ABSTRACT
Mycobacterium tuberculosis infects one third of the world's population. Due to variable efficacy of the Bacille Calmette Guerin (BCG) vaccine, development of novel TB vaccines remains a priority. Here, we demonstrate the protective efficacy of a novel multivalent DNA vaccine, which contains 15 synthetic antigens targeting the Mtb ESX secretion system.
KEYWORDS:
Tuberculosis (TB) is the leading cause of death from a single infectious agent in the world today.Citation1 The only currently-licensed vaccine against TB, M.bovis Bacille Calmette Guerin (BCG), protects against TB meningitis in infants and children, but has variable efficacy in protecting against adult pulmonary TB.Citation2 Development of a novel TB vaccine is therefore of paramount importance for the status of global health. Although a clear correlate of vaccine-induced protection has yet to be identified for TB, both interferon (IFN)-γ and interleukin-17A (IL-17) production by CD4+ T cells are targeted in vaccine-induced immunity to Mtb infection.Citation3,4 In addition, since mice lacking major histocompatibility complex (MHC)-I processing machinery or CD8+ T cells are more susceptible to TB disease,Citation5,6 CD8+ T cells have been implicated as having a role in control of Mtb infection. The novel multivalent DNA TB vaccine, RSQ-15, is designed using the pVax1 vector. The vector was used to express 15 synthetic consensus antigens of the Mtb ESX gene family (esxO, esxR, esxF, esxB, esxC, esxU, esxH, esxA, esxT, esxD, esxQ, esxE, esxV, esxS, and esxW). The antigens were selected based on diversity and cross-reactivity,Citation7 with the unique goal of inducing an immune response across a broad range of Mtb antigens, namely all 23 members of the ESX secretion system. The ESX secretion system is a family of Mtb proteins associated with mycobacterial virulenceCitation8 and contains several epitopes able to induce T cell responses in both humans and in animal models.Citation9,10 RSQ-15 is delivered intramuscularly (i.m.) followed by electroporation at the site of immunization in order to improve the immunogenicity of the DNA vector.Citation7,11 This vector/electroporation combination is approved for use in humans and is in clinical trials for treatment of Human Papilloma Virus-induced cervical disease, with good safety data thus far.Citation12 Previous results using RSQ-15 in mice have shown robust induction of multifunctional peripheral Mtb-specific CD4+ and CD8+ T cells.Citation7 On the basis of these preliminary immunogenicity results, here we investigate the protective efficacy of RSQ-15 immunization in the mouse model of TB. Our new results show that this novel multivalent TB vaccine confers protection to levels similar to BCG immunization. Thus, our study highlights novel strategies that can be targeted to design new vaccines against TB.
RSQ-15 immunization induces IFN-γ and tumor necrosis factor-α production by CD4+ and CD8+ T cells in the periphery.Citation7 Thus, we aimed to investigate whether RSQ-15 could also induce mucosal cytokine responses in the lungs. Eight week old C57BL/6 mice were immunized with 2 doses of 5µg RSQ-15, prepared as described in,Citation7 delivered intramuscularly (i.m.) followed by electroporation at the site of immunization.Citation7,11 One week after the final immunization, lungs were harvested and processed to a single cell suspension.Citation13 IFN-γ and IL-17 production was measured by antigen-driven ELISpot,Citation13 using 2.5µg/mL of a pool of all peptides represented in the RSQ-15 vaccine.Citation7 We observed robust Mtb-specific IFN-γ () but not IL-17 production (data not shown) by lung cells, indicating that RSQ-15 is a potent inducer of mucosal IFN-γ responses in the lungs, but that the antigens or DNA vaccine formulations are not permissive for induction of mucosal Th17 responses.
Figure 1. RSQ-15 induces potent IFN-γ responses in the lungs and confers protection against Mtb infection. (A) C57BL/6 mice were immunized with 2 doses at a 2 week interval of RSQ-15 followed by immediate electroporation. One week after the final immunization, lungs were harvested and antigen-specific IFN-γ production was determined by ELISpot measured as spot forming cells (SFC) per 106 cells. The pVax1 empty vector was used as a control. (B) C57BL/6 mice were immunized with 2 doses of RSQ-15, RSQ-15.IL-23, RSQ-15.pAg85B, RSQ-15.mtrIL-33, RSQ-15.pAg85B.IL-23, or RSQ-15.pAg85B.mtrIL-33 followed immediately by electroporation, with a 2 week interval between the doses. One week after the final immunization, lungs were harvested and antigen-specific cytokine production was determined by ELISpot. (C) C57BL/6 mice were immunized with either BCG, RSQ-15, RSQ-15.pAg85B.mtrIL-33, or BCG followed 4 weeks later by 2 boosts 2 weeks apart with RSQ-15.pAg85B.mtrIL-33 as described previously. Four weeks following the final immunization, mice were challenged with low dose aerosolized Mtb H37Rv, and bacterial burden in the lungs was assessed at 30 d post-infection. n = 5 ± SD, data show a combination of 2 experiments, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, assessed by one way ANOVA followed by Tukey's post-hoc test.
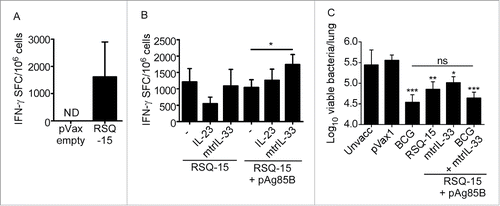
Molecular adjuvants are small molecules, such as cytokines, that can be co-administered with vaccines and can act as adjuvants. DNA constructs expressing the molecular adjuvant IL-33 have previously been shown to enhance cytokine production by T cells,Citation11,14 including responses to the Mtb antigen 85B (Ag85B).Citation11 Given the absence of IL-17 induction following RSQ-15 immunization alone, we also wanted to address whether inclusion of a construct expressing IL-23, a critical mediator of vaccine-induced IL-17 responses in Mtb infection models,Citation4,15 in the RSQ-15 vaccine could induce IL-17 responses. Finally, to further expand the antigen repertoire of RSQ-15, we also included a plasmid expressing Ag85B (pAg85B), a mycolyl transferase expressed in a system unrelated to the ESX secretion system.Citation16 Mice were immunized as described previously, with the inclusion of constructs expressing mtrIL-33, IL-23 or Ag85B. Adjuvanting RSQ-15 with mtrIL-33 or IL-23 alone did not have a significant effect on mucosal antigen-specific IFN-γ production (). Furthermore, including IL-23 in the RSQ-15 immunization regimen did not induce any detectable antigen-specific IL-17 responses (data not shown). Including pAg85B along with mtrIL-33 in the RSQ-15 vaccine, however, significantly improved mucosal antigen-specific IFN-γ production ().
Given the induction of high levels of Mtb-specific mucosal IFN-γ production in RSQ-15-immunized mice (), we next assessed the protective efficacy of the vaccine following Mtb H37Rv aerosol challenge in B6 mice. Mice were immunized with RSQ-15 as described previously. Control B6 mice received 1×106 cfu BCG delivered subcutaneously.Citation13 Four weeks after the final immunization, mice were infected with 100 cfu aerosolised Mtb H37Rv using a Glas-Col aerosol exposure system.Citation13 Infectious dose was determined at 24 hours post-infection by plating lung homogenates on 7H11 agar plates. Lungs of infected mice were harvested 30 d post-infection and homogenized and plated in serial dilutions on 7H11 agar plates to quantify bacterial burden. Subcutaneous immunization with BCG conferred ∼1 log protection over unimmunized control mice (). The RSQ-15 vaccine alone also conferred significant protection, with levels of protection not significantly different to that conferred by BCG. Despite improved induction of lung-resident antigen-specific IFN-γ responses (), however, immunization with RSQ-15.pAg85B.mtrIL-33 had no improved effect on vaccine efficacy compared that conferred by RSQ-15 alone (). The RSQ-15 vaccine has previously been demonstrated to boost BCG-induced immunogenicity.Citation7 Due to this, as well as the ability of pAg85B to enhance cytokine production in the lungs (), we aimed to determine whether boosting BCG with RSQ-15.pAg85B.mtrIL-33 would enhance the protective efficacy of BCG. We found, however, that mice immunized with BCG + RSQ-15.pAg85B.mtrIL-33 did not improve protection when compared to BCG alone ().
In this study, we sought to investigate the protective efficacy of RSQ-15, a novel synthetic vaccine expressing 15 consensus antigens of the ESX secretion system, with the goal of inducing T cell responses to all 23 Mtb ESX members. The antigens are expressed in a plasmid vector, the immunogenicity of which is enhanced by administering electroporation to the site of immunization. With the exception of BCG-based vaccines, such a broad antigen repertoire in a TB vaccine is unprecedented. A striking feature of RSQ-15-mediated immunogenicity is the induction of potent antigen-specific IFN-γ-producing cells in the lungs of immunized mice. Unless a vaccine is delivered mucosally, pre-clinical vaccine studies rarely assess the immunogenicity and induction of cytokine-producing cells in the lungs. In a study using adenovirus 5 expressing Ag85A (Ad5Ag85A) as a boost to intradermally (i.d.)-delivered BCG, i.d.-delivered AdAg85A induced cytokine-producing cells in the lungs, although not to levels induced by intranasal (i.n.) administration.Citation17 In a similar study comparing BCG administered s.c. followed by either i.m. or i.n.-administered AdAg85A, IFN-γ production in the bronchoalveolar lavage fluid was significantly lower in mice receiving a parenteral boost compared to those receiving a mucosal boost.Citation18 Thus, the ability of parenterally-delivered RSQ-15 to induce potent IFN-γ production in the lungs is, to our knowledge, unique.
The pVax1 expression system along with electroporation has primarily been used for immunization against viruses, and as such has been shown to be a potent inducer of CD8+ T cells.Citation12,14,19 RSQ-15 is also a potent inducer of CD8+ T cells, although CD4+ T cells are also induced, albeit to a lesser extent.Citation7 Although we did not assess which T cell subset was responsible for cytokine production in the lungs, it is likely, based on T cell subsets induced in the periphery, that the major cytokine-producing subset is CD8+ T cells. RSQ-15 is therefore unique in that it is the first multi-antigen TB vaccine to induce predominantly CD8+ T cells. Indeed, even protection conferred by the recombinant BCG ΔureChly+ vaccine, initially designed to induce CD8+ T cells,Citation20 was later thought to be mediated by CD4+ T cells.Citation21,22 The molecular adjuvant IL-33 has previously been shown to boost cytokine responses by both CD8+ and CD4+ T cells in a pVax1-based immunization regimen using Mtb, LCMV and HIV antigens.Citation11,14 Although administering RSQ-15 with mtrIL-33 alone had no significant effect on vaccine immunogenicity, co-administration of RSQ-15 + mtrIL-33 along with a plasmid expressing Ag85B greatly enhanced cytokine production in the lungs. Similar results were observed following immunization with plasmids expressing Ag85B alone adjuvanted with mtrIL-33,Citation11 suggesting that some feature of the Ag85B protein makes it a good candidate for being adjuvanted by mtrIL-33, perhaps due to the signaling mechanism of the protein through toll-like receptors or other pattern recognition receptors.Citation23,24 ESAT-6, a member of the ESX secretion system, and one of the proteins expressed in RSQ-15, has been shown to inhibit cytokine production by T cells;Citation25 it is therefore possible that the presence of ESAT-6 or other ESX proteins interferes with mtrIL-33 signaling through its receptor, ST2, and that the presence of Ag85B is required to overcome this.
Although RSQ-15 induces potent IFN-γ production, we detected no IL-17 induction. Given the importance of IL-17 in vaccine-induced protection against TB disease,Citation4,15 we aimed to induce the cytokine using a plasmid expressing IL-23, a cytokine essential to the proliferation of Th17 cells. Previous studies using DNA immunization and electroporation for the treatment of tumors used a plasmid expressing IL-23 to enhance Th17 responses.Citation26 Here, however, our attempt to induce IL-17 following RSQ-15 immunization using a plasmid expressing IL-23 failed. The reason for this is unclear, and could be due to a number of factors. It is possible that the antigens used are not conducive for the induction of Th17 cells, however this is unlikely, as we have previously shown that both ESAT-6 and Ag85B are able to induce IL-17 production.Citation27 Alternatively, it could be that the dose and length of electroporation either does not target Th17-inducing antigen-presenting cells, or induces non-responsiveness of antigen-presenting cell subsets responsible for Th17 induction. Finally, it is possible that RSQ-15 does not induce cytokines such as IL-1β or IL-6, which are important for the induction of Th17 cells,Citation28 and that IL-23 therefore needs to be administered along with other Th17-polarizing cytokines required for induction of Th17 cells for successful induction of IL-17-producing cells.
Electroporation as a method for adjuvanting DNA vaccine responses is growing in use, with promising results. The principle of the approach is to a) increase the uptake of the administered plasmid by cells at the site of injection, and b) possibly to influence local inflammation and the infiltration of antigen-presenting cells to the site of immunization.Citation29 Previous results using DNA vectors expressing TB antigens conferred limited protection against Mtb Citation30,31 or BCG challenge,Citation32 with the level of DNA vaccine-induced protection not equalling that induced by BCG. In contrast to these studies, following aerosol challenge with Mtb H37Rv, RSQ-15 conferred ∼1 log protection in the lungs. In spite of the addition of Ag85B and IL-33 to enhance the immunogenicity of the vaccine, however, protective efficacy was not improved. In an attempt to improve protection over BCG alone, RSQ-15.pAg85B.mtrIL-33 was administered as a boost to BCG, but again did not improve protection over levels induced by BCG alone. Given previous data showing the importance of IL-17 in control of Mtb infection,Citation4,15 the lack of IL-17 induction by RSQ-15 could account for the fact that even with the molecular adjuvant mtrIL-33 enhancing IFN-γ responses in this study, based on the immunization scheme tested, RSQ-15 did not improve on the protection conferred by BCG. It would therefore be worth exploring the use of a mucosal boost in an attempt to induce IL-17-producing cells in the lungs of RSQ-15-immunized mice, and hence improve vaccine-induced protection.
The synthetic DNA vaccine studied here delivered with electroporation represents a promising avenue to pursue for development of a novel multivalent TB vaccine, not least due to the fact that the approach has already been shown to be safe and very immune potent in humans.Citation12,19 Furthermore, given that pVax1 is a non-replicating vector, RSQ-15 could be safely administered to immunocompromised individuals. This is in contrast to BCG, which is contraindicated in infants exposed to HIV due to the risk of developing ‘BCG-osis’. This, along with the novel approach for inducing immunogenicity against a broad range of immunodominant and non-immunodominant antigens, suggests RSQ-15 is a promising vaccine candidate for future TB vaccines.
Abbreviations
| AdAg85A | = | adenovirus expressing Ag85A |
| Ag85B | = | antigen 85B |
| BCG | = | Mycobacterium bovis Bacille Calmette Guerin |
| cfu | = | colony forming units |
| ESAT-6 | = | early secretory antigenic target 6 |
| i.d. | = | intradermal |
| i.m. | = | intramuscular |
| i.n. | = | intranasal |
| IFN-γ | = | interferon gamma |
| IL-1β | = | interleukin 1β |
| IL-6 | = | interleukin 6 |
| IL-17 | = | interleukin 17A |
| IL-23 | = | interleukin 23 |
| MHC | = | major histocompatibility complex |
| mtrIL-33 | = | interleukin 33 |
| Mtb | = | Mycobacterium tuberculosis |
| RSQ-15 | = | pVax expressing 15 Mtb ESX antigens |
| TB | = | tuberculosis |
| Th17 | = | T helper 17 |
Disclosure of potential conflicts of interest
D.B.W. has grant funding, participates in industry collaborations, has received speaking honoraria, and fees for consulting. This service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments or stock or stock options and in the interest of disclosure therefore he notes potential conflicts associated with this work with Inovio, where he is a member of the SAB, Bristol Myers Squibb, Roche, Ferring, Touchlight, oncosec, Merck, VGXI, and possibly others. Licensing of technology from his laboratory has created over 100 jobs in the private sector in the biotech/pharma industry. The other authors declare no competing financial interests.
Acknowledgments
D.O.V. and D.B.W. would like to thank Penn CFAR and ACC core facilities for support of these studies. In addition the authors would like to thank Inovio Pharmaceuticals for their support of these studies, which include the final DNA formulations of RSQ-15 as well as the use of the cellectra EP system for synthetic DNA immunization.
Funding
Work presented here was supported by Washington University in St Louis and NIH grant HL105427 to S.A.K., American Lung Association Senior Research Training Fellowship RT-30592 and Alexander and Gertrude Berg Fellowship to K.L.G. D.O.V was supported by the National Institutes of Health (U19 AI078675; R01 AI092843; U19 AI109646).
References
- WHO. Global Tuberculosis Control. 2015
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. Jama 1994; 271:698-702; PMID:8309034; http://dx.doi.org/10.1001/jama.1994.03510330076038
- Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med 1993; 178:2243-7; PMID:8245795; http://dx.doi.org/10.1084/jem.178.6.2243
- Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after immunization and during Mycobacterium tuberculosis challenge. Nat Immunol 2007; 8:369-77; PMID:17351619; http://dx.doi.org/10.1038/ni1449
- Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med 2001; 193:271-80; PMID:11157048; http://dx.doi.org/10.1084/jem.193.3.271
- Behar SM, Dascher CC, Grusby MJ, Wang CR, Brenner MB. Susceptibility of mice deficient in CD1D or TAP1 to infection with Mycobacterium tuberculosis. J Exp Med 1999; 189:1973-80; PMID:10377193; http://dx.doi.org/10.1084/jem.189.12.1973
- Villarreal DO, Walters J, Laddy DJ, Yan J, Weiner DB. Multivalent TB vaccines targeting the esx gene family generate potent and broad cell-mediated immune responses superior to BCG. Hum Vaccin Immunother 2014; 10:2188-98; PMID:25424922; http://dx.doi.org/10.4161/hv.29574
- Stanley SA, Raghavan S, Hwang WW, Cox JS. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc Natl Acad Sci U S A 2003; 100:13001-6; PMID:14557536; http://dx.doi.org/10.1073/pnas.2235593100
- Brodin P, Rosenkrands I, Andersen P, Cole ST, Brosch R. ESAT-6 proteins: protective antigens and virulence factors? Trends Microbiol 2004; 12:500-8; PMID:15488391; http://dx.doi.org/10.1016/j.tim.2004.09.007
- Jones GJ, Gordon SV, Hewinson RG, Vordermeier HM. Screening of predicted secreted antigens from Mycobacterium bovis reveals the immunodominance of the ESAT-6 protein family. Infect Immun 2010; 78:1326-32; PMID:20086089; http://dx.doi.org/10.1128/IAI.01246-09
- Villarreal DO, Siefert RJ, Weiner DB. Alarmin IL-33 elicits potent TB-specific cell-mediated responses. Hum Vaccin Immunother 2015; 11(8):1954-60
- Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 2012; 4:155ra38; http://dx.doi.org/10.1126/scitranslmed.3004414
- Gopal R, Lin Y, Obermajer N, Slight S, Nuthalapati N, Ahmed M, Kalinski P, Khader SA. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG immunization. Eur J Immunol 2012; 42:364-73; PMID:22101830; http://dx.doi.org/10.1002/eji.201141569
- Villarreal DO, Svoronos N, Wise MC, Shedlock DJ, Morrow MP, Conejo-Garcia JR, Weiner DB. Molecular adjuvant IL-33 enhances the potency of a DNA vaccine in a lethal challenge model. Vaccine 2015; 33(35):4313-20; PMID:25887087
- Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol 2013; 6:972-84; PMID:23299616; http://dx.doi.org/10.1038/mi.2012.135
- Kremer L, Maughan WN, Wilson RA, Dover LG, Besra GS. The M. tuberculosis antigen 85 complex and mycolyltransferase activity. Lett Appl Microbiol 2002; 34:233-7; PMID:11940150; http://dx.doi.org/10.1046/j.1472-765x.2002.01091.x
- Forbes EK, Sander C, Ronan EO, McShane H, Hill AV, Beverley PC, Tchilian EZ. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 2008; 181:4955-64; http://dx.doi.org/10.4049/jimmunol.181.7.4955
- Santosuosso M, McCormick S, Zhang X, Zganiacz A, Xing Z. Intranasal boosting with an adenovirus-vectored vaccine markedly enhances protection by parenteral Mycobacterium bovis BCG immunization against pulmonary tuberculosis. Infect Immun 2006; 74:4634-43; PMID:16861651; http://dx.doi.org/10.1128/IAI.00517-06
- Morrow MP, Tebas P, Yan J, Ramirez L, Slager A, Kraynyak K, Diehl M, Shah D, Khan A, Lee J, et al. Synthetic consensus HIV-1 DNA induces potent cellular immune responses and synthesis of granzyme B, perforin in HIV infected individuals. Mol Ther 2015; 23:591-601; PMID:25531694; http://dx.doi.org/10.1038/mt.2014.245
- Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, Mann P, Goosmann C, Bandermann S, Smith D, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest 2005; 115:2472-9; PMID:16110326; http://dx.doi.org/10.1172/JCI24617
- Desel C, Dorhoi A, Bandermann S, Grode L, Eisele B, Kaufmann SH. Recombinant BCG DeltaureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis 2011; 204:1573-84; PMID:21933877; http://dx.doi.org/10.1093/infdis/jir592
- Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, Kaufmann SH. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine's superior protection against tuberculosis. J Infect Dis 2014; 210:1928-37; PMID:24943726; http://dx.doi.org/10.1093/infdis/jiu347
- Chatterjee S, Dwivedi VP, Singh Y, Siddiqui I, Sharma P, Van Kaer L, Chattopadhyay D, Das G. Early secreted antigen ESAT-6 of Mycobacterium tuberculosis promotes protective T helper 17 cell responses in a toll-like receptor-2-dependent manner. PLoS Pathog 2011; 7:e1002378; PMID:22102818; http://dx.doi.org/10.1371/journal.ppat.1002378
- Beamer GL, Cyktor J, Carruthers B, Turner J. H-2 alleles contribute to antigen 85-specific interferon-gamma responses during Mycobacterium tuberculosis infection. Cell Immunol 2011; 271:53-61; PMID:21714962; http://dx.doi.org/10.1016/j.cellimm.2011.06.002
- Wang X, Barnes PF, Dobos-Elder KM, Townsend JC, Chung YT, Shams H, Weis SE, Samten B. ESAT-6 inhibits production of IFN-gamma by Mycobacterium tuberculosis-responsive human T cells. J Immunol 2009; 182:3668-77; http://dx.doi.org/10.4049/jimmunol.0803579
- Kaiga T, Sato M, Kaneda H, Iwakura Y, Takayama T, Tahara H. Systemic administration of IL-23 induces potent antitumor immunity primarily mediated through Th1-type response in association with the endogenously expressed IL-12. J Immunol 2007; 178:7571-80; http://dx.doi.org/10.4049/jimmunol.178.12.7571
- Monin L, Griffiths KL, Slight S, Lin Y, Rangel-Moreno J, Khader SA. Immune requirements for protective Th17 recall responses to Mycobacterium tuberculosis challenge. Mucosal Immunol 2015; 8(5):1099-109; PMID:25627812
- Chung Y, Chang SH, Martinez GJ, Yang XO, Nurieva R, Kang HS, Ma L, Watowich SS, Jetten AM, Tian Q, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 2009; 30:576-87; PMID:19362022; http://dx.doi.org/10.1016/j.immuni.2009.02.007
- Flingai S, Czerwonko M, Goodman J, Kudchodkar SB, Muthumani K, Weiner DB. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol 2013; 4:354; PMID:24204366; http://dx.doi.org/10.3389/fimmu.2013.00354
- Kamath AT, Feng CG, Macdonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect Immun 1999; 67:1702-7; PMID:10085007
- Tanghe A, D'Souza S, Rosseels V, Denis O, Ottenhoff TH, Dalemans W, Wheeler C, Huygen K. Improved immunogenicity and protective efficacy of a tuberculosis DNA vaccine encoding Ag85 by protein boosting. Infect Immun 2001; 69:3041-7; PMID:11292722; http://dx.doi.org/10.1128/IAI.69.5.3041-3047.2001
- Huygen K, Content J, Denis O, Montgomery DL, Yawman AM, Deck RR, DeWitt CM, Orme IM, Baldwin S, D'Souza C, et al. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med 1996; 2:893-8; PMID:8705859; http://dx.doi.org/10.1038/nm0896-893
