ABSTRACT
An observational post-licensure (Phase IV) retrospective large-database safety study was conducted at Kaiser Permanente, a US integrated medical care organization, to assess the safety of Tetanus Toxoid, Reduced Diphtheria Toxoid and 5-Component Acellular Pertussis Vaccine (Tdap5) administered as part of routine healthcare among adolescents and adults. We evaluated incidence rates of various clinical events resulting in outpatient clinic, emergency department (ED), and hospital visits during various time intervals (windows) following Tdap5 vaccination using 2 pharmacoepidemiological methods (risk interval and historic cohort) and several screening thresholds. Plausible outcomes of interest with elevated incidence rate ratios (IRRs) were further evaluated by reviewing individual patient records to confirm the diagnosis, timing (temporal relationship), alternative etiology, and other health record details to discern possible relatedness of the health events to vaccination. Overall, 124,139 people received Tdap5 vaccine from September 2005 through mid-October 2006, and 203,154 in the comparison cohort received a tetanus and diphtheria toxoid adsorbed vaccine (and no live virus vaccine) during the year prior to initiation of this study. In the outpatient, ED and hospital databases, respectively, we identified 11/26, 179/700 and 187/700 unique health outcomes with IRRs significantly >1.0. Among the same unique health outcomes in the outpatient, ED, and hospital databases, 9, 146, and 385, respectively, had IRRs significantly <1.0. Further scrutiny of the outcomes with elevated IRRs did not reveal unexpected signals of adverse outcomes related to vaccination. In conclusion, Tdap5 vaccine was found to be safe among this large population of adolescents and adults.
Introduction
In the United States, approximately 95% of children receive 4 or 5 doses of diphtheria toxoid, tetanus toxoid, and acellular pertussis (DTaP)-containing vaccines by the time they enter kindergarten.Citation1 Although infants experience the greatest burden of mortality from pertussis, adolescents and adults can experience prolonged coughing, paroxysms, post-tussive vomiting, and a variety of complications from the disease (which often goes undiagnosed) and they serve as sources of infection for younger family members.Citation2-5 Despite high vaccine coverage in infants and children, since the 1980s the US has experienced periodic epidemics of pertussis, with reported incidence increasing over time.Citation6-10 Adacel® (Tetanus Toxoid, Reduced Diphtheria Toxoid and 5-component Acellular Pertussis Vaccine Adsorbed, Sanofi Pasteur, Swiftwater, PA; hereafter abbreviated as Tdap5) was designed to boost immunity against tetanus, diphtheria, and pertussis among adolescents and adults.
In 2005, Tdap5 vaccine was licensed in the United States for use in those aged 11 through 64 y and that same year the US Advisory Committee on Immunization Practices (ACIP) recommended a single dose of Tdap for all adolescents and adults aged 11–64 y.Citation11 In 2012, ACIP extended its recommendation to include all those aged 65 y and older.Citation12 As part of a post-licensure requirement with the US Food and Drug Administration, this study aimed at evaluating the safety of Tdap5 vaccine when used as part of routine health care in a large cohort of US adolescents and adults during the first year after its licensure.
Results
From September 2005 through mid-October 2006, a total of 124,139 people received Tdap5 vaccine and the comparison cohort consisted of 203,154 individuals who received a tetanus and diphtheria toxoid adsorbed (Td) vaccine (but no live virus vaccine) during the prior year. The number of Tdap5 recipients by age group was: younger than 11 years, 1,049; 11–17 years, 49,165; 18–39 years, 25,566; 40–64 years, 45,295; older than 64 years, 3,064.
Demographic and baseline characteristics
A summary of demographic characteristics of the Tdap5 vaccine cohort and Td historical comparison cohort, including sex, age, race, and time/season of vaccination, is provided in . Among the Tdap5 cohort, 57,072 were male (46.0%), 67,067 female (54.0%), the mean age was 32.1 years, and 43.0% were White. A total of 65,838 people were administered one or more vaccines concomitantly with Tdap5 vaccine, the most frequent of which were hepatitis A vaccine and meningococcal vaccine. The demographic profile of the Td historical comparison cohort was generally similar to that of the population of Tdap5 vaccine recipients.
Table 1. Summary of demographic characteristics.
ED and hospital
For the ED and hospital settings, there were no pre-determined outcomes of interest. When the 2 statistical methods (risk-interval cohort and historical cohort) were applied to calculate IRRs within predefined age group and risk-interval strata, the total number of IRRs (comparisons) calculated from each database was 20,328.
Of the 20,328 comparisons in the ED and hospital settings, 349 (1.72%) and 588 (2.89%) respectively had IRRs that were significantly (p < 0.05) >1.0, representing 179 and 187 unique outcomes. Conversely, 227 (1.12%) and 594 (2.92%) had IRRs significantly <1.0 in the ED and hospital databases, respectively, representing 146 and 385 unique outcomes ().
Figure 1. Steps in focusing reviews of outcomes and comparisons in outpatient (OP), emergency department (ED) and inpatient (IP) databases. aIRR >1.0 and its 95% CI LB excludes 1.0 on risk-interval cohort and/or historical cohort comparison(s). bIRR >1.0 on Td historical cohort comparison; IRR >2 and n ≥ 3 or IRR 95% CI LB >1.5 and n ≥ 5 on risk-interval comparison. cPI discretion. dPI classified outcomes with statistically elevated IRRs into 4 groups: likely due to confounding by indication; implausible; non-specific; and all other (i.e., potentially plausible). eIRR >1.0 and its 95% CI LB excludes 1.0 on Td historical cohort comparison. fPer PI discretion, added back 1 outcome (hepatitis) that had not been retained at previous screening step. CI, confidence interval; ED, emergency department; IP, inpatient; IRR, incidence rate ratio; LB, lower bound; n, number of people exposed to Adacel vaccine; OP, outpatient; PI, principal investigator.
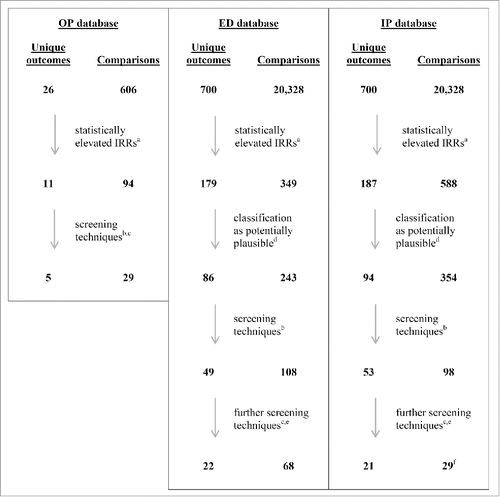
Selection of outcomes in the ED and hospital databases for further scrutiny
The list of outcomes classified as likely due to confounding by indication included all fractures, injuries, open wounds, and contusions. These likely represented situations when a trauma event led to an ED or hospital encounter with subsequent administration of vaccine for tetanus prophylaxis.
The list of outcomes classified as implausible included 58 diagnoses (e.g., codes associated with acquired and congenital deformities, burns, neoplasms, and infections [Table S1 supplemental material S1]) that were often pre-existing or had a clear alternative (i.e., non-vaccine related) explanation.
The list of outcomes classified as non-specific included 22 diagnoses (e.g., codes associated with immunization, medical examination, substance abuse, device complications, ill-defined conditions, and factors influencing health status [Table S2 supplemental material S1) for which exact diagnoses were not clear; therefore, it was determined that no further investigation of these outcomes was feasible.
The following sections focused on the remaining potentially plausible outcome categories.
Potentially plausible outcomes with elevated IRRs in the ED and hospital
Of the 366 unique outcomes in the ED and hospital settings combined, we identified 180 unique outcomes with elevated IRRs that were deemed potentially plausible. Restrictive criteria, as outlined in the methods, resulted in retaining 102 unique outcomes (49 in the ED and 53 in the hospital databases), as shown in Tables S3 and S4 (supplemental material S1).
Of these 102 unique outcomes, 21 were retained for further scrutiny because they met the screening criteria (i.e., IRR > 2 and n ≥ 3 or LB 95% CI of the IRR >1.5 and n ≥ 5) in the 6-month historical cohort comparisons. Of the remaining 81 unique outcomes, 9 were retained for further scrutiny because the IRR in the post-hoc Td comparison was >1 and statistically significant and 13 more were retained at the discretion of the investigators. These 43 unique outcomes (22 from ED, 21 from hospital databases) were selected for further scrutiny. The selected outcomes are shown in Tables S5 and S6 (supplemental material S1), with reason(s) as to why they were selected.
Further scrutiny of these 43 outcomes was performed as described in Methods. In many cases, the diagnoses were pre-existing or were associated with other non-vaccine conditions. In time plots, the cases were spread out evenly over the 60- or 180-day surveillance period or cases were more prevalent late (60–180 d post-vaccination); the category contained diverse or non-specific diagnoses; or the number of cases were so few or the rates of illness so low that no conclusions could be drawn. Specifics of the investigations are detailed in supplemental material S2. This further scrutiny did not reveal any new or unexpected adverse outcomes associated with the vaccine.
Outpatient analyses: Prespecified health outcomes of interest (HOI)
Prespecified outcomes are detailed in Methods. Of 606 comparisons, 94 (15.5%) had elevated IRRs that were significantly >1.0 (see ). These involved 11 unique HOI (arthritis, arthralgia, or arthropathy; Bell's palsy; diabetes; encephalopathy; febrile illness; hypersensitivity; multiple sclerosis; neuralgia; neuritis; neuropathy; and severe local reaction), as listed in Table S7 (supplemental material S1). Application of the criteria described in Methods led to the following results:
Arthritis, arthralgia or arthropathy; neuritis; neuropathy: none of the IRRs for the protocol-specified risk interval cohort analyses were >2, nor were any of their LB 95% CIs >1.5, with most IRRs close to or <1 in the Td historical cohort comparisons, indicating the rates of these conditions were similar to or lower than those of Td-exposed cases.
Diabetes: Although IRRs were >2 and most of their LB 95% CIs were >1.5 in the risk interval cohort analyses, all IRRs were closer to 1 and most were <1 in the Td historical cohort comparisons, indicating the rates of diabetes outcomes were similar to or lower than those of Td-exposed cases.
Neuralgia: LB 95% CI was <1.5 and there were only 11 Tdap5-exposed cases.
Severe local reaction: Although IRRs were > 2 and all had LB 95% CIs that were > 1.5 in the risk interval cohort analyses, these were expected findings. Also, all IRRs were closer to 1 and most were < 1 in the Td historical cohort comparisons, indicating the rates of reactions were similar to or lower than those of Td-exposed cases.
Five of the HOI (Bell's palsy, encephalopathy, febrile illness, hypersensitivity, and multiple sclerosis) were selected for further scrutiny because the IRRs were also elevated in the Td historical comparison analysis. Details of the 5 outcomes and reasons for further scrutiny are listed in Table S8 (supplemental material S1).
Further scrutiny of the 5 HOI above was performed using methods that varied with the clinical findings and availability of data. These included chart review, review of whether the diagnosis was primary or secondary, and time plots. The additional investigation revealed similar results as described for the ED and hospital outcomes (e.g., the diagnoses were pre-existing), or the association was not unexpected (e.g., febrile illness). The methods and results of this further scrutiny are detailed in the supplemental material S3. This further scrutiny did not reveal any new or unexpected adverse effects of the vaccine.
Review of mortality data identified 65 people who at various times prior to their deaths had received Tdap vaccine. The majority of cases were 50 y of age and older. Cause of death was determined for all cases based on hospital records, death certificates, and other information. No clustering by the onset of death was observed. Causes were multiple and included cancers, poisoning, trauma, suicide, alcoholism, HIV, coronary artery disease, and sepsis. None of the deaths were considered by the investigators to be related to vaccination. A formal analysis of causes of death was not undertaken. Four serious adverse events possibly related to vaccination were identified in the course of data review. These included 2 cases of Bell's palsy, one case of epilepsy, and one case of Guillain-Barré syndrome (GBS). The GBS case occurred 25 d after receiving a dose of Tdap vaccine and was classified as a level 2 of diagnostic certainty based on the Brighton Collaboration Case Definition. For detail, see supplemental material S4.
Discussion
This large, population-based study of over 120,000 recipients of Tdap5 vaccine verified the results of clinical trials and showed the vaccine to be safe in this population. No new signals of previously unrecognized vaccine-related adverse events (AEs) were identified. Known or expected outcomes requiring contact with the health care system occurred in the populations studied at a very low rate, confirming findings in clinical trials.
This study confirms findings of an earlier observational study of Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine safety conducted as part of the Vaccine Safety Datalink (VSD) project.Citation13 Between 7 August 2005 and 17 May 2008, data from 8 VSD sites were prospectively monitored using sequential analyses (a method known as the Maximized Sequential Probability Ratio Test). HOI included encephalopathy/encephalitis/meningitis, paralytic syndromes, seizure, cranial nerve disorders (including Bell's palsy), and Guillain-Barré Syndrome. The authors found no evidence of an association between Tdap vaccine and any of the 5 predefined AEs in a surveillance period that included 660,245 doses of Tdap vaccine over the course of 145 weeks. Most Tdap vaccine used in adults in this study was likely Tdap5 vaccine because the other Tdap vaccine (Boostrix®, GlaxoSmithKline Biologicals, Rixensart, Belgium) was initially indicated for ages 10 through 18 y and received US Food and Drug Administration approval of an expanded age indication only in December of 2008, well after the observation period.
The risk-interval cohort method, despite its advantages (e.g., implicit control for individual-level unmeasured confounding), cannot avoid all forms of bias.Citation14 One such bias to which a risk-interval analysis may be more susceptible is an unmasking bias.Citation15 Specifically, events on the vaccination date (e.g., laboratory tests ordered, appointments scheduled with specialists) drive future diagnoses in observation windows after the vaccination date. Indeed, this was borne out when review of all available multiple sclerosis cases showed that they were all prevalent cases, and that visits on the day of vaccination generated follow-up visits with specialists (during which the patients received the multiple sclerosis diagnosis). Similar findings were observed with encephalopathy cases, with the majority of these being prevalent migraine cases referred on the day of vaccination for procedures or follow-up examinations by specialists. As visits to specialists are generally scheduled within a short time frame after a well-care visit, this can show up as a cluster of visits after vaccination. These findings are similar to allergic reaction visits appearing after vaccination, when mainly these are referrals from the medicine department to the allergy clinic or the dermatology department based on a complaint of a pre-existing condition during an exam made on the day of vaccination.
A number of outcomes with elevated IRR, e.g., ‘Complications’ and ‘Respiratory arrest’, were classified in this study as “non-specific.” Because these were not manually reviewed, no possible causality conclusions can be drawn for these events.
One limitation of the historical cohort comparison method used in this study is its inability to dismiss the possible causal association of chronic diseases when the rates of the outcomes are similar in both vaccine comparison cohorts (Tdap and Td). A different study design, e.g., a large randomized trial with long-term follow-up, would be required to further evaluate a possible causal association.
An observational database screening study of this size will generate many findings based on chance alone. There was no correction for multiple comparisons in this study, and over 40,000 comparisons were made. Overall, it was found that 2.50% of the 41,262 total comparisons had elevated IRRs, and 2.03% had decreased IRRs. Using methods tailored to the findings, we examined outcomes with significantly elevated IRRs; no new or unexpected safety signals were found.
Secular trends may also play a part in generating study findings. Although Tdap and Td vaccines are both given in the ED, Tdap vaccine is more likely to be given preventively as part of “well care.” As discussed above in regard to unmasking, well care generates follow-up in a way that is likely different than with emergency care. Vaccine given at an emergency visit for a wound of some sort might be more likely to be associated later with a visit for (post-wound) cellulitis, for example. In other words, although these populations are generally comparable, they cannot be expected to be perfectly comparable. Similarly, people who are well may be more likely to “self-select” for receipt of Tdap vaccine as preventive care than people who are not well.
In conclusion, Tdap5 vaccine was found to be safe in the population studied. No new or unexpected safety signals were found.
Methods
Study setting
Kaiser Permanente (KP) is an integrated healthcare organization with comprehensive well maintained electronic medical record databases capturing all aspects of medical care received by enrollees. This study was a collaboration between 3 regions of the organization, KP Northern California region, KP Northwest region, and KP Colorado region, coordinated by the Kaiser Permanente Vaccine Study Center (KPVSC) in the Northern California region. Data were pooled from electronic patient records of these 3 regions into a common data structure. Each KP member has a unique medical record number that links information across services for the same individual over time. Electronic records are maintained for all outpatient clinic visits, emergency department (ED) visits, and hospitalizations. These records include vaccinations, medical diagnoses, laboratory tests, medications, and procedures, as well as demographic and membership information. Over 90% of hospitalizations and ED visits for members occur within the KP system, and medical encounters occurring outside of the system are captured through database tracking of referrals and claims for reimbursement.
Study design
This was a retrospective observational large database screening study (ClinicalTrials.gov; NCT00258882). The study protocol and the study design were reviewed and approved by the FDA prior to the study launch. All people receiving Tdap5 vaccine at any of the study sites from September 2005 through mid-October 2006 were identified and included in the analyses. There were no exclusion criteria. No pre-specified hypotheses were formulated prior to the start of the analyses. Instead, all health outcomes for the period of up to 6 months following vaccination, including state death reports, were captured and reviewed. To be able to screen for both short-term and long-term outcomes in outpatient clinic, emergency department and hospital databases, 2 analytical methods were used in conjunction: a risk-interval cohort method and an historical cohort comparison method.
Risk interval cohort analyses: This method has been utilized in similar vaccine post-licensure safety studies.Citation16-18 Only individuals vaccinated with Tdap5 vaccine were included in the analyses. Rates of medically attended events (termed below as “outcomes”) occurring during the risk intervals following immunization (7, 14, 30, and 60 days) were compared to rates of events occurring in the same individuals during days 61 to 120 following vaccination. Multiple risk intervals were chosen, as different outcomes might be more likely to occur within different time frames after immunization.Citation19 Incidence rate ratios (IRRs) were calculated for the time intervals being compared, along with 95% confidence intervals (CIs) and unadjusted 2-sided p-values estimated using the exact conditional method with mid-probability adjustment.Citation20,21 Note that IRRs are also referred to as “comparisons.”
Historical cohort analyses: This method was applied to screen for possible new-onset chronic illnesses during the 6 months following vaccination. We identified a comparison cohort of KP members who received Td vaccine, without a concomitant live virus vaccine, during the year prior to initiation of this study (September 2004 through November 2005). Event rates during the 6 months following vaccination with Tdap5 vaccine were compared to event rates during the 6 months following Td vaccine. IRRs were calculated for the time intervals compared, along with 95% CIs and 2-sided p-values. Analyses were performed separately for each age subgroup (<11 years, 11–17 years, 18–39 years, 40–64 years, and ≥65 years), and for all ages combined.
Outcomes
ED, hospital, and outpatient outcomes were analyzed separately. In ED and hospital databases, all unique health outcomes (ICD-9 codes) were considered, whereas for the outpatient database, only prespecified outcomes of interest, selected prior to the study, were analyzed ().
Table 2. Prespecified outcomes of interest for the outpatient database.
No formal statistical adjustment for multiple comparisons was applied. Because the number of associations with elevated IRRs in the ED and hospital databases was expected to be large, given the number of outcomes, settings, risk intervals, and age groups compared, it was necessary to limit the number of outcomes for further review to those that were clinically important. Seven hundred unique outcomes (ICD-9 codes) were manually reviewed by the investigators (RB and VP) and classified into 4 groups: (1) outcomes likely due to confounding by indication (e.g., Tdap5 or Td vaccination associated with an injury), (2) implausible, (3) non-specific, and (4) potentially plausible. Complete lists of outcomes for each of these groups are available in supplemental material S1.
In order to refine this list to be more clinically and statistically relevant, we applied more restrictive post-hoc criteria. First, we compared rates for all Group 4 outcomes with significantly elevated IRR in the risk-interval comparisons with rates in the historical Td cohort, using the same time window (0–7, 0–14, 0–30, or 0–60 days) and age group (“post-hoc Td comparison”) to see if rates of adverse events following Tdap5 vaccine were higher than seen after Td. Outcomes that had an elevated IRR in both the risk-interval and post-hoc historical Td analyses received additional consideration (e.g., chart review). Second, for all significantly elevated IRRs in either the risk-interval or historical cohort comparison, we gave greater scrutiny to outcomes where either 1) the lower bound of the 95% CI (LB 95% CI) of the IRR was >1.5 and the number of Tdap5-vaccinated recipients with the given diagnosis in the given age group and the specific risk window was ≥5, or (2) the value of the IRR was >2 and the number of Tdap5-vaccinated people with the given diagnosis in the given age group and specific risk window was ≥3. In addition to the aforementioned techniques, all outcomes with significantly elevated IRRs were considered for further scrutiny by one of the Investigators (RB), judging biological plausibility and consistency of outcomes (i.e., an outcome being statistically significant in multiple analyses). The investigator used this discretion to add outcomes for further scrutiny that did not meet the above criteria; however, there was no discretion to ignore or exclude any outcomes from scrutiny.
For outcomes that met criteria for greater scrutiny, we then required the IRR of the post-hoc Td comparison to be statistically significant (p < 0.05). After applying the above criteria, a variety of methods, which varied with the clinical finding and availability of data, were used for further review. These included medical record review, review of whether the diagnosis was a primary or secondary diagnosis, and time plots of adverse events.
Sample size considerations
Because this was an observational study of multiple outcomes following real-life vaccine use in the general population, undertaken without prespecified hypotheses, no formal sample size calculation was feasible. Nevertheless, prior to study initiation, it was specified that at least 10,000 adolescents and 6,000 adults exposed to Tdap5 vaccine would be included.
Nested study of Tdap5-exposed pregnancies
In this study, 225 women received Tdap5 during pregnancy. Their outcomes and those of their infants will be reported in a separate article [Unpublished data].
Abbreviations
| ACIP | = | Advisory Committee on Immunization Practices |
| AE | = | adverse event |
| CI | = | confidence interval |
| DTaP | = | diphtheria toxoid, tetanus toxoid, and acellular pertussis |
| ED | = | emergency department |
| HOI | = | health outcomes of interest |
| IP | = | inpatient |
| IRR | = | incidence rate ratio |
| KP | = | Kaiser Permanente |
| KPVSC | = | Kaiser Permanente Vaccine Study Center |
| LB | = | lower bound |
| OP | = | outpatient |
| PI | = | principal investigator |
| Td | = | tetanus and diphtheria toxoid adsorbed |
| Tdap | = | Tetanus toxoid, reduced diphtheria toxoid and acellular pertussis |
| Tdap5 | = | Tetanus Toxoid, Reduced Diphtheria Toxoid and 5-Component Acellular Pertussis Vaccine |
| VSD | = | Vaccine Safety Datalink |
Disclosure of potential conflicts of interest
RB has received research funding from GSK, MedImmune, Novartis and Sanofi Pasteur. VP, DPG, DRJ and MDD are employees of Sanofi Pasteur. RB, JH and JT are employees of Kaiser Permanente.
Authors' contributions
RB, VP, DPG, DRJ and MDD conceived and designed the study. JH and JT acquired the data and performed the statistical analysis. All authors analyzed and interpreted the data, drafted the manuscript, critically revised the manuscript for important intellectual content, and have seen and approved the final manuscript for submission. No external writing support was used for the preparation of the manuscript.
Supplementary files
Download MS Word (63.9 KB)Acknowledgments
The authors would like to acknowledge Eric France, MD, Kaiser Permanente Colorado, and Sheila Weinmann, PhD, Kaiser Center for Health Studies for their assistance with study management and oversight in their respective regions.
Funding
This research was sponsored by Sanofi Pasteur. Kaiser Permanente authors did not receive compensation for their consultancy work on this manuscript.
References
- Seither R, Masalovich S, Knighton CL, Mellerson J, Singleton JA, Greby SM. Vaccination coverage among children in kindergarten - United States, 2013-14 school year. MMWR Morb Mortal Wkly Rep 2014; 63:913-20; PMID:25321068
- Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, Grimprel E, Greenberg D, Halperin S, Liese J, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J 2007; 26:293-9; PMID:17414390; http://dx.doi.org/10.1097/01.inf.0000258699.64164.6d
- Cherry JD. Epidemiology of pertussis. Pediatr Infect Dis J 2006; 25:361-2; PMID:16567990; http://dx.doi.org/10.1097/01.inf.0000210478.60841.69
- Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, Rebmann CA, Gabel J, Schauer SL, Lett SM. Infant pertussis: who was the source? Pediatr Infect Dis J 2004; 23:985-9; PMID:15545851; http://dx.doi.org/10.1097/01.inf.0000145263.37198.2b
- Lavine J BH, Harvill ET, Bjornstad ON. Imperfect vaccine-induced immunity and whooping cough transmission to infants. Vaccine 2011; 29:11-6; http://dx.doi.org/10.1016/j.vaccine.2010.10.029
- Edwards K, Decker M. Pertussis Vaccines. In: Plotkin S, Orenstein WA, Offit P, eds. Vaccines, Elsevier, 2008:467-517
- Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics 2005; 115:1422-7; PMID:15867059; http://dx.doi.org/10.1542/peds.2004-2648
- Centers for Disease Control and Prevention. Pertussis epidemic - Washington, 2012. MMWR 2012; 61:517-22; PMID:22810264
- Nennig ME, Shinefield HR, Edwards KM, Black SB, Fireman BH. Prevalence and incidence of adult pertussis in an urban population. JAMA 1996; 275:1672-4; PMID:8637142; http://dx.doi.org/10.1001/jama.1996.03530450062034
- Tan T, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatr Infect Dis J 2005; 24:S10-8; PMID:15876918; http://dx.doi.org/10.1097/01.inf.0000160708.43944.99
- Centers for Disease Control and Prevention. Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR 2006; 55:1-37
- Centers for Disease Control and Prevention. Updated Recommendations for Use of Tetanus Toxoid, Reduced Diphtheria Toxoid, and Acellular Pertussis (Tdap) Vaccine in Adults Aged 65 Years and Older — Advisory Committee on Immunization Practices (ACIP), 2012. MMWR 2012; 61:468-70; PMID:22739778
- Yih WK, Nordin JD, Kulldorff M, Lewis E, Lieu TA, Shi P, Weintraub ES. An assessment of the safety of adolescent and adult tetanus-diphtheria-acellular pertussis (Tdap) vaccine, using active surveillance for adverse events in the Vaccine Safety Datalink. Vaccine 2009; 27:4257-62; PMID:19486957; http://dx.doi.org/10.1016/j.vaccine.2009.05.036
- Andrews NJ. Statistical assessment of the association between vaccination and rare adverse events post-licensure. Vaccine 2001; 20(Suppl 1):S49-53; PMID:11587812; http://dx.doi.org/10.1016/S0264-410X(01)00280-8
- Jacobsen SJ, Sy LS, Ackerson BK, Chao CR, Slezak JM, Cheetham TC, Takhar HS, Velicer CM, Hansen J, Klein NP. An unmasking phenomenon in an observational post-licensure safety study of adolescent girls and young women. Vaccine 2012; 30:4585-7; PMID:22580356; http://dx.doi.org/10.1016/j.vaccine.2012.04.103
- Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, Lewis N, Wu X, Ambrose CS. A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in adults 18-49 years of age. Vaccine 2012; 30:3053-60; PMID:22425787; http://dx.doi.org/10.1016/j.vaccine.2012.02.080
- Baxter R, Toback SL, Sifakis F, Hansen J, Bartlett J, Aukes L, Lewis N, Wu X, Ambrose CS. A postmarketing evaluation of the safety of Ann Arbor strain live attenuated influenza vaccine in children 5 through 17 years of age. Vaccine 2012; 30:2989-98; PMID:22386746; http://dx.doi.org/10.1016/j.vaccine.2012.02.039
- Baxter R, Tran TN, Hansen J, Emery M, Fireman B, Bartlett J, Lewis N, Saddier P. Safety of Zostavax–a cohort study in a managed care organization. Vaccine 2012; 30:6636-41; PMID:22963800; http://dx.doi.org/10.1016/j.vaccine.2012.08.070
- Rowhani-Rahbar A, Klein NP, Dekker CL, Edwards KM, Marchant CD, Vellozzi C, Fireman B, Sejvar JJ, Halsey NA, Baxter R. Biologically plausible and evidence-based risk intervals in immunization safety research. Vaccine 2012; 31:271-7; PMID:22835735; http://dx.doi.org/10.1016/j.vaccine.2012.07.024
- Flanders WD, Dash C. Exact logistic regression: an extension of Barnard's approach for continuous covariates. Int J Stat Manag Syst 2009; 4:82-95
- Mehta CR, Walsh SJ. Comparison of xxact, mid-p, and Mantel-Haenszel confidence intervals for the common odds ratio across several 2 × 2 contingency tables. Am Stat 1992; 46:146-50
