ABSTRACT
The Bacillus Calmette-Guerin (BCG) vaccine is not a single organism, but consists of substrains that vary in genotypes and phenotypes. Actually, BCG is the common name given to a family of vaccines created in 1921 by the in vitro attenuation of a virulent Mycobacterium bovis in France. Even nearly a century of use, the BCG vaccine lingers generating confusion and debate due to its diversity and failure to protect against tuberculosis (TB). That is probably owing to the enduring lack of standardization during production, distribution and administration procedures. Since the 1940s, substantial sequence modifications among the BCG substrains have been described. To increase the level of complexity, even though that the prolific generation of recombinant BCG vaccines has been promising, the relationships between those candidates used in current clinical trials and their parental substrains are either unsatisfactorily connected or have been never fully delineated. Consequently, the use of the most protective BCG substrain as the background or platform in the development of all recombinant BCG vaccine candidates has not been standardized. In order to schematize and to clarify the subject regarding substrains commonly used to generate those novel vaccines, a sequential emergence of the parental BCG vaccine substrains and their matching recombinant ones, if any, was built. Hence, for a total of 24 BCG substrains currently in circulation worldwide, 9 have been used to sustain one or more genetic modifications, resulting in around 21 novel recombinant BCG vaccines. Although this is a remarkable success, only 2 out of the 21 recombinant BCG substrains harbor a background representative of the most immunogenic group. Systematizing the novel BCG vaccines and their parental strains may facilitate our understanding of protection provided by BCG immunizations.
The Bacillus Calmette-Guerin (BCG) is the only licensed tuberculosis (TB) vaccine in use at present, with an estimated 100 million children receiving BCG every year globally. The BCG vaccine is not a single organism, but consists of substrains that vary in genotype and phenotype.Citation1 BCG is the common name given to a family of vaccines created in 1921 by the in vitro attenuation of a virulent Mycobacterium bovis in France. Like many other currently employed vaccines, BCG was developed empirically. After approximately a century of use, the BCG vaccine continues to generate confusion and debate due to its diversity and failure to protect against TB. This is probably owing to the lack of standardization during production, distribution and administration procedures.
Several reasons explain the inability of BCG vaccine to protect against adult pulmonary TB, such as those associated with the substrain, as well as dose, site and route of administering the vaccine. Concerning the former, BCG has partial nitrogen metabolic capacity, which limits the multiplication and persistence of this live vaccine within the host.Citation2 Additionally, evidence strongly indicates that over time, protection wanes with aging. BCG is clearly affected by unknown host and/or environmental variables that modify the ability of certain groups of vaccinees to react effectively.Citation3 Despite being a broadly used vaccine in human history, the deficiency of a model allied to the mechanisms by which this vaccine protects against TB and its effects on the immune system remain unknown. On the one hand, there are no conceivable studies on the effectiveness of different BCG routes of administration. On the other hand, comparative genetic studies of BCG vaccines used worldwide have shown that the currently employed substrains are diverse.Citation4
Since the 1940s, substantial sequence modifications among BCG substrains have been described. Despite the promising prolific generation of recombinant BCG vaccines, the relationships between candidates currently used in clinical trials and their parental substrains are either unsatisfactorily connected or not fully defined. Consequently, there are no standards defining the use of the most protective BCG substrain as the background or platform for the development of all recombinant BCG vaccine candidates. Some experts in the field are confident that original TB vaccines based upon a given BCG background, or novel approaches using BCG for priming or boosting protocols against adult forms of TB, will be invaluable in the future. Hence, selections of viral, bacterial and parasitic antigens expressed in BCG have been used as a primary platform, and these recombinant mycobacteria are able to induce immune responses. Previously, our team revisited the latest advances in the TB vaccine focusing on understanding the immune response necessary to control this pathogen.Citation5 At that time, major emphasis was given to the route of administration, either mucosa or parenteral. It was anticipated that there was going to be some progress toward the improvement of the BCG vaccine. Since then, there have been concerns regarding intrinsic discrepancies in parental substrains of recombinant BCG vaccines used in pre-clinical and clinical trials. However, these issues remain unresolved and there is a need to update this topic.
A database using sequential emergence of the parental BCG vaccine substrains and their matching recombinant ones (if any) was built as an attempt to categorize the substrains commonly used to generate novel vaccines (). For a total of 24 BCG substrains (excluding BCG Copenhagen) currently in circulation worldwide, roughly a third (total of 9) have been used for one or more genetic modifications, resulting in around 21 novel recombinant BCG vaccines. [Note: Beijing. Chanchung, Lanzhou and Shanghai BCG were not clustered as China BCG]. Out of these, VPM1002 has been shown to be safe and immunogenic for B- and T-cell responses, and it is currently facing phase IIb clinical trials. VPM1002 is also the most protective vaccine in terms of reducing lung bacteremia.Citation6 Although this is a remarkable success, only 2 out of the 21 recombinant BCG substrains harbor a background representative of the most immunogenic group (Group I): a recombinant BCG Moreau expressing Ag85B-ESAT6 fusion protein Citation7 and a recombinant BCG Japan with a frameshift mutation in the l-alanine dehydrogenase gene (ald).Citation8 Yet, none of these candidates have advanced to clinical trials.
Figure 1. Main features of all BCG vaccine substrains. Footnote: Common names: a. China; b. Actual. Note: Related names and culture cycles (no. passages, if any) of the BCG vaccine substrains: Moreau or Brazil; Polish or Poland; Russia or Moscow (3522); Sofia or Bulgaria (222); Japan or Tokyo (172); Romanian or Romania (192); Sweden or Gothenburg; Prague or Czech (725); Connaught or Toronto; Frappier or Montreal; Glaxo or London (1077); Tice or Chicago; Phipps or Philadelphia; Denmark/Copenhagen (?) or Danish (1331); Madras or India (809).
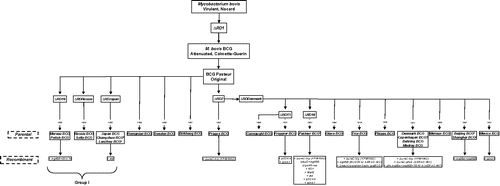
Different BCG vaccines induce different immune responses in humans. It has been hypothesized that under-representation of BCG substrains from group I (Moreau, Russia and Japan) may reduce the efficacy of the recombinant BCG vaccines. This is possibly due to the fact that they do not have the high immunogenicity inherent to group I background, despite the fact that there are virtually no surrogate markers to predict vaccine efficacy.Citation9 Besides safety records and other supportive features, serious adverse reactions associated to the BCG vaccine substrains from group I are rare and local complications may be related to good delayed-type hypersensitivity skin test response (at least for the BCG Moreau). The most compelling safety evidence comes from the central register of adverse events of BCG vaccination, which was established in 1994 by the Polish National Tuberculosis Center.Citation10 Before 1955, when either BCG Pasteur or BCG Danish was used, there were reports of cases of osteitis media. Since then, the mass BCG vaccination in Poland has been relying on the Brazilian-derived BCG Moreau strain instead, and osteitis media has no longer been observed after the adoption of this strain.Citation10 BCG Moreau very rarely produces complications and has outstanding safety records in Brazil.Citation5 Even though, nearly 90% of routine vaccination campaigns worldwide use BCG Denmark, BCG Russia and BCG Japan. In addition, systemic disorder and lethal dissemination are also unusual for the BCG vaccine, except in cases of cutaneous manifestations of disseminated BCG-induced diseases (BCGosis and BCGitis) in children with severe combined immunodeficiency.Citation11 However, it should be noticed that BCG Moreau may be clinically heterogeneous and leads to degree of virulence in animal models.Citation12 It still remains to be determined whether the current Citation7 or future stable, genetically engineered substrain will improve the efficacy of BCG Moreau without affecting its safety. Recently, BCG Moreau showed superior ability to induce in vitro monocyte apoptosis in adult healthy donors when compared with BCG Pasteur and BCG Danish (unpublished results). Apart from preliminary data, there have been very few comparative studies of BCG strains in recent years, despite the fact that inconsistencies in BCG vaccine efficacy may vary according to the substrain. Alternatively, BCG strain performance may also depend on the setting, but there is no evidence that phenotypic differences relate to differences in protective immunity.
M. tuberculosis is an extremely well-adapted and an enormously successful human pathogen. It has co-existed with the human host for millennia and it can infect the host for decades without triggering the clinical disease. Reactivation only happens when the host's immunity is depressed. This pathogen has learned how to modulate potentially protective host responses to ensure its own survival. Although there has been a great deal of progress in TB vaccine development over the past decade, this infection presents distinctive challenges to vaccine development which are not faced in other diseases. Candidates for novel vaccines against TB based on diverse BCG backgrounds are valuable tools for TB control. The most promising TB vaccine candidate currently undergoing clinical trials was derived from the original BCG vaccine. This candidate, however, has background from groups III and IV. One can hypothesize that greater representation of BCG substrains from the most immunogenic group (Group I) may be responsible for a highest efficacy of this candidate. Systematizing novel BCG vaccines and their parental substrains may facilitate our understanding of protection mechanisms provided by BCG immunization. In order to develop a BCG vaccine capable of generating an immune response that eradicates TB in the host, it is necessary to characterize both protective and pathogenic immune responses against M. tuberculosis infection. The more we know of the natural and vaccine-induced response to this pathogen, the better placed we will be to improve immune-interventions. Advances in the fields of immunology and molecular biology have stimulated research into new vaccination techniques for TB and alternative approaches to develop more reliable tools to induce a protective immune response against this disease are warranted.
Disclosure of potential conflicts of interest
The author has no commercial association that poses a conflict of interest.
Acknowledgments
The author is grateful to Mr. Patrick Salinas and to Mrs. Veronica Antas for text editing.
Funding
This work was supported by FAPERJ-JCNE and CNPq-PQ-2 research fellowships, and Fiocruz.
References
- Behr MA, Small PM. A historical and molecular phylogeny of BCG strains. Vaccine 1999 Feb 26; 17(7–8):915-22; PMID:10067698; http://dx.doi.org/10.1016/S0264-410X(98)00277-1
- Chen JM, Alexander DC, Behr MA, Liu J. Mycobacterium bovis BCG vaccines exhibit defects in alanine and serine catabolism. Infect Immun 2003 Feb; 71(2):708-16; PMID:12540549; http://dx.doi.org/10.1128/IAI.71.2.708-716.2003
- Smith D, Wiegeshaus E, Balasubramanian V. An analysis of some hypotheses related to the Chingelput bacille Calmette-Guerin trial. Clin Infect Dis 2000 31:S77-S80; PMID:11010828; http://dx.doi.org/10.1086/314073
- Brosch R, Gordon SV, Billault A, Garnier T, Eiglmeier K, Soravito C, Barrell BG, Cole ST. Use of a Mycobacterium tuberculosis H37Rv bacterial artificial chromosome library for genome mapping, sequencing, and comparative genomics. Infect Immun 1998 May; 66(5):2221-9; PMID:9573111
- Antas PR, Castello-Branco LR. New vaccines against tuberculosis: lessons learned from BCG immunisation in Brazil. Trans R Soc Trop Med Hyg 2008 Jul; 102(7):628-30; PMID:18440575; http://dx.doi.org/10.1016/j.trstmh.2008.03.014
- da Costa C, Walker B, Bonavia A. Tuberculosis vaccines-state of the art, and novel approaches to vaccine development. Int J Infect Dis 2015 Mar; 32:5-12; PMID:25809749; http://dx.doi.org/10.1016/j.ijid.2014.11.026
- Clark SO, Kelly DL, Badell E, Castello-Branco LR, Aldwell F, Winter N, Lewis DJ, Marsh PD. Oral delivery of BCG Moreau Rio de Janeiro gives equivalent protection against tuberculosis but with reduced pathology compared to parenteral BCG Danish vaccination. Vaccine 2010 Oct 8; 28(43):7109-16; PMID:20708695; http://dx.doi.org/10.1016/j.vaccine.2010.07.087
- Chen JM, Alexander DC, Behr MA, Liu J. Mycobacterium bovis BCG vaccines exhibit defects in alanine and serine catabolism. Infect Immun 2003 Feb; 71(2):708-16; PMID:12540549; http://dx.doi.org/10.1128/IAI.71.2.708-716.2003
- Saikolappan S, Estrella J, Sasindran SJ, Khan A, Armitige LY, Jagannath C, Dhandayuthapani S. The fbpA/sapM double knock out strain of Mycobacterium tuberculosis is highly attenuated and immunogenic in macrophages. PLoS One 2012; 7(5):e36198; PMID:22574140; http://dx.doi.org/10.1371/journal.pone.0036198
- Lotte A, Wasz-Hockert O, Poisson N, Dumitrescu N, Verron M, Couvet E. BCG complications. Estimates of the risks among vaccinated subjects and statistical analysis of their main characteristics. Adv Tuberc Res 1984; 21:107-93; PMID:6475644
- Lee WI, Huang JL, Yeh KW, Jaing TH, Lin TY, Huang YC, Chiu CH. Immune defects in active mycobacterial diseases in patients with primary immunodeficiency diseases (PIDs). J Formos Med Assoc 2011 Dec; 110(12):750-8; PMID:22248828; http://dx.doi.org/10.1016/j.jfma.2011.11.004
- Bunch-Christensen K, Ladefoged A, Guld J. The virulence of some strains of BCG for golden hamsters. Further studies. Bull World Health Organ 1970; 43:65-70; PMID:4921093
