.10.032
- Wheatley K, Ives N, Hancock B, Gore M, Eggermont A, Suciu S. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev 2003; 29:241-52; PMID:12927565; http://dx.doi.org/10.1016/S0305-7372(03)00074-4
- Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015; 16:522-30; PMID:25840693; http://dx.doi.org/10.1016/S1470-2045(15)70122-1
- Bakdash G, Schreurs I, Schreibelt G, Tel J. Crosstalk between dendritic cell subsets and implications for dendritic cell-based anticancer immunotherapy. Expert Rev Clin Immunol 2014; 10:915-26; PMID:24758519; http://dx.doi.org/10.1586/1744666X.2014.912561
- Lou Y, Liu C, Kim GJ, Liu YJ, Hwu P, Wang G. Plasmacytoid dendritic cells synergize with myeloid dendritic cells in the induction of antigen-specific antitumor immune responses. J Immunol 2007; 178:1534-41; PMID:17237402; http://dx.doi.org/10.4049/jimmunol.178.3.1534
- Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015; 348:69-74; PMID:25838375; http://dx.doi.org/10.1126/science.aaa4971
- Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015; 348:803-8; PMID:25837513; http://dx.doi.org/10.1126/science.aaa3828
- Delamarre L, Mellman I, Yadav M. Neo approaches to cancer vaccines. Science 2015; 348:760-1; PMID:25977539; http://dx.doi.org/10.1126/science.aab3465
- Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol 2008; 26:5275-83; PMID:18838703; http://dx.doi.org/10.1200/JCO.2008.17.8954
- Sznol M, Chen L. Antagonist antibodies to PD-1 and B7-H1 (PD-L1) in the treatment of advanced human cancer. Clin Cancer Res 2013; 19:1021-34; PMID:23460533; http://dx.doi.org/10.1158/1078-0432.CCR-12-2063
