Zika virus (ZIKV) has raised global public health concerns due to its rapid transmission and unexpected clinical manifestations.Citation1 No vaccine or antiviral treatment is currently available, and the development of animal model of ZIKV infection is of priority.Citation2,3 The mouse model of ZIKV infection was recently developed using A129 and AG129 mice.Citation4-7 However, both mice are deficient in critical innate immune signaling, and more importantly, these animals are limited resource, and are not available for most labs in ZIKV-endemic areas. Thus, a more relevant animal mode in immunocompetent animals is urgently needed.Citation8 Here, we establish an immunocompetent murine model using a contemporary American ZIKV strain GZ01 (GenBank nos. KU740184) that was isolated from a patient returned from Venezuela in 2016.Citation9 ZIKV stocks were propagated in mosquito C6/36 cells and titrated by plaque forming assay on BHK-21 cells.Citation10
We firstly inoculated 4-week-old 129Sv/Ev male mice with 105 PFU of ZIKV by the intraperitoneal (ip) route. Upon infection, no animal showed any clinical signs and all survived with a gradual increase on body weight (). Transient viremia were observed by RT-qPCR assay, peaked at day 1 post infection (pi) and decreased below detection limit at day 3 pi (). At days 1, 2 and 3 pi, mice were anesthetized and selected organs were dissected for RT-qPCR assay. The results showed that viral RNAs were disseminated into all tested organs at day 1 pi and the highest viral RNA loads were detected in testis, which was similar to that in A129 mice.Citation4 At day 3 pi, viral RNAs were cleared in most tissues except for spleen and testis (). Additionally, hematoxylin and eosin staining showed that no obvious histological lesions were observed in brain, heart, kidney and testis tissues at day 1 pi. Slight loose deformation of liver cells with focal necrosis and partial central venous dilatation were found in liver, and multinucleated giant cells were observed within the membrane and red pulp of spleen (). Similar viremia and viral RNA kinetics were also observed in ZIKV-infected Balb/C mice (data not shown).
Figure 1. Characterization of ZIKV strain GZ01 in 129Sv/Ev mice. Group of 4-week-old 129Sv/Ev mice were infected ip with 105 PFU of ZIKV strains GZ01/2016. The animals were observed daily for weight change (A). At days 1, 2, 3, 5 and 7 pi, blood was collected and analyzed by RT-qPCR for viremia (B). At days 1, 2 and 3 pi, 2 anesthetized mice were harvested, weighed, homogenized, and analyzed by RT-qPCR (C). Histopathology findings in indicated tissue of ZIKV-infected mice at day 1 pi. Therapeutic effects of human convalescent phase serum in ZIKV-infected 129Sv/Ev mice. PBS treatment was set as mock group. Viremia at days 1, 2, and 3 pi were determined by RT-qPCR (E). Viral RNA loads in spleen and testis at day 3 pi were determined by RT-qPCR (F). Viremia in 8-week-old 129Sv/Ev mice infected with GZ01 (G). Viral loads in serum or tissue are expressed as RNA copies per milliliter or RNA copies per gram, respectively. Results are shown as the mean ± standard error of the mean and statistical analysis was performed using the unpaired, 2-tailed t-test.
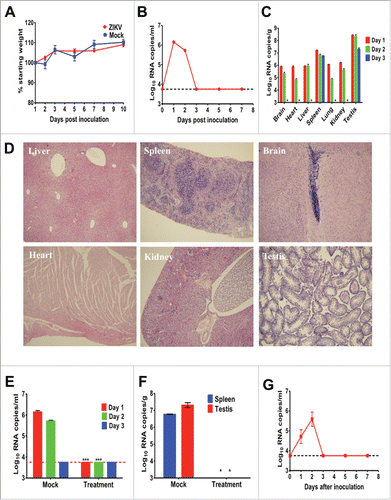
Further, to utilize the animal model described here for potential antiviral tests, the therapeutic effects of human convalescent phase serumCitation9 with the neutralization antibody titer of 1/80 were assayed. Briefly, group of 129Sv/Ev mice infected with 105 PFU of ZIKV were administrated ip with 150 μl of antibody at 4 and 24 h pi, and mock group were treated with PBS control. Blood was collected for viremia and autopsy were performed for viral dissemination. The result showed that passive transfer of convalescent phase serum completely eliminated viremia at day 1 and 2 in all ZIKV-infected mice compared with mock treatment (). Importantly, viral RNAs were undetectable in the indicated organs (spleen and testis) in mice treated with convalescent phase serum ().
Finally, we tested the infectivity of ZIKV in 8-week-old 129 Sv/Ev mice. The result showed that that ZIKV could also cause short viremia in these old mice (), and the peak viremia was slightly lower than that in young mice. This finding lays the foundation for potential vaccine efficacy test using this animal model.
Overall, our results showed that ZIKV infection resulted in transient viremia and dissemination in multiple central and visceral organs in immunocompetent mice, albeit no clinical symptoms developed. Passive transfer of ZIKV-specific neutralization antibody significantly prevents viremia and viral dissemination in specific tissues. The reduction of viremia could serve as endpoints for protection in vaccine or antiviral efficacy tests. Therefore, the mouse model described here provides a universal platform to test potential weapons against ZIKV.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by the State Key Laboratory of Pathogen and Biosecurity (SKLPBS1601), the National Key Research and Development Project of China (2016YFD0500304), and the National Natural Science Foundation (NSFC) of China (31270974 and 31470265). CFQ was supported by the Excellent Young Scientist Program from the NSFC of China (No.81522025) and the Newton Advanced Fellowship from the Academy of Medical Sciences, UK and the NSFC of China (81661130162).
References
- Fauci AS, Morens DM. Zika virus in the Americas-Yet another arbovirus threat. N Eng J Med 2016; 374:601-4; PMID:26761185; http://dx.doi.org/10.1056/NEJMp1600297
- Lazear HM, Diamond MS. Zika virus: new clinical syndromes and its emergence in the western hemisphere. J Virol 2016; 90:4864-75; PMID:26962217; http://dx.doi.org/10.1128/JVI.00252-16
- Li XF, Han JF, Shi PY, Qin CF. Zika virus: a new threat from mosquitoes. Sci China Life Sci 2016; 59:440-2; PMID:26944581; http://dx.doi.org/10.1007/s11427-016-5020-y
- Lazear HM, Govero J, Smith AM, Platt DJ, Fernandez E, Miner JJ, Diamond MS. A mouse model of Zika virus pathogenesis. Cell Host Microbe 2016; 19:720-30; PMID:27066744; http://dx.doi.org/10.1016/j.chom.2016.03.010
- Rossi SL, Tesh RB, Azar SR, Muruato AE, Hanley KA, Auguste AJ, Langsjoen RM, Paessler S, Vasilakis N, Weaver SC. Characterization of a novel murine model to study Zika Virus. Am J Tropical Med Hygiene 2016; 94(6):1362-9; PMID:27022155
- Aliota MT, Caine EA, Walker EC, Larkin KE, Camacho E, Osorio JE. Characterization of Lethal Zika Virus Infection in AG129 Mice. PLoS Neglected Tropical Dis 2016; 10:e0004682; PMID:27093158; http://dx.doi.org/10.1371/journal.pntd.0004682
- Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, et al. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 2016; 165:1081-91; PMID:27180225; http://dx.doi.org/10.1016/j.cell.2016.05.008
- Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi PY, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res 2016; 130:69-80; PMID:26996139; http://dx.doi.org/10.1016/j.antiviral.2016.03.010
- Zhang FC, Li XF, Deng YQ, Tong YG, Qin CF. Excretion of infectious Zika virus in urine. Lancet Infect Dis 2016; 16:641-2; PMID:27184420; http://dx.doi.org/10.1016/S1473-3099(16)30070-6
- Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, et al. Structures of the Zika virus envelope protein and its complex with a Flavivirus broadly protective antibody. Cell Host Microbe 2016; 19:696-704; PMID:27158114; http://dx.doi.org/10.1016/j.chom.2016.04.013
