ABSTRACT
Unprecedented clinical success has recently been achieved in cancer immunotherapy using cytotoxic T cells armed with activating tumor-specific Chimeric Antigen Receptors (CARs). Natural killer (NK) cells, potent cytotoxic effectors, also hold potential to be effectively harnessed for immunotherapy. The anti-tumor efficacy of NK cell therapies has been limited by a lack of antigen specificity and the poor persistence of NK cells in vivo. To address these limitations, Vallera and colleagues developed novel Trispecific Killer cell Engagers (TriKEs), reported in the Feb. 2016 issue of Clinical Cancer Research.Citation1 The novel TriKE immunomodulator evolved from the Bispecific Killer cell Engager (BiKE), a precursor developed by the same team. BiKEs comprise 2 antibody fragments, a first recognizing a tumor antigen and a second directed against CD16 on NK cells, which together trigger antibody-dependent cell-mediated cytotoxicity. IL-15 was further integrated to create TriKEs in order to drive NK cell expansion. Compared to BiKEs, TriKEs elicit far superior NK cytotoxicity and NK cell persistence in a xenograft tumor model in vivo, and are proposed to be effective adjuncts to existing NK transfer protocols. Importantly, TriKEs provide a versatile and cost-effective platform onto which novel targeting ligands can be incorporated and hold the potential to stimulate endogenous NK cells in order to circumvent the need for cell transfers altogether, heralding a new generation of immunotherapeutics.
NK cell killing of tumors
Natural Killer (NK) cells are known to play a role in cancer immune surveillance.Citation2 They are potent cytotoxic effectors that were first identified in the 1970s as being able to lyse tumors without prior antigen sensitization.Citation3,4 NK cells can kill via several mechanisms: by delivering lytic granules containing perforin and granzymes (degranulation),Citation5 by secreting effector cytokines Citation6 and by engaging death-inducing receptors through surface ligands such as FasL and TRAIL to induce target cell apoptosis.Citation7 NK cells also express CD16 (or FcγRIII), an activating receptor for the conserved Fc portion of IgG. When IgG-coated target cells bind to CD16, target cell killing is triggered via antibody-dependent cell-mediated cytotoxicity (ADCC).Citation8 NK cell-mediated ADCC is potent and can occur without the need for co-activation from other receptors ().
Figure 1. Natural killer cell-mediated killing of target cells. (A) No killing of healthy cells due to a balance of activating and inhibitory signals. (B) Killing of targets due to down-regulation of MHC molecules (“missing self”); killing due to overexpression of stress ligands (“induced self”) that can override inhibitory signals; killing due to the recognition of antibodies bound to target cells by CD16 on NK cells (“antibody-dependent cell-mediated cytotoxicity” (ADCC). (C) BiKEs and TriKEs facilitate NK cell interaction with tumor targets via CD16, (D) triggering degranulation and cytokine production via the ADCC pathway.
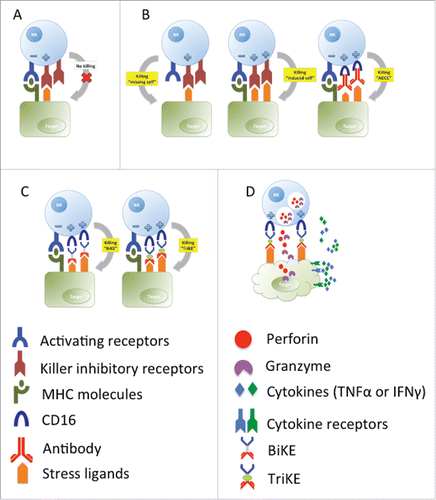
Unlike T cells that rearrange gene segments to generate antigen-specific receptors, NK cells recognize their target ligands using an array of germ-line encoded receptors. These include both activating and inhibitory receptors, from which signals are integrated to determine NK cell function (reviewed inCitation9). Activating receptors recognize a number of stress ligands that may be overexpressed by tumors.Citation10 Tumors may also downregulate MHC molecules recognized by the NK inhibitory receptors. It is believed that NK cells are therefore activated to kill tumors due to overriding activating signals and/or lack of inhibitory signals due to ‘missing-self’Citation11 (). This mode of target cell recognition lacks specificity however, and a key challenge in harnessing NK cells for cancer immunotherapy has been to develop strategies that enhance their specificity for tumors. Another challenge of NK cell-based therapies is to maintain NK cell numbers and function in vivo. Citation12-14 Therefore, many genetic and pharmacological approaches are currently being pursued to re-direct NK cells, augment their function and sustain their in vivo expansion and persistence (reviewed in Citation9,15).
BiKEs and TriKEs effectively drive NK cell anti-tumor effects
The TriKE reagent builds upon previous versions of engineered bispecific molecules designed to crosslink tumors and NK cells, termed Bispecific Killer cell Engagers (BiKEs) (). An example of a BiKE created by Vallera and colleagues is one that comprises a single-chain variable fragment (scFv) domain specific for CD16 on NK cells and a second scFv specific for the acute myeloid leukemia (AML) antigen CD33 (known as 1633 BiKE).Citation16 In vitro, 1633 BiKEs could overcome inhibitory killer-cell immunoglobulin-like receptor (KIR) signaling to stimulate NK cells to kill AML blasts.Citation16 Stimulation of NK cells with 1633 BiKEs in vitro also restored NK cell functions that had been inhibited in patients with myelodysplastic syndrome (MDS).Citation17
In the current study, Vallera and colleagues integrated IL-15 into the existing BiKE to create the novel 161533 TriKECitation1 (). The TriKE therefore performs 3 key functions: (a) to direct NK cells to tumors by facilitating formation of intracellular synapses; (b) to bind CD16 on NK cells to trigger ADCC; and (c) to drive in vivo NK cell expansion. IL-15 was selected (instead of IL-2) to promote NK cell activation, expansion and survival in order to avoid problems associated with the use of IL-2: the risk of systemic vascular leak Citation18 and the concurrent activation of CD25+ T regulatory cells that could inhibit NK cell function.Citation12,19
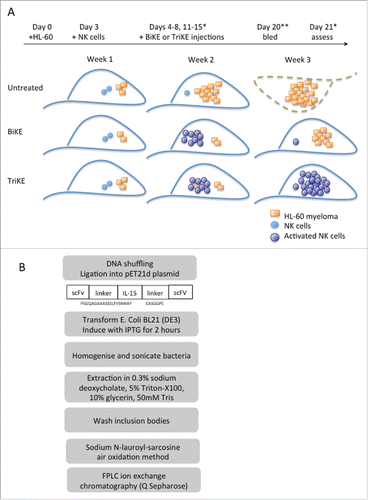
When compared to its predecessor BiKE, the TriKE elicited superior NK cell killing of CD33+ myeloma cell lines and primary AML blasts, by enhancing NK cell degranulation, cytokine production (), proliferation and survival in vitro.Citation1 It was also superior to the BiKE in restoring NK proliferation and killing functions in samples obtained from patients who had recently undergone haematopoietic stem cell transplantation (HSCT)Citation1. The significance of this capacity is discussed in the subsequent section. In vivo efficacy of the TriKE was further demonstrated in a xenograft model where human NK cells were adoptively transferred into mice to eradicate engrafted human CD33+ myeloma cells Citation1 (). Tumor load was reduced significantly 3 weeks after NK cell infusion when the transferred NK cells were stimulated with TriKE but not with BiKE, and at this time point, significant increases in NK cells in the blood after TriKE stimulation was recorded. Together, this study showed that TriKE elicited superior anti-tumor responses from NK cells and supported their in vivo persistence.
Figure 2. Production of TrIKEs and functional testing in a xenograft model.Citation1 (A) TrIKEs are expressed as recombinant proteins in bacteria before refolding and purification. Peptide linkers flanking IL-15 are indicated. (B) TriKEs and BiKEs promote NK cell-mediated killing of engrafted HL-60. TriKEs further support NK cell expansion and persistence that is associated with tumor clearance. A luciferase-expressing HL-60 myeloma cell line (7.5 × 105) was delivered intravenously 3 d before adoptive transfer of 1 × 106 CD3/CD19-depleted NK cells that were stimulated overnight with IL-15. TriKEs or BiKES were injected subcutaneously (50 μg each injection) for 10 d. *Tumor load was assessed by chemiluminesence after luciferin administration on days 14 and 21. **NK cells in peripheral blood were enumerated.
TriKEs complement NK transfer therapies for haematological malignancies
Allogeneic HSCT is a current standard treatment for acute myeloid leukemia (AML) and for myelodysplastic syndrome (MDS) but is compromised by treatment-associated mortality and high relapse rates.Citation20,21 It is thought that defective NK function early after HSCT may play a role in these relapses.Citation22 Therefore, infusion of fully functional NK cells is an option for consolidation therapy while patients are in remission.Citation19 Since TriKEs were found to restore NK function in samples from recipients that had undergone HSCT in vitro, it is expected that TriKEs may also find clinical application when administered in vivo to activate reconstituting NK cells early after HSCT. It was noted in the current study (1) that TriKEs activated NK cells but did not induce T cell proliferation within the same post-HSCT samples. The mechanism for this differential activation is unknown but a strategy that avoids expanding T cells (that could mediate graft-versus-host responses) while being able to potentiate the graft-vs.-leukemia responses of NK cells warrants further investigation.
Apart from HSCT, the infusion of haploidentical NK cells (without aiming for donor haematopoesis) has also been trialled for treating AML or ALL. Pilot studies have produced good outcomes with increased safety profiles when improved NK purification protocols and reduced IL-2 doses have been used.Citation13,14,23 The use of IL-2 is still not ideal, however, and by selecting an IL-15-containing TriKE to support NK cell expansion, Vallera and colleagues hope to avoid IL-2-mediated toxicities and to avoid the expansion of regulatory T cells.Citation24 It was also hypothesized that incorporating IL-15 within a TriKE would reduce the risk of systemic toxicity as this restricts IL-15 availability to local NK-target cell synapses (Citation1 and personal communication, D. Vallera). Further, IL-15 may be more relevant than IL-2 in this clinical setting, as it was noticed in 2 clinical trials that a transient surge in IL-15 production correlated with NK reconstitution,Citation13,14 suggesting that the presence of IL-15-stimulated NK cells may be associated with leukemic remission.
TriKEs are versatile and amenable to further optimization
The TriKE is a versatile platform amenable to incorporation of unique combinations of targeting ligands by straightforward molecular cloning approaches () and,Citation25 with IL-15 integrated, flanked by linkers, in between the dual targeting scFv domains. The TriKE is then readily produced as a recombinant protein in bacteria and purified by chromatography. Relevant scFv domains against clinically tested tumor-associated antigens, including those from solid tumors such as EpCAM Citation25 can be expressed within a TriKE. It is also possible to target 2 tumor antigens simultaneously, as described in a tri-specific molecule comprising scFv against CD19 and CD22 as well as against CD16.Citation26
As TriKEs can be generated with different specificities, they provide the option to re-target NK cells in accordance with the emergence of tumor-associated antigens during tumor escape and immunoediting, which can impede the long-term success of cancer therapies. A remaining question pertinent to repeated TriKE stimulation, however, is whether TriKE-expanded NK cells sustain CD16 expression and whether their progeny will express unoccupied CD16 available for further stimulation (personal communication, D. Vallera). To overcome the problem of CD16 shedding upon NK cell activation, Vallera and colleagues had previously investigated if concurrent administration of an ADAM17 inhibitor with TriKES to prevent CD16 shedding might be useful.Citation16
Apart from altering targeting specificities, intrinsic TriKE function might be enhanced by simple engineering of scFv variants with higher affinity for CD16. For instance, modifying the Fc fragment of humanised antibodies to increase CD16 binding enhances ADCCCitation27,28 a similar strategy could work in the context of TriKEs.
While a CD16-specific TriKE (and BiKE) primarily takes advantage of ADCC, additional NK cell receptors might also be suitable for TriKE-mediated targeting. A particularly promising candidate is the activating receptor Natural Killer Group 2D (NKG2D), which has important roles in tumor surveillance. The NKG2D pathway is the target of several tumor escape mechanisms,Citation29 including shedding of NKG2D ligands by tumors, and/or de-sensitization and down-regulation of NKG2D due to chronic stimulation. Of particular interest, in 2015, a soluble murine-UL16-binding protein-like transcript (MULT)-1 ligand was identified. This soluble MULT1 was found to, contrary to expectations, increase NK cell activity by binding to NKG2D.Citation30 It is thought that soluble MULT1 stabilises NKG2D on the cell surface instead of inducing its downregulation, in part due to its higher affinity for NKG2D compared to previously described ligands. This mode of engagement could overcome inhibitory signals delivered via a second NKG2D ligand, retinoic acid early inducible (RAE)-1, resulting in maintenance of NK functions. A targeting modality based on soluble MULT1 could be tested within the context of a TriKE.
The TriKE platform not only provides options to target different receptors, or novel tumor ligands, but also provides a way to explore new targeting strategies based upon newly discovered molecules reasonably rapidly.
Could TriKEs become a cost-effective off-the-shelf alternative to NK cell transfer?
Rapid improvements are being made in technologies for producing clinical-grade NK cells suitable for adoptive transfer. The sources of NK cells are diverse, including cell lines, peripheral and cord blood, or pluripotent stem cells (reviewed in Citation31). Similarly, many protocols for ex vivo primary NK cell expansion using cytokine cocktails and/or feeder cells are also being developed for clinical use.Citation32,33 TriKEs could be used to potentiate these NK expansion protocols by conferring antigen specificity, by boosting ADCC and by supporting NK cell expansion, pre- or post-infusion. For instance, in addition to in vivo applications, TriKEs may also be useful for stimulating and expanding NK cells in vitro in the presence of appropriate ligands (email discussion with D. Vallera).
At this stage, however, it is unclear if TriKEs could be used to bypass the need of NK cell transfer altogether. TriKE activity has thus far only been tested on transferred human NK cells in a xenograft model. While TriKES could drive dramatic levels of NK cell expansion (up to 350 fold compared to BiKE) for up to 3 weeks,Citation1 it is not known if in vivo administration of TriKEs alone will be sufficient to stimulate expansion of endogenous NK populations and their activity to therapeutically relevant levels (personal communication, D. Vallera). It will be interesting to see the outcomes of further preclinical testing of TriKEs: for instance, to determine whether they can expand and redirect a physiologically relevant quantity and diversity of human NK cells in mice with humanised immune systems. The development of humanised models able to support full human NK cell reconstitution and suitable for testing NK cell functions, including ADCC, would further these investigations.Citation34
Safety and efficacy considerations
Clinical trials for haematological malignancies using patient T cells armed with activating, tumor-specific chimeric antigen receptors (CARs) have achieved unprecedented remission rates (recently reviewed in Citation35). CAR T cells targeting CD19 have brought 70 to 90% of patients with refractory or relapsed ALL into remission.Citation36,37 Despite these spectacular successes, there have been reports of treatment-related deaths and severe side effects associated with cytokine-release syndrome after infusion of CAR T-cells.Citation38-40 By virtue of IL-15's potential to stimulate and expand both T cells and NK cells, TriKEs could present similar risks of activating systemic immune responses by triggering of cytokine cascades.Citation1 Indeed, the recent report of unexpected and severe acute graft-versus-host responses in several patients who had been given infusions of NK cells (pre-activated with IL-15 and TNFSF9), cautions that depending on their mode of activation, NK cells could initiate damaging immune responses.Citation33 It was encouraging that Vallera and colleagues showed more than 10 doses of 20μg/day of IL-15-incorporating-TriKE administered to xenografted SCID immunodeficient mice for 2 weeks did not cause observable detrimental effects despite successful activation and expansion of NK cells. However, longer-term safety evaluations in additional model systems are required to monitor for TriKE-mediated immune toxicities, or their separate biological effects on tumors. One such system could be tumor-engrafted immunocompetent mice, since TriKE reacts with the mouse IL-15 receptor (personal communication, D. Vallera).
Another important risk after cancer immunotherapy is the untoward killing of healthy cells, as tumor antigens can be shared with healthy cells. Indeed, persisting CAR T cells specific for CD19 or CD38 have been reported to cause long-term B cell depletion or bone marrow suppression in patients, respectively. While the shorter lifespan of NK cells are among several properties that led researchers to consider them “better CAR drivers” (discussed in Citation41), leading to genetic modifications of NK cell lines (NK-92 in particular) and primary NK cells with CARs to target different tumors,Citation42,43 TriKEs provide a simpler alternative for re-targeting NK cells in vivo without genetic modification. However, further characterisations of the proliferative and functional responses of NK cells under various TriKE dosing regimens are required. These studies would provide insight into the biological effect of TriKEs on NK cells, in regard to their activation and maturation and conversely, exhaustion; their activities upon cessation of TriKE administration; and their potential to develop into long-lived “memory” populations.Citation44
The road ahead for cancer immunotherapy
Cancer immunotherapy has been hailed as the biggest breakthrough in modern cancer treatment. While the field is currently dominated by CAR T cell therapies and antibody therapies that aim to reverse immunosuppression of T cells, NK cell-based approaches are beginning to move to the forefront. TriKES and BiKES, with their potential to enable tumor-specific targeting, to boost NK functions and to drive cellular expansion hold promise as versatile, cost-effective and widely accessible off-the-shelf solutions, when compared to more complex and costly genetic modifications, ex vivo expansion protocols or more personalised therapies. TriKES and BiKES can be used in conjunction with existing therapies and also have the potential to be used in lieu of NK cell transfer. We are optimistic that positive outcomes from further preclinical evaluations of the safety and efficacy profiles of TriKEs and BiKEs will support progress toward clinical testing, and herald potent novel avenues for immunotherapeutic treatments for cancers.
Disclosure of potential conflicts of interest
The authors whose names are listed above have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers' bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- Vallera DA, Felices M, McElmurry RT, McCullar V, Zhou X, Schmohl J, Zhang B, Lenvik A, Panoskaltsis-Mortari A, Verneris MR, et al. IL-15 Trispecific Killer Engagers (TriKEs) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing In Vivo Expansion, and Enhanced Function. Clin Cancer Res 2016; PMID:26847056 [Epub ahead of print]
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000; 356:1795-9; PMID:11117911; http://dx.doi.org/10.1016/S0140-6736(00)03231-1
- Herberman RB, Nunn ME, Holden HT, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 1975; 16:230-9; PMID:1080480; http://dx.doi.org/10.1002/ijc.2910160205
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer 1975; 16:216-29; PMID:50294; http://dx.doi.org/10.1002/ijc.2910160204
- Krzewski K, Coligan JE. Human NK cell lytic granules and regulation of their exocytosis. Front Immunol 2012; 3:335; PMID:23162553; http://dx.doi.org/10.3389/fimmu.2012.00335
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44-9; PMID:21212348; http://dx.doi.org/10.1126/science.1198687
- Wallin RP, Screpanti V, Michaelsson J, Grandien A, Ljunggren HG. Regulation of perforin-independent NK cell-mediated cytotoxicity. Eur J Immunol 2003; 33:2727-35; PMID:14515256; http://dx.doi.org/10.1002/eji.200324070
- Farag SS, Caligiuri MA. Human natural killer cell development and biology. Blood Rev 2006; 20:123-37; PMID:16364519; http://dx.doi.org/10.1016/j.blre.2005.10.001
- Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer 2015; 16:7-19; http://dx.doi.org/10.1038/nrc.2015.5
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31:413-41; PMID:23298206; http://dx.doi.org/10.1146/annurev-immunol-032712-095951
- Karre K. Immunology. A perfect mismatch. Science 2002; 295:2029-31; PMID:11896262; http://dx.doi.org/10.1126/science.1070538
- Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood 2014; 123:3855-63; PMID:24719405; http://dx.doi.org/10.1182/blood-2013-10-532531
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005; 105:3051-7; PMID:15632206; http://dx.doi.org/10.1182/blood-2004-07-2974
- Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol 2010; 28:955-9; PMID:20085940; http://dx.doi.org/10.1200/JCO.2009.24.4590
- Childs RW, Carlsten M. Therapeutic approaches to enhance natural killer cell cytotoxicity against cancer: the force awakens. Nat Rev Drug Discov 2015; 14:487-98; PMID:26000725; http://dx.doi.org/10.1038/nrd4506
- Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, et al. Targeting natural killer cells to acute myeloid leukemia in vitro with a CD16 x 33 bispecific killer cell engager and ADAM17 inhibition. Clin Cancer Res 2013; 19:3844-55; PMID:23690482; http://dx.doi.org/10.1158/1078-0432.CCR-13-0505
- Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, et al. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood 2014; 123:3016-26; PMID:24652987; http://dx.doi.org/10.1182/blood-2013-10-533398
- Baluna R, Vitetta ES. Vascular leak syndrome: a side effect of immunotherapy. Immunopharmacology 1997; 37:117-32; PMID:9403331; http://dx.doi.org/10.1016/S0162-3109(97)00041-6
- Stern M, Passweg JR, Meyer-Monard S, Esser R, Tonn T, Soerensen J, Paulussen M, Gratwohl A, Klingebiel T, Bader P, et al. Pre-emptive immunotherapy with purified natural killer cells after haploidentical SCT: a prospective phase II study in two centers. Bone Marrow Transplant 2013; 48:433-8; PMID:22941380; http://dx.doi.org/10.1038/bmt.2012.162
- Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol 2010; 3:429-41; PMID:21083034; http://dx.doi.org/10.1586/ehm.10.32
- Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk acute leukaemia. Semin Immunol 2014; 26:173-9; PMID:24613727; http://dx.doi.org/10.1016/j.smim.2014.02.004
- Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood 2011; 118:2784-92; PMID:21757615; http://dx.doi.org/10.1182/blood-2011-04-347070
- Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R, Balduzzi A, Owoc-Lempach J, Fagioli F, Or R, et al. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood 2010; 115:3437-46; PMID:20040760; http://dx.doi.org/10.1182/blood-2009-03-207001
- Miller JS, Rooney CM, Curtsinger J, McElmurry R, McCullar V, Verneris MR, Lapteva N, McKenna D, Wagner JE, Blazar BR, et al. Expansion and homing of adoptively transferred human natural killer cells in immunodeficient mice varies with product preparation and in vivo cytokine administration: implications for clinical therapy. Biol Blood Marrow Transplant 2014; 20:1252-7; PMID:24816582; http://dx.doi.org/10.1016/j.bbmt.2014.05.004
- Schmohl JU, Felices M, Taras L, Miller JS, Vallera DA. Enhanced ADCC and NK cell activation of an anti-carcinoma bispecific antibody by genetic insertion of a modified IL-15 cross-linker. Mol Ther 2016.
- Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Mol Cancer Ther 2012; 11:2674-84; PMID:23075808; http://dx.doi.org/10.1158/1535-7163.MCT-12-0692
- Romain G, Senyukov V, Rey-Villamizar N, Merouane A, Kelton W, Liadi I, Mahendra A, Charab W, Georgiou G, Roysam B, et al. Antibody Fc engineering improves frequency and promotes kinetic boosting of serial killing mediated by NK cells. Blood 2014; 124:3241-9; PMID:25232058; http://dx.doi.org/10.1182/blood-2014-04-569061
- Bowles JA, Wang SY, Link BK, Allan B, Beuerlein G, Campbell MA, Marquis D, Ondek B, Wooldridge JE, Smith BJ, et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood 2006; 108:2648-54; PMID:16825493; http://dx.doi.org/10.1182/blood-2006-04-020057
- Belting L, Homberg N, Przewoznik M, Brenner C, Riedel T, Flatley A, Polic B, Busch DH, Rocken M, Mocikat R. Critical role of the NKG2D receptor for NK cell-mediated control and immune escape of B-cell lymphoma. Eur J Immunol 2015; 45:2593-601; PMID:26151313; http://dx.doi.org/10.1002/eji.201445375
- Deng W, Gowen BG, Zhang L, Wang L, Lau S, Iannello A, Xu J, Rovis TL, Xiong N, Raulet DH. Antitumor immunity. A shed NKG2D ligand that promotes natural killer cell activation and tumor rejection. Science 2015; 348:136-9; PMID:25745066; http://dx.doi.org/10.1126/science.1258867
- Dahlberg CI, Sarhan D, Chrobok M, Duru AD, Alici E. Natural Killer Cell-Based Therapies Targeting Cancer: Possible Strategies to Gain and Sustain Anti-Tumor Activity. Front Immunol 2015; 6:605; PMID:26648934; http://dx.doi.org/10.3389/fimmu.2015.00605
- Choi I, Yoon SR, Park SY, Kim H, Jung SJ, Jang YJ, Kang M, Yeom YI, Lee JL, Kim DY, et al. Donor-derived natural killer cells infused after human leukocyte antigen-haploidentical hematopoietic cell transplantation: a dose-escalation study. Biol Blood Marrow Transplant 2014; 20:696-704; PMID:24525278; http://dx.doi.org/10.1016/j.bbmt.2014.01.031
- Shah NN, Baird K, Delbrook CP, Fleisher TA, Kohler ME, Rampertaap S, Lemberg K, Hurley CK, Kleiner DE, Merchant MS, et al. Acute GVHD in patients receiving IL-15/4-1BBL activated NK cells following T-cell-depleted stem cell transplantation. Blood 2015; 125:784-92; PMID:25452614; http://dx.doi.org/10.1182/blood-2014-07-592881
- Shiokawa M, Takahashi T, Murakami A, Kita S, Ito M, Sugamura K, Ishii N. In vivo assay of human NK-dependent ADCC using NOD/SCID/gammac(null) (NOG) mice. Biochem Biophys Res Commun 2010; 399:733-7; PMID:20696130; http://dx.doi.org/10.1016/j.bbrc.2010.07.145
- Frey NV, Porter DL. CAR T-cells merge into the fast lane of cancer care. Am J Hematol 2016; 91:146-50; PMID:26574400; http://dx.doi.org/10.1002/ajh.24238
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, Fry TJ, Orentas R, Sabatino M, Shah NN, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 2015; 385:517-28; PMID:25319501; http://dx.doi.org/10.1016/S0140-6736(14)61403-3
- Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371:1507-17; PMID:25317870; http://dx.doi.org/10.1056/NEJMoa1407222
- van den Berg JH, Gomez-Eerland R, van de Wiel B, Hulshoff L, van den Broek D, Bins A, Tan HL, Harper JV, Hassan NJ, Jakobsen BK, et al. Case Report of a Fatal Serious Adverse Event Upon Administration of T Cells Transduced With a MART-1-specific T-cell Receptor. Mol Ther 2015; 23:1541-50; PMID:25896248; http://dx.doi.org/10.1038/mt.2015.60
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther 2010; 18:843-51; PMID:20179677; http://dx.doi.org/10.1038/mt.2010.24
- Minagawa K, Zhou X, Mineishi S, Di Stasi A. Seatbelts in CAR therapy: How Safe Are CARS? Pharmaceuticals (Basel) 2015; 8:230-49; PMID:26110321
- Klingemann H. Are natural killer cells superior CAR drivers? Oncoimmunology 2014; 3:e28147; PMID:25340009; http://dx.doi.org/10.4161/onci.28147
- Boissel L, Betancur-Boissel M, Lu W, Krause DS, Van Etten RA, Wels WS, Klingemann H. Retargeting NK-92 cells by means of CD19- and CD20-specific chimeric antigen receptors compares favorably with antibody-dependent cellular cytotoxicity. Oncoimmunology 2013; 2:e26527; PMID:24404423; http://dx.doi.org/10.4161/onci.26527
- Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, Tonn T. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother 2016; 65:485–92.
- Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, `Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119:2665-74; PMID:22180440; http://dx.doi.org/10.1182/blood-2011-10-386995
