ABSTRACT
Changing epidemiology of Hepatitis A virus (HAV) has led to an increased susceptibility of adolescents and adults to the infection. Vaccination can remarkably reduce the incidence and associated morbidity of HAV infection. This review is focused on the safety and efficacy of H2 strain derived live attenuated Hepatitis A vaccine. We found the vaccine to be highly immunogenic with minimal or negligible safety issues. Moreover, a single dose of live attenuated vaccine persists a long term immune response and can be a preferred option for developing countries. In 2014, Indian Academy of Paediatrics (IAP) also updated their recommendations for H2 vaccine as a single dose as against the previous 2 dose schedule. A focused approach to include the vaccine in national immunization program should be explored.
Introduction
Hepatitis A is the most common form of acute viral hepatitis worldwide. Outbreaks of hepatitis A have been recognized for centuries, affecting both military and civilian populations. It was first described by Hippocrates as an epidemic jaundice.Citation1
For some time after its identification, Hepatitis A virus (HAV) was thought to be an enterovirus. In 1991, it was sub classified as a member of the Hepato virus genus of the family Picornaviridae. HAV has a small non-enveloped structure with a single-stranded RNA. It is thermostable (up to 60°C) and acid-resistant. The virus has only one serotype but multiple genotypes.Citation2
HAV is commonly reported in conditions of poor hygiene and sanitation. It spreads through the fecal-oral route. After ingestion, the virus enters the blood stream through the epithelium of the gastro-intestinal tract and reaches the liver. It replicates in the hepatocytes and Kupffer cells and the virion is excreted into the intestine through bile. The replication process of HAV alters the liver function, causing an immune response and liver inflammation.Citation3
The relative frequency of symptomatic hepatitis and asymptomatic infection has been reasonably well characterized and appears to be strikingly age dependent.Citation4 About 50% of the children with HAV infection aged less than 6 y fail to show noticeable symptoms of the disease although remaining have mild manifestation, often not evaluated as hepatitis. It was reported that children with hepatitis A aged below 4 y (5%) and 6 y (10%) have less tendency of developing jaundice, while this tendency increases by 75% when an adolescent moves into adulthood. However, sporadic infection with HAV can lead to acute liver failure (in approximately 0.2% of clinical cases).Citation5
Epidemiology of HAV
There has been a significant change in the epidemiology of hepatitis A virus (HAV) infection over the past few years. The epidemiological studies demonstrated that the sero-prevalence of anti- HAV antibodies ranged from15% (in the Nordic countries) to about 100% in different parts of the world. Globally, 1.5 million cases of HAV infection are reported each year.Citation6
In other parts of Europe and Australia, Japan and in the United States, 40%–70% of the adult population has demonstrable antibodies to HAV. Practically, all the adults living in the developing areas of the world have a serological evidence of past infection. For practical purposes, the world can be divided into areas of very low, low, intermediate and high endemicity based on sero-prevalence where high implies ≥90% seropositive individuals by age 10 years; intermediate ≥50% seropositive individuals by age 15 years, with <90% seropositive individuals by age 10 years; low ≥50% seropositive individuals by age 30 years, with <50%seropositive individuals by age 15; and very low <50% seropositive individuals by age 30 y.Citation7 As per the position paper published by the World Health Organization (WHO) in 2012, the frequency of hepatitis A occurrence and socioeconomic development are directly related to each other.Citation6
The improvement in public health measures, socioeconomic status (SES) and sanitation measures are the main causes that has led to a shift in the age at which the infection occurs. There is an increase in the average age of infection making older children and adolescents more susceptible to infection.Citation8,9 Recent epidemiological studies conducted across India and China also show a trend of change in the epidemiology of HAV. In the recent years, there is a shift from high to intermediate endemicity of HAV infection owing to local SES and improvement in public health parameters.Citation10-12 The sero-prevalence in China and India as reported in different studies.Citation13-26 In China, a decrease in HAV incidence was reported from 359.7/100,000 (1992) to 17.7/100,000 (2009), which is approximately a 93% decrease from 1992 to 2009.Citation27
In India, Arankalle and colleagues studied age-related sero-prevalence of HAV across 4 different metros of the country and concluded that the sero-prevalence in children of age group 6–10 (50.3%) year was significantly higher (P = 0.000) than in 18 months to 6 y (30.3%) age group. Further, the study found that SES and educational status of the parents were significantly associated with HAV seropositivity (P = 0.000 for both). An increase in sero-prevalence was observed with an increase in age in all SESs (P<0.000–0.001 for all) except upper SES (P = 0.124).Citation28
Thus, it is summarized that even though countries moving quickly from high to intermediate endemicity may be a good marker of SES development, it may pose a risk to the pockets of seronegative subjects leading to outbreaks. While HAV infection does not have a mainstay treatment and is managed supportively, vaccination stands out as the best possible preventive measure.
Numerous hepatitis A vaccines have been introduced in the market after successful isolation of HAV in 1979. Broadly, there are 2 types of hepatitis A vaccines available globally; formaldehyde inactivated vaccines (commonly used) and live attenuated vaccines (manufactured in China).Citation29 Many research studies have been published about inactivated vaccines in the international literature and inactivated vaccines are well accepted and acknowledged. The live attenuated H2 strain vaccine (H2 vaccine) apart from its country of origin, China, has been available in India for over a decade and is now available in Thailand, Philippines, Guatemala and Bangladesh. The vaccine is available by the brand name of Zhepu in China and Biovac-A (Wockhardt Ltd.) in India. While this vaccine has proven to be safe and effective even with a single dose and supported with a lot of short term and long-term studies, much of the data is either scattered or published in Chinese. Moreover, there is no single article that reviews the studies on live attenuated H2 strain vaccine from India. The objective of this article is to summarize the various landmark studies on this vaccine establishing its safety and efficacy. This is important for the clinicians as Indian Academy of Paediatrics (IAP) has recently updated their recommendations for H2 vaccine as a single dose as against the previous 2 dose schedule.
Vaccine development and properties
A program aiming to develop a live attenuated vaccine was set up in China in 1980. The attenuated H2- strain of HAV was isolated from the feces of a 12 y old patient with Hepatitis A and cultured in a monolayer of newborn monkey kidney (NMK) cells in 1982.Citation30 The virus was passed serially in monolayer NMK cells and preserved in liquid nitrogen for 15 passages at 35°C, followed by 5 passages at 32°C. It was subsequently adapted to grow in a culture of human embryonic lung fibroblast tissue (KMB17) at 32°C and was carried out through an additional 4 passages in KMB17 cells at that temperature. The experimental candidate vaccine was made from the master seed virus (H2 attenuated strain) by 2 additional passages in KMB17 cells. The vaccine was then evaluated with a series of preclinical studies, in-vitro and animal studies in monkeys, for sterility, safety and immunogenicity.Citation31 The data of the studies was reviewed by the experts committee on Certification of Biological Products, Beijing and the vaccine finally received an approval for testing through human trials.Citation30
Immunogenicity and efficacy of live attenuated Hepatitis A Vaccine
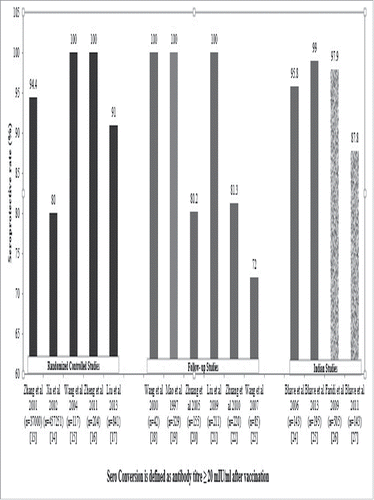
One of the earliest human trials was conducted in a small sample size; wherein a total of 12 subjects (8 women and 4 men), 18–27 y old, seronegative for HAV and Hepatitis B at baseline and termed healthy based on physical examination and liver enzymes {serum glutamic-pyruvic transaminase (SGPT), type 5 isoenzyme of lactate dehydrogenase (LDH5) and iso citrate dehydrogenase (ICD)} were recruited for the study. The subjects received a subcutaneous single dose of vaccine (106.5 TCID50/mL) in 1 mL volume. These subjects were monitored daily with physical examination for up to 6 weeks and blood samples were monitored until 20 weeks since vaccination. Except for one subject who recorded a temperature of 37.6°C on day 10 post-vaccination, all the other subjects recorded a body temperature of <37.5°C throughout the follow-up. The serum samples also revealed liver enzymes within the normal range. All the 12 subjects were found to have seroconverted with a mean onset at 3 weeks post-vaccination. The enzyme linked immunosorbent assay (ELISA) competitive test titers of antibodies ranged from 1:2 to 1:8 at 20 weeks (Geometric Mean Titers, GMT at 20 weeks = 3.48 + 1.35 mIU/mL). A follow up study of one year in 10 of these subjects revealed that the GMT continued to be persistent above the minimum requisite levels. Stool samples (n = 30) from 4 of these subjects were collected from day 8–30 post-vaccination and after concentration were found to be negative for hepatitis A antigen (HAA). The samples were then cultured in KMB17 and the virus could be recovered from 3 of the 4 subjects, indicating that the attenuated HAV is excreted in stools although not as much as the wild type virus.Citation30
Following the above study, the same dose of vaccine was then administered subcutaneously to 127 children (4–12 years old) in a 0.5 mL volume. During 8 weeks follow up, none of the children showed any abnormal findings on physical examination and their liver enzymes continued to be within the normal range. All the children showed a seroconversion of antibody to HAV at fourth week, when the first blood sample was drawn. These two studies suggested a good immunogenicity of this vaccine with minimal side effects in humans ().Citation30
Mao et al. conducted a series of studies trying to identify the possibility of transmission of the live attenuated vaccine from vaccinated to the non-vaccinated subjects. In the first such study, 141 primary school children were vaccinated with a single dose of the vaccine and 87 close contacts (classmates) were followed up for any sign of seroconversion at fifth and eleventh months. During follow up, while there was 100% seroconversion seen in the vaccinated children, the antibody titer remained negative for the close contacts.Citation25 In the second part of the study, 222 seronegative students from a primary school were randomly allocated to receive a single dose (106.5 TCID50) of live attenuated (H2) strain HAV vaccine either orally (n = 101) or subcutaneously (n = 121). At three months of follow up, there was a seroconversion of 99.2% (120/121) in the subcutaneous group whereas not a single subject seroconverted in the oral group (0/101) indicating a significant difference in the route of administration (P < 0.001).Citation25
In 1987, one of the largest safety studies was initiated by Zhang et al.; a total of 3089 healthy subjects between the ages of 4–27 y were vaccinated with a single subcutaneous dose. None of the recipients developed any local or systemic reaction during a 42-day follow-up after vaccination. The serum enzyme activities, including SGPT/ALT and LDH5, were within normal range during the 4 to 16 weeks of serial tests after vaccination. Seroconversion occurred at a mean time of 2 to 5 weeks after inoculation, and the seropositive rate was 95.6%.Citation32
In one of the large immunogenicity studies conducted by Cheng et al. 11,451 subjects were vaccinated subcutaneously with a single dose of H2 strain vaccine. A quick antibody response was noted within 2–5 weeks with a seroconversion rate of 92.9% indicating a good immunogenicity of the vaccine.Citation33 Based on the rigorous phase I to phase III trials from 1989 to 1991 conducted in China, the H2 strain vaccine received a marketing authorization from the Ministry of Health of People's Republic of China in 1992.Citation30
Since the launch of the vaccine, long term follow-up studies that ranged in duration from 4 to 15 y have been conducted across various regions of China. One of the first of its kind, conducted by Mao et al. aimed to evaluate the protective efficacy of H2-strain vaccine over the duration of 4 y. The study was carried out in Shaoxing County of Zhejiang province which reported morbidity associated with hepatitis A of around 150/100000 during 1980s. From September 1989 to September 1993, 6298 children from 11 primary schools were voluntarily vaccinated with the H2 strain vaccine. The controls in this study were the unvaccinated subjects from these 11 schools as well as other schools in the country. During the 4 y, no case of Hepatitis A was reported among the vaccinated group where as a significant number of cases (n = 495) were reported in the control group (P < 0.001).Citation25 Another long-term study was conducted by Zhuang et al. to observe the immunogenicity of the vaccine. Overall, 220 children aged 1 to 3 y were vaccinated with a single dose of live attenuated hepatitis A vaccine and followed up for 10 y for immunogenicity. The seroconversion at 6 months was observed to be 98.6% and was 80.2% after 10 y.Citation34 The GMT levels were 128 mIU/mL even 5 y after follow up. This cohort reported a seroconversion of 81.3% after 15 y that was significantly higher than the required protective level of 20 mIU/mL as recommended by the WHO experts.Citation24
Zhao et al. studied the protective efficacy of H2-strain vaccine during the hepatitis A outbreaks. Following the informed consent from the parents, 5551 children (cohort of pre-school and grade 1–3 primary school age group) from Hebei Province of China, were vaccinated with a single dose of H2 strain vaccine in May 1997. Another 6485 children of the same age and similar demographics were observed as controls. In May 1998, an outbreak of hepatitis A in the province which lasted for about 80 d was reported. The protective efficacy of H2 strain vaccine during this outbreak was 95.27%.Citation35
In 2004, H2 strain vaccine received a marketing authorization in India based on the robust clinical data that supported it. Since the launch of the vaccine, there have been 3 single arm studies conducted; of which 2 were long term.Citation17,36,37
Bhave et al. in 2004 initiated an open labeled, non-comparative study of a live attenuated H2 strain in 143 healthy Indian children aged 1 to 12 y (mean age 4.87 + 2.76 years; 88 boys, 55 girls). Children were assessed for antibodies 2 months after the single dose of the vaccine. Overall, 137 children (95.8 %) developed protective antibodies > 20 mIU/mL.Citation20 In the follow up of this study, 131 evaluable subjects were monitored for anti-HAV antibodies 30 months after vaccination. The sero-protective antibody levels were found to be >20 mIU/mL with an overall GMT of 92.02 mIU/mL. It was reported that there were no hepatitis like infection observed in any of the patient. In the 10 y follow-up of the same cohort, 106 of 108 evaluable children had anti-HAV titres >20 mIU/mL, i.e., sero-protection rate was 98.15% (95% CI: 93.47%, 99.77%). Only 2 subjects had anti-HAV titres <20 mIU/mL (i.e., 11.5 mIU/mL and 13.5 mIU/mL). The GMT of anti-HAV antibodies in 2014 was 100.46 mIU/mL (95% CI: 87.44 mIU/mL, 115.43 mIU/mL).Citation37
Faridi et al. in a multi-centric study of 505 children aged 1.5 to 5 y conducted across 4 centers in India (Delhi, Mumbai, Kolkata and Chennai) concluded that the H2 vaccine was immunogenic and tolerable with minimal reactogenecity in a single dose schedule. At 6 weeks, 95.1 % of the children seroconverted and at the end of 6 months, 97.9 % had seroconverted. The authors while analyzing the age-wise seroconversion at 6 weeks and 6 months following single dose administration of the vaccine, further observed that the GMT at 6 weeks and 6 months were maximum in the age group 18–24 months. This indicated that the vaccine is highly immunogenic at an early age. Safety profile was also satisfactory in the study population. Both solicited and unsolicited vaccine induced local and systemic adverse events (SAEs) were insignificant at all the centers, except swelling and induration in a few subjects.Citation17 As an extension to this study, a follow up study was initiated further at first, second, third, fourth and fifth year post vaccination that demonstrated a sero-protection rate of 98.3%, 96.2%, 97.8%, 92.6% and 97.3%, respectively. The geometric mean concentration (GMC) over the years was recorded as 135.2 mIU/mL at 1 year, 124.6 mIU/mL at 2 years, 137.6 mIU/mL at 3 years, 127.4 mIU/mL at 4 y and 127.1 mIU/mL at 5 y. The follow up study concluded that the vaccine was well tolerated and conferred long-term immunogenicity in Indian children.
In 2010, there was a change in the manufacturing site of the H2 vaccine with a change in the inactive stabilizers. Based on the requirements of the Indian regulatory authorities (Drug Controller General of India, DCGI), Bhave et al. initiated a bridging study across 2 centers in India (Pune and Kolkata). This open labeled, non-comparative, non-randomized study involving 137 children with a mean age of 4.09 +2.5 y concluded that live attenuated H2 strain hepatitis A vaccine is immunogenic and safe in Indian children. Eight weeks after a single dose of the vaccine, 136 subjects from both the centers developed protective antibodies >20 mIU/mL. The overall seroconversion rate was 99% (Kolkata: 100%, Pune: 98%). The haematological and biochemical parameters remained within normal limits. All the adverse events (AEs) were non-serious and mild in severity.Citation26
Safety and tolerability of live attenuated Hepatitis A vaccine
Majority of the studies have reported live attenuated Hepatitis A vaccine to be safe and well tolerated. None of the studies reported any serious AE related to the vaccine. No patient was withdrawn from any of the study due to an AE. Long-term follow up studies also did not report any significant AEs. The most commonly observed AEs with the live attenuated HAV vaccine were fever, pain, redness, and swelling at the injection site which resolve within few hours or days.
In India, Bhave et al. reported mild fever in one child that lasted a few hours and subsided without any treatment.Citation16 Faridi et al. reported mild swelling and slight indurations in some children.Citation17,20 Mitra et al. reported that 28 (20%) subjects in their study experienced at least one AE during the study period. All the AEs were mild in severity. In the 48 hours post vaccination observation period, systemic AEs were seen in 6 children (mild fever: 4; cough: 2) and local AEs in 4 children (local pain: 3; local swelling: 1). The AEs reported during the remaining study period were respiratory tract infection (5.1%), fever (6.5%), vomiting (0.7%), and gastroenteritis (0.7%). In the 5-year follow-up study of the same cohort, no significant AE was observed.
Comparison between live and inactivated vaccines
Previous studies have been conducted to compare the inactivated vaccines and live attenuated vaccines and found them to be similar in efficacy. Zhang et al. compared live attenuated and inactivated vaccine in 211 children aged 3 to 13 y randomly allocated to Group A injected with 3 doses at 0, 6 and 12 months; Group B was administrated 2 doses of live attenuated Hepatitis A vaccine at, and Group C was immunized with inactivated vaccine at 0 and 6 months. In all the groups, 100% of the children were seroconverted after the second dose.Citation38 In another study, Zheng et al. randomly allocated 841 children in 4 groups (H2 vaccine-204, Healive- 208, Havrix- 208, Control- 217). On Day 7, the sero-conversion proportions were 25%, 35%, 27% and 2% (P < 0.0001) with GMC of 6 mIU/ml, 8 mIU/ml, 6 mIU/ml and 3 mIU/ml, respectively for the 4 groups. At 28 days, sero-conversion proportions were 98%, 100%, 93% and 3% (P < 0.0001) with GMC of 47 mIU/ml, 71 mIU, 67 mIU/ml and 3 mIU/ml, respectively.Citation21
Liu et al. compared immunogenicity among an inactivated hepatitis A vaccine with one-dose and 2 dose regimens, and 3 kinds of live attenuated vaccines in 924 children aged 1.5 to 6 y. It was observed that after 6 months the seroconversion with one dose of inactivated vaccine was 92.5 % and seroconversion with one dose of live attenuated vaccines ranged from 96.8% to 100%. After 12 months of vaccination, the seroconversion dropped to 91% with inactivated vaccine and ranged from 77% to 84% with live attenuated vaccines, the highest (84%) being with Biovac-A.Citation18 All these studies showed similar immunogenicity of live attenuated and inactivated hepatitis A vaccines, some of them showing a higher seroconversion rate with live attenuated vaccine after a single dose.
Discussion
Presence of anti-HAV antibodies or humoral response in an individual alone does not confer life-long immunity. Rather, long-term protection against HAV infection is related to the cellular immunity that persists even after the anti-HAV antibodies become undetectable. It implies that when vaccinated individuals are re-exposed to the HAV infection, an anamnestic response may prevent them from the disease.Citation14 Inactivated vaccines elicit a humoral and cellular response against the HAV and generally require a booster dose.Citation39 The main advantage of live attenuated vaccines is the activation of all phases of immune system; humoral response and cell-mediated response. The live vaccines have a widespread repertoire of antigens which may stimulate CD4 and CD8 T- cells and the non-classical γδ and DN αβ-T cells which mediate active protection against the infection.Citation40 Previous studies have suggested that a single dose of live attenuated HAV vaccine could induce both humoral and cell mediated immune response.Citation13,23,41-43
Further, live attenuated HAV vaccine is safe and effective and can provide long term protection against Hepatitis A with a single dose. There is an abundance of evidence in literature that supports the fact that live attenuated vaccine can provide long-term immunogenicity with a single dose.Citation25,26,34,44 The long-term immunogenicity and effectiveness of live attenuated HAV (H2 strain) after one dose injection could last as long as for 15 y.Citation45
Global data showed that hepatitis A vaccination programs have remarkably decreased the incidence of HAV infection in countries like USA (rate of HAV infection per 1,000,000 population reduced from 12 in 1995 to 1.5 in 2005), Israel (resulted in sero-protective antibody concentrations among 100% of children), and Argentina.Citation28 The incidence of Hepatitis A reduced dramatically in the Chinese regions where the vaccine was used in mass and routine public immunization programs.Citation27,46 Biovac–A (Zhepu) has been used in China from a long time, and has shown remarkable safety, immunogenicity, and long-term protection to millions of subjects.Citation47 With the available evidence, WHO in their 2012 position paper on hepatitis A, has recommended the use of H2 vaccine as a single dose. The WHO recommends that routine immunization programs in middle income countries like India are likely to be cost effective and should therefore be encouraged. Further, vaccination against HAV should be integrated into the national immunization schedule for children aged ≥1 year for countries with change in the endemicity from high to intermediate.Citation47
In 2014, Indian Academy of Paediatrics (IAP), updated their recommendations for H2 vaccine as a single dose as against the previous 2 dose schedule. This update was included in the recommendations of IAP after reviewing the published and unpublished long-term follow up data on immunogenicity and safety of the H2 vaccine from Indian studies.Citation49 The vaccination for hepatitis A is also recommended by the Advisory Committee on Immunization Practices (ACIP) for all children at age of 12–23 months and persons with high risk of hepatitis A such as travelers, drug addicts, homosexualsCitation50 and for any person wishing to obtain immunity.Citation51 However, large-scale hepatitis A vaccination is apt for cost-effectiveness and should be encouraged. Beside these, continued monitoring of anti-HAV antibodies is needed for a rational hepatitis A immunization strategy in developing countries like India.
Last but not the least, given the benefits of live attenuated Hepatitis A vaccine and considering epidemiological shift, fear of outbreaks and morbidity involved, a strategy should be designed to include the vaccine in the national immunization schedule.
Table 1. Summary of studies conducted across India and China.
Disclosure of potential conflicts of interest
SR, SMR, SM, GK are the salaried employees of Wockhardt Ltd. JSM are the employee of Institute of Viral Diseases and FCZ are the employee of Zhejiang Academy of Medical Sciences and declare no conflict of interest. Biovac A is manufactured by Zhejiang Pukang Biotechnology Co. Ltd and Wockhardt Ltd has license to market Biovac A in India.
Acknowledgment
The author acknowledges Knowledge Isotopes (www.knowledgeisotopes.com) for editing this article and subsequently revising it by addressing author comments.
References
- Krugman S. The gordon Wilson lecture. The ABC's of viral hepatitis. Trans Am Clin Climatol Assoc 1992; 103:145-56; PMID:1413374
- Costa-Mattioli M, Di Napoli A, Ferre V, Billaudel S, Perez-Bercoff R, Cristina J. Genetic variability of hepatitis A virus. J Gen Virol 2003; 84:3191-201; PMID:14645901; http://dx.doi.org/10.1099/vir.0.19532-0
- Koff RS. Hepatitis A. Lancet 1998; 351:1643-9; PMID:9620732; http://dx.doi.org/10.1016/S0140-6736(98)01304-X
- Hadler SC, McFarland L. Hepatitis in day care centers: epidemiology and prevention. Rev Infect Dis 1986; 8:548-57; PMID:3018889; http://dx.doi.org/10.1093/clinids/8.4.548
- Jacobsen KH. The global prevalence of Hepatitis A virus infection and susceptibility: a systematic review. 2010. Available at: http://apps.who.int/iris/bitstream/10665/70180/1/WHO_IVB_10.01_eng.pdf; Accessed on August 10, 2016.
- World Health Organization. Immunization, vaccines and biologicals. IVB Catalogue. 2016. Available at: http://archiveswhoint/vaccines/en/hepatitisashtml; Accessed on August 10, 2016.
- Weekly Epidemiological Record (WER). WHO position paper on hepatitis A vaccines. 2012. Available at: http://www.who.int/wer/2012/wer8728_29.pdf?ua=1. Accessed on August 10, 2016.
- Campagna M, Siddu A, Meloni A, Basciu C, Ferrai L, Pettinau A, Cardia C, Masia G, Coppola RC. Changing pattern of hepatitis a virus epidemiology in an area of high endemicity. Hepatitis Monthly 2012; 12:382-5; http://dx.doi.org/10.5812/hepatmon.5940
- WHO position paper on hepatitis A vaccines June 2012. Available at: http://wwwwhoint/wer/2012/wer8728_29pdf. Accessed on 23 November, 2015; 2012:261-76
- Pham B, Duval B, De Serres G, Gilca V, Tricco AC, Ochnio J, Scheifele DW. Seroprevalence of hepatitis A infection in a low endemicity country: a systematic review. BMC Infec Dis 2005; 5:56; PMID:16001978; http://dx.doi.org/10.1186/1471-2334-5-56
- Chitambar SD, Chadha MS, Joshi MS, Arankalle VA. Prevalence of hepatitis a antibodies in western Indian population: changing pattern. Southeast Asian J Tropical Med Public Health 1999; 30:273-6; PMID:10774693
- Dhawan PS, Shah SS, Alvares JF, Kher A, Shankaran, Kandoth PW, Sheth PN, Kamath H, Kamath A, Koppikar GV, et al. Seroprevalence of hepatitis A virus in Mumbai, and immunogenicity and safety of hepatitis A vaccine. Indian J Gastroenterol 1998; 17:16-8; PMID:9465507
- Mao JS, Chai SA, Xie RY, Chen NL, Jiang Q, Zhu XZ, Zhang SY, Huang HY, Mao HW, Bao XN, et al. Further evaluation of the safety and protective efficacy of live attenuated hepatitis A vaccine (H2-strain) in humans. Vaccine 1997; 15:944-7; PMID:9261939; http://dx.doi.org/10.1016/S0264-410X(96)00304-0
- Zhuang FC, Mao ZA, Jiang LM, Wu J, Chen YQ, Jiang Q, Chen NL, Chai SA, Mao JS. [Long-term immunogenicity and effectiveness of live attenuated hepatitis A vaccine (H2-strain)-a study on the result of 15 years' follow up]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2010; 31:1332-5; PMID:21223658
- Zhuang FC, Qian W, Mao ZA, Gong YP, Jiang Q, Jiang LM, Chen NL, Chai SA, Mao JS. Persistent efficacy of live attenuated hepatitis A vaccine (H2-strain) after a mass vaccination program. Chinese Med J 2005; 118:1851-6; PMID:16313838
- Faridi MM, Shah N, Ghosh TK, Sankaranarayanan VS, Arankalle V, Aggarwal A, Sathiyasekaran M, Bhattacharya N, Vasanthi T, Chatterjee S, et al. Immunogenicity and safety of live attenuated hepatitis A vaccine: a multicentric study. Indian Pediatr 2009; 46:29-34; PMID:19179715
- Bhave S, Ghosh A, Sapru A, Mitra M, Chatterjee S, Bhattacharya N, Kadhe G, Mane A, Roy S. Immunogenicity and safety of live attenuated hepatitis A vaccine (Biovac-A™) in healthy Indian children. Vaccine: Dev Ther 2013; 4:1-6
- Zheng H, Chen Y, Wang F, Gong X, Wu Z, Miao N, Zhang X, Li H, Chen C, Hou X, et al. Comparing live attenuated and inactivated hepatitis A vaccines: an immunogenicity study after one single dose. Vaccine 2011; 29:9098-103; PMID:21875638; http://dx.doi.org/10.1016/j.vaccine.2011.08.078
- Liu XE, Wushouer F, Gou A, Kuerban M, Li X, Sun Y, Zhang J, Liu Y, Li J, Zhuang H. Comparison of immunogenicity between inactivated and live attenuated hepatitis A vaccines: a single-blind, randomized, parallel-group clinical trial among children in Xinjiang Uighur Autonomous Region, China. Hum Vaccin Immunother 2013; 9:1460-5; PMID:23571173; http://dx.doi.org/10.4161/hv.24366
- Wang XY, Xu ZY, Ma JC, von Seidlein L, Zhang Y, Hao ZY, Han OP, Zhang YL, Tian MY, Ouyang PY, et al. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: results from 8-year follow-up. Vaccine 2007; 25:446-9; PMID:16949710; http://dx.doi.org/10.1016/j.vaccine.2006.08.004
- Wang XY, Xu Z, Yao X, Tian M, Zhou L, He L, Wen Y. Immune responses of anti-HAV in children vaccinated with live attenuated and inactivated hepatitis A vaccines. Vaccine 2004; 22:1941-5; PMID:15121306; http://dx.doi.org/10.1016/j.vaccine.2003.11.007
- Zhang Y, Liu X, Ma J. [A field evaluation of the epidemiological efficacy of an attenuated live hepatitis A vaccine (H2 strain)]. Zhonghua yu fang yi xue za zhi [Chinese J Prevent Med] 2001; 35:387-9; PMID:11840766
- Xu Z, Wang X, Li R, Meng Z, Zhang Y, Gong J, Ma J, Li Y, Zhao S, Li Y, et al. [Immunogenicity and efficacy of two live attenuated hepatitis A vaccines (H(2) strains and LA-1 strains)]. Zhonghua yi xue za zhi 2002; 82:678-81; PMID:12133465
- Liu HF, Zhang XJ, Zhang JL. [Comparison of antibody persistence between live attenuated and inactivated hepatitis A vaccines]. Zhongguo yi miao he mian yi 2009; 15:300-3; PMID:20077725
- Wang X, Ma J, Zhang Y, Zhang Y, Han C, Xing Z, Chen J, Zhang Y, Zhao S, Gu H, et al. [Primary study on immunologic effect of live attenuated hepatitis A vaccine (H2 strain) after booster dose]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2000; 21:124-7; PMID:11860772
- Bhave S, Bavdekar A, Madan Z, Jha R, Bhure S, Chaudhari J, Pandit A. Evaluation of immunogenicity and tolerability of a live attenuated hepatitis a vaccine in Indian children. Indian Pediatr 2006; 43:983-7; PMID:17151402
- Bhave S, Bavdekar A, Sapru A, Bawangade S, Pandit A. Immunogenicity of single dose live attenuated hepatitis a vaccine. Indian Pediatr 2011; 48:135-7; PMID:21169655; http://dx.doi.org/10.1007/s13312-011-0039-4
- Fangcheng Z, Xuanyi W, Mingding C, Liming J, Jie W, Qi J, Yuanping G, Wen Q, Yajuan X, Jiangsen M. Era of vaccination heralds a decline in incidence of hepatitis A in high-risk groups in China. Hepatitis Monthly 2012; 12:100-5; PMID:22509186; http://dx.doi.org/10.5812/hepatmon.4907
- Arankalle VMM, Bhave S, Ghosh A, Balasubramanian S, Chatterjee S, Choudhury J, Chitkara A, Kadhe G, Mane A, Roy S. Changing epidemiology of hepatitis A virus in Indian children. Dove Press J 2014
- WHO position paper on hepatitis A vaccines: June 2012-Recommendations. Vaccine 2013; 31:285-6; PMID:23142134; http://dx.doi.org/10.1016/j.vaccine.2012.10.102
- Mao JS, Dong DX, Zhang HY, Chen NL, Zhang XY, Huang HY, Xie RY, Zhou TJ, Wan ZJ, Wang YZ, et al. Primary study of attenuated live hepatitis A vaccine (H2 strain) in humans. J Infect Dis 1989; 159:621-4; PMID:2538518; http://dx.doi.org/10.1093/infdis/159.4.621
- Mao JS, Xie RY, Huang HY, Chai SA, Chen NL, Yu PH, Wan XZ, Liu CJ, Cao YY, Dong DX, et al. Studies in monkeys of attenuated hepatitis A variants. Scientia Sinica Series B, Chem, Biol, Agricultural, Med Earth Sci / Chung-kuo k'o hsueh yuan, chu pan 1988; 31:338-43
- Zhang S. [Safety observation of attenuated live hepatitis B vaccine (H2 strain) in humans]. Zhonghua yi xue za zhi 1990; 70:682-4, 48; PMID:1963372
- Cheng NL. [Immunological effects of live attenuated hepatitis A vaccine]. Zhonghua yi xue za zhi 1992; 72:581-3, 638; PMID:1338501
- Zhuang FC, Qian W, Mao ZA, Gong YP, Jiang Q, Jiang LM, Chen NL, Chai SA, Mao JS. Presistent efficacy of live attenuated hepatitis A vaccines H2-strian: after a mass vaccination. Chin Med J 2005; 118:1851-6; PMID:16313838
- Zhao YL, Meng ZD, Xu ZY, Guo JJ, Chai SA, Duo CG, Wang XY, Yao JF, Liu HB, Qi SX, et al. H2 strain attenuated live hepatitis A vaccines: protective efficacy in a hepatitis A outbreak. World J Gastroenterol: WJG 2000; 6:829-32; http://dx.doi.org/10.3748/wjg.v6.i6.829
- Bhave S, Sapru A, Bavdekar A, Kapatkar V, Mane A. Long-term immunogenicity of single dose of live attenuated Hepatitis A vaccine in Indian children. Indian Pediatr 2015; 52:687-90; PMID:26388627; http://dx.doi.org/10.1007/s13312-015-0697-8
- Mitra M, Shah N, Faridi M, Ghosh A, Sankaranarayanan VS, Aggarwal A, Chatterjee S, Bhattacharyya N, Kadhe G, Vishnoi G, et al. Long term follow-up study to evaluate immunogenicity and safety of a single dose of live attenuated hepatitis a vaccine in children. Hum Vaccin Immunother 2015; 11:1147-52; PMID:26018443; http://dx.doi.org/10.4161/21645515.2014.979646
- Zheng H, Cui FQ. [The immunogenicity and impact factors of hepatitis A attenuated live vaccine and inactivated vaccine]. Zhongguo yi miao he mian yi 2009; 15:371-4
- Schmidtke P, Habermehl P, Knuf M, Meyer CU, Sanger R, Zepp F. Cell mediated and antibody immune response to inactivated hepatitis A vaccine. Vaccine 2005; 23:5127-32; PMID:16054733; http://dx.doi.org/10.1016/j.vaccine.2005.06.022
- Lu M, Xia ZY, Bao L. A mycobacterium bovis BCG-naked DNA prime-boost vaccination strategy induced CD4+ and CD8+ T-cell response against mycobacterium tuberculosis. Immunogens. J Immunol Res 2014; 2014:1-8; PMID: 24741595.
- Berger R, Just M, Althaus B. Time course of hepatitis A antibody production after active, passive and active/passive immunisation: the results are highly dependent on the antibody test system used. J Virol Methods 1993; 43:287-97; PMID:8408443; http://dx.doi.org/10.1016/0166-0934(93)90147-J
- Wang X, Ma J, Zhang Y. [Immunogenicity and long-term persistence of anti-HAV in groups with different attenuated and inactived hepatitis A vaccine dosage]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2001; 22:111-3; PMID:11860857
- Wang X, Ma J, Zhang Y, Han C, Xing Z, Chen J, Zhao S, Gu H, Xu Z. [Primary study on immunologic effect of live attenuated hepatitis A vaccine (H2 strain) after booster dose]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2000; 21:124-7; PMID:11860772
- Zaho YL MZ, Xu YZ, et al. H2 strain attenuated live hepatitis A vaccines: protective efficacy in a hepatitis A outbreak” World J Gastroentero 2000; 6:829-32; http://dx.doi.org/10.3748/wjg.v6.i6.829
- Cui F, Liang X, Wang F, Zheng H, Hutin YJ, Yang W. Development, production, and postmarketing surveillance of hepatitis A vaccines in China. J Epidemiol / Japan Epidemiol Assoc 2014; 24:169-77; http://dx.doi.org/10.2188/jea.JE20130022
- Wang H, Sui H. The meta analysis of protective efficacy of attenuated live Hepatitis A vaccine. Chinese J Vaccines and Immunization 2008; 14:1-6.
- Mao JS. Development of live, attenuated hepatitis A vaccine (H2-strain). Vaccine 1990; 8:523-4; PMID:1965075; http://dx.doi.org/10.1016/0264-410X(90)90001-3
- WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec 2012; 87:261-76; PMID:22905367
- Vashishtha VM, Choudhury P, Kalra A, Bose A, Thacker N, Yewale VN, Bansal CP, Mehta PJ. Indian Academy of Pediatrics (IAP) recommended immunization schedule for children aged 0 through 18 years–India, 2014 and updates on immunization. Indian Pediatr 2014; 51:785-800; PMID:25362009; http://dx.doi.org/10.1007/s13312-014-0504-y
- World Health Organization. Hepatitis A. Available at: http://www.who.int/mediacentre/factsheets/fs328/en/; Accessed on August 11, 2016.
- Zhuang F, Jiang Q, Gong Y. [Epidemiological effects of live attenuated hepatitis A vaccine (H(2)-strain): results of A 10-year observation]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2001; 22:188-90; PMID:11860874