ABSTRACT
A government-funded trivalent influenza vaccine (TIV) program to prevent seasonal influenza was implemented in Taiwan since 1998. However, mismatch between the vaccine and circulating strains may occur. Alternatively, a quadrivalent influenza vaccine (QIV) includes all 4 influenza lineages could minimize the risk of mismatches. Therefore, QIV could be considered as an alternative strategy to enhance protection against seasonal influenza. The objective of the study was to analyze, from a governmental perspective, the cost-effectiveness of using QIV vs. TIV as a vaccination strategy in Taiwan.
A lifetime multi-cohort, static Markov model was constructed with 9 age groups to assess the costs and effectiveness of QIV vs. TIV. Direct costs were obtained from a database released by the Ministry of Health and Welfare. Outcomes included life-years gained, quality-adjusted life years (QALYs) gained, influenza cases avoided and incremental cost-effectiveness ratios (ICERs). The discount rate of costs and effectiveness was set at 3.5% and the time horizon used in the model was 100 y.
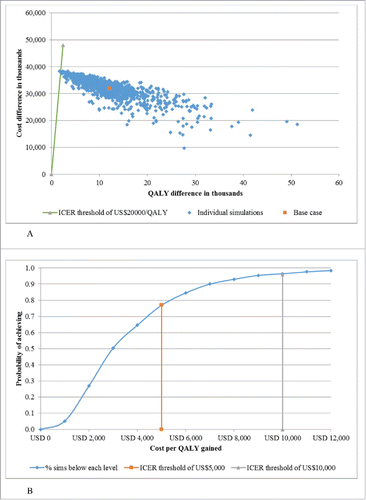
Results show that a vaccination strategy utilizing QIV instead of TIV would bring an additional 10,557 QALYs at an extra cost of US$39.4 million, yielding an ICER of US$3,015.07 per QALY gained. When setting the willingness-to-pay threshold at US$10,000, compared to TIV, the probability that QIV would be cost-effective was 98%. Sensitivity analyses show that ICER was sensitive to the changes of circulation of influenza virus subtypes and vaccine mismatch.
From a governmental perspective, the QIV vaccination could be considered as a cost-effective strategy within the context of public health in Taiwan.
Introduction
Influenza pandemics incur a heavy economic toll that strain existing public health services. The prevalence rates of influenza in Taiwan increased from 4.9 in 2009 per 100,000 to 6.4 in 2011 with an attack rate of 10–20% among the general population and 40–50% in densely populated areas such as schools, nursing homes, and hospitals.Citation1 In 2011/2012, the fatality rate of influenza patients was 9.0%.
Vaccination is currently recommended by the WHO as the most effective means to prevent infection from influenza viruses. Influenza vaccines have been shown to prevent influenza among 70% to 90% of healthy adults.Citation2 In 1998, Taiwan initiated a government-funded trivalent influenza vaccination (TIV) program as a first-line strategy for high-risk populations to prevent seasonal influenza. These priority groups included the elderly aged 65 and over, children aged 6 months to 6 y old, school-aged children, residents of nursing care facilities, patients with rare disorders, patients with catastrophic illness (including cancer, congenital factor disorder, chronic renal failure, congenital hypothyroidism and other rare diseases that are officially defined by Taiwan's National Health Insurance AdministrationCitation3), high-risk subjects aged 60–64 y old (patients with diabetes, chronic liver disease, chronic respiratory disease, chronic heart disease, chronic neurological disease, or chronic renal disease), health care workers, workers in livestock breeding, and workers involved in prevention of animal epidemics. The coverage rates of influenza vaccine were 61.9% in children aged 6 months - 6 y and 37.3% among the elderly.Citation4
The annual TIV comprises 2 strains of influenza A virus, A/H1N1 and A/H3N2, and one of the 2 lineages of influenza B to protect against the influenza viruses expected to circulate in the upcoming influenza season. The B viruses include 2 antigenically distinct lineages, Yamagata and Victoria, which co-circulate among humans. One of the strains is selected for the annual trivalent vaccine based on viral surveillance data.
However, it is highly possible that the B-lineage strain included in the vaccine does not match the circulating B-lineage virus. When this happens, the vaccine is unable to provide optimal protection against influenza B. For example, the predominantly circulating influenza B lineage differed from that selected for the vaccine during 2000/2001, 2004/2005, and 2010/2011 in the US.Citation5 Similarly in Taiwan, between July 2000 and June 2010, the vaccine match rates for the B virus was estimated at 62.19%. During the same period, the circulating strains of influenza B compared to A varied from 1.3% to 56.2%. Influenza B was predominant over influenza A in 3 of the 10 influenza seasons, namely 2000/2001 (B/Yamagata), 2004/2005 (B/Victoria and B/Yamagata co-circulating), and 2006/2007 (B/Victoria) seasons. In the seasons in which influenza B was predominant, mismatches between vaccines and influenza B occurred during 2004/2005 and 2006/2007 seasons,Citation6 which would have reduced the protection provided by the vaccines.
An alternative strategy to increase the protection against influenza would be to provide a quadrivalent influenza vaccine (QIV). A QIV has all 4 influenza lineages, including a second influenza B strain, so that it could minimize the chance of a mismatch in lineage between the vaccines and circulating strain. However, the increased antigen content of QIV could also increase the chances of reactogenicity and adverse events. Reed et al.Citation7 estimated the potential impact of QIV on public health in the US. Their results indicated that QIV would result in a modest reduction in influenza-associate outcomes, compared to TIV. This reduction would vary season to season according to vaccine coverage, the circulation of the 2 influenza B viruses, and the rate of influenza-associated illnesses. Lee et al.Citation8 compared the economic value of a QIV vaccine with that of TIV over 10 influenza seasons (1999–2009) in the US. They reported that the implementation of QIV could save $3.1 billion in societal costs and $292 million in the costs assumed by third party payers. The aim of this study was to analyze the cost-effectiveness of QIV vs. TIV as an alternative influenza vaccination strategy for all population from a governmental perspective.
Results
presents the results of base-case analysis. Compared to the number of cases prevented by TIV, additional influenza-associated cases avoided by QIV throughout the estimated lifetime are as follows: 529,874 cases of influenza, 476,886 cases requiring medical advice for uncomplicated influenza, 93,157 cases requiring antiviral treatment, 60,387 cases with influenza complications, 52,261 outpatient visits, 8,126 inpatients receiving treatment for complications, and 3,590 deaths due to influenza. QIV can increase QALYs, but with additional costs. The incremental gain in QALYs was 10,557 at an additional cost of US$31,829,269, yielding an ICER of US$3,015.07 per QALY gained. Table S1 shows the number of influenza cases, number of seeking medical treatment, number of influenza complication and deaths predicted to be averted by QIV compared with TIV. In the first year, QIV was expected to avert 14,368 additional influenza cases, 226 influenza complication and 23 deaths.
Table 1. Base case results.
Total costs included vaccination costs (US$39,425,208), followed by the cost of post-exposure prophylaxis (PEP) treatment (US$10,260). The additional cost of vaccination associated with QIV vaccination strategy can be partially offset by savings in treatment for uncomplicated influenza, hospitalizations, and outpatient treatment for complications of influenza.
The sensitivity of estimates related to cost-effectiveness was evaluated by varying the main costs and effectiveness drivers across plausible ranges on the ICER. One-way sensitivity analyses showed that only 2 parameters, the distribution of influenza A and B and the proportion of vaccine mismatch, had the greatest impacts on the results. shows the cost-effectiveness plane from the probabilistic sensitivity analysis. compares TIV and QIV using the cost-effectiveness acceptability curve (CEAC) at given WTP threshold per QALY gained. The probability that QIV would be cost-effective was 98% and 100% when the WTP threshold was set at US$10,000 and US$20,000, respectively.
Discussion
Previous studies indicated that, comparing with no intervention, TIV was cost-effective when used in healthy children,Citation9 healthy adults aged 50–64 y old,Citation10,11 and the elderly.Citation12,13 However, when analyzing the cost-effectiveness of QIV, it should be compared with current vaccination program instead of no intervention. This study used a lifetime, multi-cohort Markov model to compare the impact of QIV and TIV on the disease burden of influenza in Taiwan from the governmental perspective. The base-case results demonstrate that QIV would yield significant health benefit, in addition to cutting down the number of influenza cases by 529,874, the number of medical visits by 476,886, the number of complications by 60,387, the number of hospitalizations for complications by 8,126, and the number of deaths by 3,590, compared to TIV. Furthermore, the reduced cost of treating influenza-related complications, as a result of QIV, would also partially offset the increased cost of QIV compared with TIV. Overall, QIV was estimated to be a cost-effective strategy compared with TIV, with an ICER estimated at US$3,015.07/QALY. This is far below the threshold range of GB€20,000 to 30,000 considered cost-effective by the National Institute for Health can Clinical Excellence (NICE)Citation14, CAD$50,000–100,000 suggested by the Canadian Agency for Drugs and Technologies in Health in Canada,Citation15 or US$50,000 which is commonly used as the threshold in the USA.Citation16
In terms of health gains from QIV, our results are consistent with finding from previous studies which demonstrated that QIV can provide public health benefits, including reducing the number of influenza cases, hospitalizations, and deaths,Citation7 and cost saving.Citation8 Van Bellinghen et al. used a multi-cohort static model to estimate cost-effectiveness of QIV versus TIV in the elderly and at-risk group in the UK.Citation17 The results showed that QIV could reduce the burden of influenza and would be a cost-effective intervention (ICER: GB£5,299/QALY) compared with TIV. Clements et al. estimated the cost-effectiveness of universal quadrivalent vaccine in the US.Citation18 They reported that the probability of being cost-effective is 61% when the WTP threshold was set at US$100,000 (ICER: US$90,301/QALY). You et al. using a decision model to estimate the cost-effectiveness of QIV vs. TIV from perspectives of healthcare provider and society of Hong Kong.Citation19 They found that QIV is cost-effective in Hong Kong population, except for age 15–64 years, from the societal perspective (ICER: US$12,558/QALY). Chit et al. estimated the cost-utility of quadrivalent inactivated influenza vaccine (IIV4) versus trivalent inactivated influenza vaccine (IIV3) of all population in Ontario, Canada.Citation20 They reported that the ICER was US$63,773/QALY from the societal perspective and the probability of IIV4 being cost-effective was 65% when the WTP threshold was set at US$100,000/QALY gained. Meier et al updated the cost-effectiveness analysis of QIV in at-risk adults and the elderly in the UK.Citation21 After excluding the influenza seasons with mismatching, the ICER was GB£14,645/QALY from the governmental perspective and was GB£13,497/QALY from the societal perspective.
The controversial results of previous studies may due to different perspectives, target patients and theoretical models. Some studies found that elderly patients and those in a clinical risk group received QIV would be cost-effectiveness.Citation17,19,21 Other studies using all population as target patient showed lower probability of being cost-effectiveness.Citation20,22 The theoretical models applied among studies varied. Two of these 5 studies using decision tree model to estimate cost-effectiveness of QIV in 1 y.Citation18,19 However, decision tree models were not designed to handle the fact that individuals could have more than one influenza in 1 y. Other two studies using static multi-cohort lifetime model only estimated the elderly and those clinical at-risk.Citation17,21 Although there is a study using multi-cohort model and all population,Citation20 it still lacks information about complications caused by influenza which could underestimate the effectiveness of QIV. Compared with previous studies, our study is the first study applying a static multi-cohort lifetime model incorporating influenza-related complications to estimate the cost-effectiveness of QIV using on all population.
Comparing with previous studies, the clinical outcomes of present study are consistent with the reported findings that QIV reduced clinical event. The results indicated that the ICER was most sensitive to changes in annual influenza circulation and the matching of vaccines with the influenza B virus. The prevention of general practitioners (GPs) visits and hospitalization could be expected to result in significant net cost savings in the provision of health services. Our results demonstrate that replacing the current TIV vaccination program with QIV in the government funded vaccination program for priority groups and all population would be cost-effective in Taiwan. Results showed that the ICER per QALY gained is cost-effective while the overall vaccination rate is below 50%, it would decrease to $2,908.7 when the coverage rate increase by 10%. This suggest that QIV would be more cost-effectiveness when increasing the cover rate of vaccination.
However, this study is subject to a number of limitations, most of which were related to uncertainty with regard to modeling assumptions. First, we included data inputs as a 10 y average for the distribution of influenza A and B. We recognize that this does not necessarily capture all of the variability that exists among seasons and may underestimate the effects of QIV. Second, the estimated proportion of influenza-like illness (ILI) diagnosed as influenza was based on expert opinion, which may not necessarily capture all of the influenza cases. Nonetheless, we addressed these issues through probability sensitivity analysis. Third, the static models may underestimate the external benefits resulted by QIV and may have higher ICER value than dynamic models. However, this study demonstrated that using a static multi-cohort model can also capture the potential effect at different cohorts. Fourth, the governmental perspective was used in the study, we only included the direct medical costs reimbursed by the NHIA and vaccine costs paid by the government. Other costs including out-of-pocket paid by patients were not included. Further research taken from the societal perspective may be warranted. Finally, since this study assumed the governmental perspective, indirect costs, such as out-of-pocket payment and productivity losses, were not included in this study. The inclusion of such costs would no doubt increase the differences in costs between QIV and TIV, because the adoption of QIV would result in fewer GP visits and hospitalizations, which would reduce losses in productivity. Future research analyzing the data from a societal perspective is warranted.
Conclusions
Under a series of plausible assumptions and from the perspective of government, this multi-cohort simulation shows that the adoption of QIV as a replacement for TIV for the total population could save 10,557 QALYs over a 100 y time horizon yielding an ICER of US$3,015.07 per QALY gained. Therefore, compared to current practices (TIV), the adoption of QIV would be a cost-effective vaccination strategy from a governmental perspective.
Methods
Model structure and assumptions
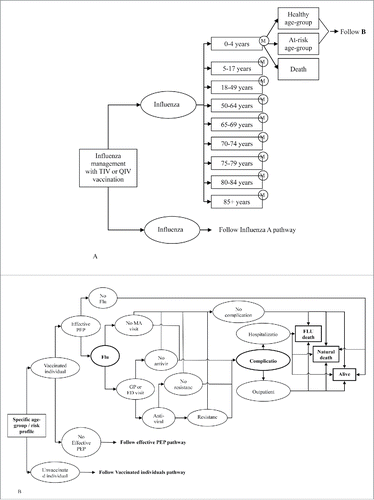
This study employed a static lifetime multi-cohort Markov model which has been published and validated.Citation17 Two vaccination strategies were evaluated: TIV and QIV. This static multi-cohort model simulates the impact of vaccination on seasonal influenza through annual cycles over a life-time horizon of 100 y (). At time zero, the total 2010 Taiwan population was represented in the aforementioned multi-cohort model according to the Taiwan population distribution of 9 distinct age cohorts. Influenza A and B were accounted for separately, to allow the model to evaluate differences in vaccine protection. In each cycle, a number of events could occur, including receiving vaccination, receiving PEP, infected with influenza, seeking medical advice (either from a GP or emergency department), receiving antiviral treatment, becoming treatment resistant, developing influenza-related complications, having hospitalization due to complication, having outpatient visit due to complications or died from influenza or no-influenza-related causes. The rest surviving individuals would move to the next annual cycle.
Figure 2. A: Overview of model structure; B: Overview of possible event pathways. ED, emergency department; Alive=Healthy or ar-risk gropu; GP, gerenal practitioner; MA, medical advice; PEP, post-exposure prophylaxis; QIV, quadrivalent vaccine; TIV, trivalent vaccine.
Costs used in the model included vaccination costs, neuraminidase inhibitors (NI) medication costs, medical costs (costs due to ambulatory visits, emergency visits, and hospitalizations) as well as other costs resulting from complications. Effectiveness included life-years (LY) and quality-adjusted life-years (QALY). Incremental values of the 2 strategies were compared.
Demographic data were obtained from government statistics and cost data were obtained from National Health Insurance claims data, released through the Health and Welfare Data Science Center (HWDC) of the Ministry of Health and Welfare (MOHW) in Taiwan. Transition probabilities were obtained from previous studies. The model was run within Microsoft Excel (2003, Microsoft Corp., Redmond, WA). Cost and effectiveness were discounted at 3.5% and costs are expressed in 2010 US dollars with the exchange rate of 1USD: 30 New Taiwan Dollars (NTD).
Several assumptions were made in accordance with the opinions of experts and suggestions proposed in previous studies. First, according to estimates from previous studies, the probability of a patient having influenza when diagnosed with an ILI was assumed to be 50%.Citation23 Second, the probability that individuals with symptomatic influenza would seek medical advice was assumed to be 90%.Citation24 Third, the probability of a patient receiving NI after a GP or emergency visit was assumed to be 50%.
Model input and assumptions
Vaccine efficacy and coverage
Vaccine efficacy and coverage rate are shown in . The overall impact of efficacy difference between the QIV and TIV was affected on the proportion of circulating influenza B among all influenza cases, which varies by years. Base-case estimates of the average distribution of influenza cases between influenza A (61.00%) and influenza B (39.00%) were calculated from 2000–2010 from official reports and previous studies.Citation25,26 The average 62.19% (±13.82%) match between the TIV and the circulation influenza lineage was used in the analysis.
Table 2. Input data for vaccine efficacy and coverage.
Base on the distribution of influenza type and matching, the efficacy of TIV and QIV against influenza A was 59.0% in the 0–17 y old group, 61.0% in the 18–64 y old group, and 58.0% in the group 65 y and older.Citation27,28 We assumed that efficacy of QIV against influenza A was identical to that of TIV. In addition, the efficacy of QIV against influenza B was assumed to be equal to TIV when the lineages of the circulating influenza B matched the annual vaccine.Citation23
According to vaccination guidelines issued by the Center for Disease Control in Taiwan, people aged 65 or older, patients with catastrophic illnesses as defined by the National Health Insurance Administration (NHIA), poultry and livestock workers, health care workers, people aged 50–64 with chronic illnesses, children aged 0.5–6 y and elementary school-aged children are included as priority groups eligible to receive government-financed vaccinations. Based on governmental reports, the vaccination coverage rates were as follows: 0–4 y (39.23%), 5–17 y (39.49%), 18–49 y (3.24%), 50–64 y (4.21%), and 65 y and older (37.30%)Citation1,4,29,30
Population distribution
Nine age groups were included in the model (0–4, 5–17, 18–49, 50–64, 65–69, 70–74, 75–79, 80–84, ≧85 years) and were subdivided into 2 groups: healthy and at-risk. Younger age groups moved to older age groups over time. The at-risk group included individuals with respiratory disease, circulatory disease, diabetes and endocrine diseases, disease of the liver, renal disorders, malignant neoplasms, and immune compromise defined by previous study, as well as those living in residential care homes.
Incidence rate of influenza and complications
The main age-dependent probabilities are listed in . The incidence rates of influenza among different age groups were based on estimates reported by Turner et al.Citation4 Thus, the assumed incidence of 0–17 y was 19.21% at baseline with a range of 11.91%-18.35%; the incidence of 18–64 y was 6.55% at baseline with a range of 2.33%-9.44%; the incidence of 65 y and older was 6.17% at baseline with a range of 2.33%-9.44%. This model included respiratory complications, cardiac complications, renal complications, and central nervous system (CNS) complications, otitis media (OM), and gastro intestinal (GI) bleeding. The proportions of individuals with any of these complications and who did not undergo antiviral treatment were 14.05% in the healthy group and 18.29% in the at-risk group, based on estimations obtained from previous studies.Citation31 The relative risk of developing complications following antiviral treatment vs. no antiviral treatment was set at 69.45 in the 0–4 y old group, 61.87 for 5–17 y old group, 47.77 for 18–49 y old group, 47.84 for 50–64 y group, and 48.18 for the 65 y old and above.Citation23 The probability to be resistant to antivirus treatment in the 0–4 and 5–17 y old group was 5.03% and1.77% for 18 y old and above. The probabilities of having complications for patients resistant to antivirus treatment were assumed to be the same with those without effective antivirus treatment.
Table 3. Input data for age-dependent probabilities.
Proportions of GP visit, hospitalization or mortality
Relatively few hospitalizations or deaths were specifically coded as influenza-related; therefore, this study used the following methods to estimate the proportion of visits to GP and hospitalizations attributable to influenza. The proportion of subjects seeking medical advice for influenza were derived from a nationwide survey by the Center for Disease Control in Taiwan.Citation32 The proportions of subjects visiting a GP or an emergency department were computed from the Taiwan National Health Insurance Research Database (NHIRD). The NHIRD is a longitudinal, individual-based database containing all medical claims data of patients since 1995.
This study first identified individuals diagnosed with influenza (ICD-9-CM code: 487.xx) and found that the proportion of subjects visiting GPs was 98.0% and emergency room visits accounted for 2.0%. Based on estimations by Tappenden et al,Citation23 the proportion of hospitalizations for any type of complications among different groups younger than 18 y old was 10.87% in the healthy group and 15.79% in the at-risk group.
The proportion of outpatients receiving NI within 48/36 hours of a visit was 40%-60%, based on suggestions from key opinion leaders. Thus, we set the proportion of antiviral treatment following GP or emergency visits as 50% at baseline with a range of 40%-60%, and the proportions of individuals 0–17 y old who were resistant to NI as 5.03%.Citation33 The rate of fatality due to influenza-related complications in age groups younger than 17 y were set to 0% in the healthy group and 0.15% in the at-risk group, in accordance with estimations used in a previous study.Citation31 All-cause mortality rates in different age groups were obtained from the Ministry of the Interior.
Costs
lists cost data used in the base-case analysis. From a governmental perspective, we collected information related to the direct medical costs of vaccines borne by the government, patients, and the NHIA. TIV was publicly funded in Taiwan. Each year, the Centers for Disease Control (CDC) in Taiwan would process the procurement of TIV. We obtained the budget and total amount of TIV purchased by the CDC from the governmental e-procurement system during 2010–2012. The cost of TIV in the model was set at US$3.83 which is the average cost per dose. In addition, vaccination administration costs were set at US$3.33, which is subsidized by the MOHW only for the groups aged 65 y or older. The administration fee for younger age was paid for by parents thus was not included in the model. The cost of quadrivalent vaccine was assumed to be 15% higher (US$4.40) at baseline with a range of ±33%. The cost of antiviral treatment per course was set at US$31.67 per course, as obtained from the NHIA's fee schedule for medications. Unit costs of medical services, including cost of visits to a GP or emergency room, and hospitalization costs were extracted from the claims files of the NHIRD.
Table 4. Input data for costs.
Utility weight
Utility data are shown in . No previous study has estimated the utility of influenza vaccination of Taiwanese population. Therefore, we obtained values based on the estimations of previous studies.Citation12,23 Disutilities were derived from EuroQoL data reported from uncomplicated influenzaCitation34 and from assumptions presented in a previous study.Citation12 The utilities were then modified according to the duration of the disease.
Table 5. Input data for utility.
Sensitivity analyses
One-way and probabilistic sensitivity analyses were performed to assess the impact of epidemiological data, vaccine costs, unit costs of medical services, and health state utilities on model outcomes. This study determined ranges for parameters based on reports from previous studies. We also conducted Monte Carlo simulation with1000 iterations to generate estimates of the cost per QALY gained ratio, which were then plotted as a cost-effectiveness acceptability curve (CEAC) to compare the ceiling value of the cost per QALY gained with cost-effectiveness probability.Citation35 All parameters were included in the probabilistic sensitivity analysis except for a few that were considered fixed (Table S2).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_material.docx
Download ()Funding
This study was funded by GlaxoSmithKline (GSK) Far East B.V. Taiwan Branch. GSK were given the opportunity to review the manuscript for medical accuracy and protection of intellectual property, however editorial control resided fully with the authors only. None of the authors have direct or indirect financial relationship with the sponsor.
References
- Chang CW, Wu KB, Huang ZM, Chen CH. Prevalence of influenza virsus between Taiwan and other contries (in Chinese). Taiwan Epidemiol Bull 2006; 22:813-27.
- World Health Organization. Vaccine use. 2013.
- National Health Insurance Administration. Catastrophic Illness definition of National Health Insurance. 2011.
- Lee PI. Influenza Vaccine. Taipei, Taiwan: Centers for Disease Control, Department of Health, R.O.C (Taiwan); 2011.
- Centers for Disease Control and Prevention. Seasonal influenza activity surveillance reports: 1999–2000 to 2010–2011 seasons. Atlanta, USA.
- Chang C-W, Chuang R-H, Wu K-B. Epidemiological Analysis of Seasonal Influenza Epidemic in Taiwan in 2006/2007. Epidemiol Bull 2008; 24:876-89.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993-8; PMID:22226861; http://dx.doi.org/10.1016/j.vaccine.2011.12.098
- Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine 2012; 30:7443-6; PMID:23084849; http://dx.doi.org/10.1016/j.vaccine.2012.10.025
- Salo H, Kilpi T, Sintonen H, Linna M, Peltola V, Heikkinen T. Cost-effectiveness of influenza vaccination of healthy children. Vaccine 2006; 24:4934-41; PMID:16678945; http://dx.doi.org/10.1016/j.vaccine.2006.03.057
- Turner DA, Wailoo AJ, Cooper NJ, Sutton AJ, Abrams KR, Nicholson KG. The cost-effectiveness of influenza vaccination of healthy adults 50–64 years of age. Vaccine 2006; 24:1035-43; PMID:16183177; http://dx.doi.org/10.1016/j.vaccine.2004.12.033
- Newall AT, Scuffham PA, Kelly H, Harsley S, MacIntyre CR. The cost-effectiveness of a universal influenza vaccination program for adults aged 50–64 years in Australia. Vaccine 2008; 26:2142-53; PMID:18343537; http://dx.doi.org/10.1016/j.vaccine.2008.01.050
- Rothberg MB, Bellantonio S, Rose DN. Management of influenza in adults older than 65 years of age: Cost-effectiveness of rapid testing and antiviral therapy. Annal Int Med 2003; 139:321-9; http://dx.doi.org/10.7326/0003-4819-139-5_Part_1-200309020-00007
- Hoshi SL, Kondo M, Honda Y, Okubo I. Cost-effectiveness analysis of influenza vaccination for people aged 65 and over in Japan. Vaccine 2007; 25:6511-21; PMID:17681651; http://dx.doi.org/10.1016/j.vaccine.2007.05.067
- Devlin N, Parkin D. Does NICE have a cost-effectiveness threshold and what other factors influence its decisions? A binary choice analysis. Health Economics 2004; 13:437-52; PMID:15127424; http://dx.doi.org/10.1002/hec.864
- George B, Harris A, Mitchell A. Cost effectiveness analysis and the consistency of decision making - Evidence from pharmaceutical reimbursement in Australia (1991 to 1996). Pharmacoeconomics 2001; 19:1103-9; PMID:11735677; http://dx.doi.org/10.2165/00019053-200119110-00004
- Bridges JFP, Onukwugha E, Mullins CD. Healthcare Rationing by Proxy Cost-Effectiveness Analysis and the Misuse of the $50 000 Threshold in the US. Pharmacoeconomics 2010; 28:175-84; PMID:20067332; http://dx.doi.org/10.2165/11530650-000000000-00000
- Van Bellinghen LA, Meier G, Van Vlaenderen I. The Potential Cost-Effectiveness of Quadrivalent versus Trivalent Influenza Vaccine in Elderly People and Clinical Risk Groups in the UK: A Lifetime Multi-Cohort Model. Plos One 2014; 9:e98437; PMID:24905235; http://dx.doi.org/10.1371/journal.pone.0098437
- Clements KM, Meier G, McGarry LJ, Pruttivarasin N, Misurski DA. Cost-effectiveness analysis of universal influenza vaccination with quadrivalent inactivated vaccine in the United States. Hum Vaccin Immunotherap 2014; 10:1171-80; http://dx.doi.org/10.4161/hv.28221
- You JHS, Ming WK, Chan PKS. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong - A decision analysis. Hum Vaccin Immunotherap 2015; 11:564-71; http://dx.doi.org/10.1080/21645515.2015.1011016
- Chit A, Roiz J, Aballea S. An assessment of the expected cost-effectiveness of quadrivalent influenza vaccines in Ontario, Canada Using a static model. Plos One 2015; 10:e0133606; PMID:26222538; http://dx.doi.org/10.1371/journal.pone.0133606
- Meier G, Gregg M, Nautrup BP. Cost-effectiveness analysis of quadrivalent influenza vaccination in at-risk adults and the elderly: an updated analysis in the UK. J Med Econom 2015; 18:746-61; http://dx.doi.org/10.3111/13696998.2015.1044456
- You JHS, Ming WK, Chan PK. Cost-effectiveness analysis of quadrivalent influenza vaccine versus trivalent influenza vaccine for elderly in Hong Kong. BMC Infect Dis 2014; 14:618; http://dx.doi.org/10.1186/s12879-014-0618-9
- Tappenden P, Jackson R, Cooper K, Rees A, Simpson E, Read R, Nicholson K. Amantadine, oseltamivir and zanamivir for the prophylaxis of influenza (including a review of existing guidance no. 67): a systematic review and economic evaluation. Health Technol Assess 2009; 13:1-+; PMID:19215705; http://dx.doi.org/10.3310/hta13110
- Centers for Disease Control. The public poll of avian influenza immunization. e-Society Research Group 2005.
- Lin JH, Chiu SC, Shaw MW, Lin YC, Lee CH, Chen HY, Klimov A. Characterization of the epidemic influenza B viruses isolated during 2004–2005 season in Taiwan. Virus Res 2007; 124:204-11; PMID:17196288; http://dx.doi.org/10.1016/j.virusres.2006.11.005
- Lo YC, Chuang JH, Kuo HW, Huang WT, Hsu YF, Liu MT, Chen CH, Huang HH, Chang CH, Chou JH, et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011–12 season. PLoS One 2013; 8:e58222; PMID:23472161; http://dx.doi.org/10.1371/journal.pone.0058222
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Database of Systematic Reviews 2010:CD004876.
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2008:CD004879.
- Chuang JH, Huang AS, Huang WT, Liu MT, Chou JH, Chang FY, Chiu WT. Nationwide surveillance of influenza during the pandemic (2009–10) and post-pandemic (2010–11) periods in Taiwan. PLoS One 2012; 7:e36120; PMID:22545158; http://dx.doi.org/10.1371/journal.pone.0036120
- Turner D, Wailoo A, Nicholson K, Cooper N, Sutton A, Abrams K. Systematic review and economic decision modelling for the prevention and treatment of influenza A and B. Health Technol Assess 2003; 7:iii-iv, xi-xiii, 1-170; PMID:14609480; http://dx.doi.org/10.3310/hta7350
- Meier CR, Napalkov PN, Wegmuller Y, Jefferson T, Jick H. Population-based study on incidence, risk factors, clinical complications and drug utilisation associated with influenza in the United Kingdom. Eur J Clin Microbiol Infect Dis 2000; 19:834-42; PMID:11152308; http://dx.doi.org/10.1007/s100960000376
- Chuang JH, Cheng-Hua Lee P-IL, Wu CF, Liu M-T, Su WY, Huang WT. Effectiveness of influenza A (H1N1) monovalent vaccine and trivalent inactivated influenza vaccine. Centers for Disease and Control, R.O.C. (Taiwan), 2010.
- Centers for Disease Control. Practical guideline for prevention and control of seasonal influenza (in Chinese). Taipei City, Taiwan (R.O.C.): Centers of Disease and Control, Department of Health, 2008.
- Griffin AD, Perry AS, Fleming DM. Cost-effectiveness analysis of inhaled zanamivir in the treatment of influenza A and B in high-risk patients. PharmacoEconomics 2001; 19:293-301; PMID:11303417; http://dx.doi.org/10.2165/00019053-200119030-00007
- Fenwick E, Marshall DA, Levy AR, Nichol G. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. Bmc Health Serv Res 2006; 6:52; PMID:16623946
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Db Syst Rev 2008.
- Jefferson T, Di Pietrantonj C, Al-Ansary LA, Ferroni E, Thorning S, Thomas RE. Vaccines for preventing influenza in the elderly. Cochrane Db Syst Rev 2010.
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database of Systematic Reviews 2010; CD001269
- Tricco AC, Chit A, Soobiah C, Hallett D, Meier G, Chen MH, Tashkandi M, Bauch CT, Loeb M. Comparing influenza vaccine efficacy against mismatched and matched strains: a systematic review and meta-analysis. BMC Med 2013; 11:153; PMID:23800265; http://dx.doi.org/10.1186/1741-7015-11-153
- Gagliardi L, Rusconi F, Galassi C, Forastiere F. Re.: “Antibiotic exposure by 6 months and asthma and allergy at 6 years: findings in a cohort of 1,401 US children”. Am J Epidemiol 2011; 173:1343; author reply 4–5; PMID:21478296; http://dx.doi.org/10.1093/aje/kwr082