ABSTRACT
Multivalent combination vaccines have reduced the number of injections and therefore improved vaccine acceptance, timeliness of administration and global coverage. The hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus/Haemophilus influenzae type b (DTPa-HBV-IPV/Hib; Infanrix hexa™) vaccine, administered according to various schedules, is widely used for the primary vaccination of infants worldwide. In the current publication, we are presenting the immunogenicity and safety of 3 doses of DTPa-HBV-IPV/Hib vaccine when administered to Indian infants. 224 healthy infants (mean age 6.8 weeks) were vaccinated at 6–10–14 weeks (W) of age (n = 112) or 2–4–6 months (M) of age (n = 112). One month after the third vaccine dose, the seroprotection/seropositivity status against diphtheria, pertussis, tetanus, polio, hepatitis B and Hib antigens ranged from 98.6% to 100% in both groups. The vaccine response rate to the pertussis antigens ranged from 97% to 100%. Pain (6–10–14W group: 25.2%; 2–4–6M group: 13.4%) and fever (15.3% and; 15.2%, respectively) were the most frequently reported solicited local and general symptoms. Unsolicited adverse events were reported for 35.7% (6–10–14W group) and 22.3% (2–4–6M group) of subjects. No vaccine related serious adverse events were reported. In conclusion, the hexavalent DTPa-HBV-IPV/Hib vaccine was immunogenic and well tolerated, irrespective of the dosing schedule.
Introduction
Infections resulting from vaccine-preventable diseases including diphtheria, tetanus, pertussis, Haemophilus influenzae type B (Hib) and poliovirus, account for a large number of deaths in children below 5 y of age.Citation1,2 Infants and children infected with hepatitis B are at risk to become chronic carriers of HBV, with potential complications such as cirrhosis and hepatocellular carcinomas later in life.Citation3 It is estimated that 18.7 million infants worldwide and >4 million infants in India did not receive vaccination against diphtheria-tetanus-pertussis (DTP) during their first year of life.Citation4
Combination vaccines, such as DTP, have been widely adopted in pediatric vaccination programs, as the reduced number of injections and simplified administration has been associated with higher compliance and improved vaccine coverage.Citation5,6 A combined hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliomyelitis and Hib conjugate vaccine (DTPa-HBV-IPV/Hib, Infanrix hexa™, GSK Vaccines, Belgium) has been widely used for over 15 y.Citation7,8 Many studies have established the immunogenicity and safety of the combined DTPa-HBV-IPV/Hib administered according to different primary vaccination schedules and in diverse settings.Citation5,7,9-11 Furthermore, comparable efficacy and safety profiles to those of the respective monovalent components have also been established.Citation7,10
In accordance with the Expanded Program on Immunization (EPI) recommendations, the Indian infant vaccination schedule comprises primary vaccination with 3 doses of DTP at 6, 10 and 14 weeks, followed by a booster dose in the second year of life.Citation12 In addition, the Indian Academy of Pediatrics (IAP) recommends that infants are vaccinated against hepatitis B at birth, and 6 and 14 weeks of age; against Hib infections at 6, 10, and 14 weeks of age; oral poliovirus vaccine (OPV) should be given at birth and inactivated poliovirus vaccine (IPV) at 6, 10 and 14 weeks of age.Citation13 In 2015, the coverage with 3 doses of DTP, Hib, hepatitis B and polio vaccines was estimated at 87%, 45%, 87%, and 86%, respectively, in India.Citation14
Currently, only pentavalent combination vaccines are available in India (i.e. DTPw-whole cell pertussis [Pw]-HBV-Hib and DTPa-IPV/Hib).
Although the use of OPV in India has led to the country being declared polio-free in 2014,Citation15 it also contributes to the risk of vaccine-associated paralytic poliomyelitis (VAPP).Citation16,17 The incidence of VAPP has been estimated at 2–4 cases/million birth cohort per year in countries using OPVCitation18 which highlights the need to consider the risks associated with continued OPV use.Citation19 Studies have reported the incidence of VAPP cases associated with OPV in IndiaCitation16 and have recommended replacing OPV with IPV in order to avoid VAPP.Citation20,21 Moving from OPV to IPV is very important in complete global eradication of polio, and vaccines combining IPV (e.g. DTPa-HBV-IPV/Hib) appear to be the most convenient way to facilitate this change.Citation22
In order to support policy makers and healthcare providers to make informed decisions on vaccination in India, we evaluated the immunogenicity and safety of DTPa-HBV-IPV/Hib when administered to Indian infants according to either the 6–10–14 weeks (W) of age or 2–4–6 months (M) of age schedule.
Results
Study population
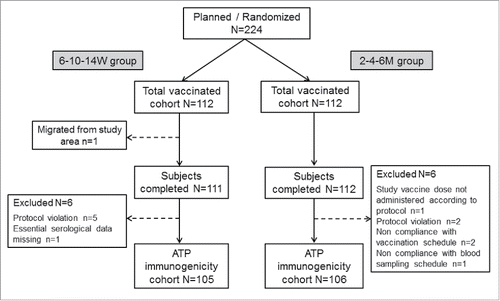
A total of 224 children were enrolled and vaccinated (total vaccinated cohort [TVC]) in this study, of whom 223 (6–10–14W group n = 111; 2–4–6M group n = 112) completed the study (). 211 subjects (6–10–14W group n = 105; 2–4–6M group n = 106) were included in according-to-protocol (ATP) immunogenicity cohort. Both groups were comparable demographically: all subjects were of Central/South Asian heritage with an overall mean age of 6.8 (±1.1) weeks; 53.6% were male.
Assessment of immunogenicity
One month after the third vaccine dose, seropositivity/seroprotection rates were similar in both groups (). All subjects in both groups were seroprotected against diphtheria and tetanus.
Table 1. Seroprotection/seropositivity rates and GMC/Ts for anti-diphtheria, anti-tetanus, anti-PRP, anti-HBs, anti-poliovirus 1, 2, 3, anti-PT, anti-FHA and anti-PRN antibodies by group before first dose and one month after the third vaccine dose (ATP cohort for immunogenicity).
In both groups, ≥99.0% of subjects were seroprotected against Hib polyribosylribitol phosphate (PRP) ().
Before the first dose study vaccination, 17.5% of the subjects in 6–10–14W group and 16.0% of the subjects in 2–4–6M group were seroprotected for hepatitis B, reflecting the hepatitis B birth vaccination that all study participants received. One month after primary vaccination, ≥99.0% of subjects were seroprotected against hepatitis B in both groups.
Before the first dose of study vaccination, at least 68.6%, 67.9% and 26.1% of the subjects were seroprotected against poliovirus type 1, 2 and 3, respectively, in both groups, reflecting the OPV vaccination all study participants received at birth. One month post-primary vaccination, all subjects were seroprotected against poliovirus 1 and 2 in both groups; 98.6% subjects in 6–10–14W group (all except one subject) and all subjects in 2–4–6M group were seroprotected against poliovirus 3 ().
One month post-primary vaccination, all subjects in both groups were seropositive for anti-pertussis toxin (PT), anti-filamentous haemagglutinin (FHA) and anti-pertactin (PRN) antibodies (≥5 EL.U/ml) and the vaccine response rates to the pertussis antigens were above 97% and 98% in the 6–10–14W and 2–4–6M groups, respectively, for at least one antigen (). At pre-vaccination, the geometric mean concentration (GMC) values were 5.0 EL.U/ml and 4.6 EL.U/ml for anti-PT; 18.7 EL.U/ml and 20.1 EL.U/ml for anti-FHA; and 3.4 EL.U/ml and 3.2 EL.U/ml for anti-PRN in the 6–10–14W and 2–4–6M groups, respectively. The levels of pertussis antibodies observed at the pre-vaccination are probably due to the natural exposure to the disease or maternal transfer of antibodies against pertussis. One month after the third dose, the GMC values were 107.3 EL.U/ml and 108.2 EL.U/ml for anti-PT; 293.7 EL.U/ml and 369.3 EL.U/ml for anti-FHA; and 224.4 EL.U/ml and 243.6 EL.U/ml for anti-PRN across the 2 groups.
Table 2. Vaccine response rate (%) to anti-PT, anti-FHA and anti-PRN antibody concentrations one month after third vaccine dose (ATP cohort for immunogenicity).
Assessment of safety and reactogenicity
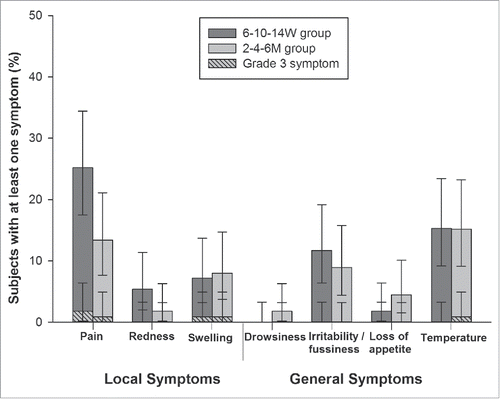
During the 4-day follow-up period, pain was the most frequently reported solicited local symptom, being reported in 25.2% (28/111) and 13.4% (15/112) subjects in the 6–10–14W and 2–4–6M groups, respectively (). Pain was also the most frequently reported grade 3 local symptom (1.8% of subjects in the 6–10–14W group; 0.9% of subjects in the 2–4–6M group). Fever (axillary temperature ≥37.5°C) was the most frequently reported solicited general symptom in both groups, with an incidence of 15.3% (17/111 subjects) and 15.2% (17/112 subjects) in the 6–10–14W and 2–4–6M groups, respectively. In all instances, fever was assessed as causally related to the vaccination. It was also the most frequently reported grade 3 (i.e., axillary temperature >39°C) solicited general symptom and was recorded in 0.9% of subjects in the 2–4–6M group. None of the subjects in the 6–10–14W group reported grade 3 fever.
Figure 2. Overall incidence of solicited local and general symptoms for 4 d after primary vaccination (Total vaccinated cohort).
During the 31-day follow-up period, at least one unsolicited adverse event (AE) was reported for 35.7% (40/112) and 22.3% (25/112) of subjects in 6–10–14W and 2–4–6M groups, respectively. Upper respiratory tract infection was the most frequently reported unsolicited AE (6–10–14W group: 16.1% [18/112]; 2–4–6M group: 9.8% [11/112]). None of the subjects reported grade 3 unsolicited AEs. Three serious AEs (SAEs; lower respiratory tract infection, pneumonia and bronchiolitis) were reported in 5 subjects in both groups (6–10–14W group, n = 2; 2–4–6M group, n = 3). None of the unsolicited adverse events or SAEs were assessed by the investigator as causally related to the vaccination. No subjects withdrew from the study due to an AE or SAE.
Discussion
Since its licensure in 2000, more than 137 million doses of the combined DTPa-HBV-IPV/Hib vaccine were distributed worldwide. Post-marketing surveillance data confirmed its clinically acceptable safety profile and its robust immuno-genicity.Citation5,8,23
We assessed the immunogenicity and safety of primary vaccination with the combined DTPa-HBV-IPV/Hib vaccine administered to Indian infants according to either a 6–10–14W or 2–4–6M schedule. One month after third vaccine dose, the seroprotection rates ranged from 98.6% to 100% for all study antigens. These results are in line with a previous study from the Philippines where seroprotection rates ranging from 90% to 100% were recorded after primary vaccination with the DTPa-HBV-IPV/Hib vaccine using the 6–10–14W schedule.Citation11 Likewise, several studies have reported comparable seroprotection/seropositivity rates one month post primary vaccination according to a 2–4–6M schedule.Citation24–26 The vaccine response to the pertussis antigens (97–100%) was also similar to previous reports.Citation24
Post-primary vaccination, ≥99.0% of the subjects were seroprotected against anti-HBs. Even though it has been established that the hepatitis B immunogenicity elicited by the combined DTPa-HBV-IPV/Hib vaccine is comparable to that elicited by the monovalent vaccine,Citation27 previous studies have demonstrated that a HBV birth dose is essential to achieve targeted seroprotection rates ≥95%, with the combined vaccine, when the accelerated 6–10–14W schedule is used.Citation11 It should be noted that, in accordance with local Indian recommendations, all subjects included in this study received a birth dose of hepatitis B vaccine.
Similarly, the pre-vaccination seroprotection rates for poliovirus 1, 2 and 3 were due to the birth dose of OPV received as per local standard of care. Post-vaccination seroprotection rates (98.6% to 100%) were in line with previous reports.Citation11 Cases of VAPP are occasionally reported in countries where wild poliovirus has been eliminated, and therefore replacing OPV by IPV in pediatric immunization schedules is an important step towards global polio eradication.Citation22 The role of IPV in poliovirus control and complete polio eradication is reflected in WHO guidelines recommending the administration of at least one IPV dose to all children, and by the growing number of countries transitioning from OPV to an all-IPV schedule.Citation18 The combined DTPa-HBV-IPV/Hib vaccine would facilitate this transition from OPV to IPV in India, which is a key step in the fight for global polio elimination.
The combined DTPa-HBV-IPV/Hib vaccine was generally well tolerated by Indian infants. This vaccine was known to be associated with low incidence of clinically significant AEs and very rare SAEs.Citation5 There were no withdrawals from the study due to any AEs or SAEs. A previous study (NCT00316147) has also confirmed the safety of the hexavalent vaccine in Indian infants when administered according to either recommended 6–10–14W or internationally adopted 2–4–6M vaccination schedules.Citation28 These findings are in line with previous reports where acceptable safety profiles have been observed across different dosing schedules.Citation11,23
Our study is the first to report the immunogenicity and safety of DTPa-HBV-IPV/Hib in India. A high level of compliance was observed, with 99.6% subjects receiving all the 3 vaccine doses and 94.2% subjects being eligible for inclusion in the immunogenicity cohort. Although our study was limited by the small sample size and exploratory nature of analysis, it did comply with the current regulatory requirements in India.
Combined vaccines are the preferred choice for infant immunization as they simplify the childhood vaccination schedule. Furthermore, they are convenient and cost-effective, with better coverage, improved compliance, and require fewer clinic visits.Citation6,29 The choice of dosing schedule differs markedly between countries, although the extended 2–4–6M schedule has the advantage of facilitating visits at the crucial 4 and 6 month stages, when infants are being weaned (from breastfeeding).Citation30 This schedule is widely adopted in Western countries, as well as in some Asian countries.Citation31
Recent resurgence in pertussis disease incidence and a shift of the disease to older age groups in some countries that switched from DTPw to DTPa vaccinationCitation32 in infancy, were partly attributed to waning of long-term Pa vaccine efficacy, which was estimated to be more rapid when compared to Pw vaccines.Citation33 Therefore, WHO endorsed the use of DTPw in settings in which additional booster immunizations or other strategies for preventing early pertussis infant mortality (e.g., pertussis maternal immunization) are not easily attained.Citation34 Although the reactogenicity of DTPa vaccines was shown to be more reduced than that of DTPw, the currently available measures of immune response against pertussis might not be predictive of long-term effectiveness, and so interchangeability of infant DTP vaccines should be further explored and cautiously approached in the context of different pediatric immunization programs.
In conclusion, the combined DTPa-HBV-IPV/Hib vaccine is immunogenic and well tolerated when administered according to either a 6–10–14W or 2–4–6M schedule to healthy Indian infants. DTPa-HBV-IPV/Hib might be considered a viable choice in the transition from OPV to IPV vaccination, which could contribute to reducing the risk of VAPP.
Materials and methods
Study design and participants
This open-label, phase III study was conducted at 4 Indian centers between 16 April 2012 and 25 February 2013 (www.clinicaltrials.gov NCT01353703). It was conducted according to Good Clinical Practice and the Declaration of Helsinki, and the study protocol was approved by the ethics committees at all participating study centers. Written informed consent was obtained from the parents/guardians of all the subjects before initiating any study-related procedure.
A target enrollment of 224 subjects (112 subjects per group) was considered, which after a 10% drop-out would still have ensured 100 subjects per group evaluable for immunogenicity. Subjects were randomized (1:1) in 2 groups to receive 3 doses of the combined DTPa-HBV-IPV/Hib vaccine according to either a 6–10–14W or 2–4–6M schedule (control group). Healthy infants aged 6 to 10 weeks, who had previously received a dose of hepatitis B vaccine within the first week of birth, and born after a gestation period of 36 weeks, were enrolled in this study. The exclusion criteria included: use of any investigational drug/vaccine (except human rotavirus) 30 d before vaccination or any immunoglobulins/blood products since birth, or immunosuppressants/immune modifying drugs within 6 months of the first vaccine dose, known hypersensitivity to the study vaccine components, confirmed/suspected immunosuppressive/immunodeficient condition, family history of congenital/hereditary immunodeficiency, major congenital defects or chronic illness and/or fever at the time of enrolment. Other reasons for exclusion included previous exposure to diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis and/or Hib vaccination or disease (with the exception of a birth dose of hepatitis B and OPV vaccination).
Study vaccine
Each 0.5 ml dose of the recombinant DTPa-HBV-IPV/Hib vaccine contained ≥30 international units (IU) of diphtheria toxoid (DT), ≥40 IU tetanus toxoid (TT), 25µg PT, 25µg FHA, 8µg PRN, 10µg recombinant hepatitis B surface antigen, 40D, 8D and 32D antigen units of poliovirus types 1, 2 and 3, respectively, and 10µg PRP conjugated to TT (20–40µg). All vaccine doses were administered intramuscularly into the upper side of the right thigh.
Immunogenicity assessment
Blood samples were collected from all subjects before the first dose (pre-vaccination) and one month after the third dose (post-vaccination). Antibodies against DT, TT, PRP, FHA, and PRN were measured using standard in-house enzyme-linked immunosorbent assays (ELISA). The anti-HBs concentrations were measured using a commercial chemiluminescence assay (ADIVA Centaur™ Anti-HBs, Siemens Healthcare, Marburg, Germany; cut-off: 6.2 mIU/mL). Antibodies against each poliovirus (type 1, 2 and 3) were measured by a virus micro-neutralization assay. Seroprotection was defined as an antibody concentration ≥0.1 IU/ml for diphtheria and tetanus, ≥10 mIU/ml for antibodies to hepatitis B (anti-HBs), ≥1:8 dilution for poliomyelitis, and ≥0.15µg/ml for PRP. Seropositivity for antibodies against pertussis antigens (PT, FHA and PRN) was defined as an antibody concentration ≥5 EL.U/ml. The primary objective of the study was to assess the vaccine response rates to the pertussis antigens and seroprotection rates against all other antigens included in the vaccine, one month after the third dose.
Assessment of safety and reactogenicity
All and grade 3 solicited local (injection site pain, redness, swelling) and general (drowsiness, fever, irritability/fussiness and loss of appetite) symptoms were recorded for 4 (Day 0–3) subsequent days after each vaccine dose. Unsolicited AEs were recorded for 31 (Day 0–30) days after vaccination. SAEs were recorded during the entire study period.
Statistical analysis
The statistical analyses were performed using SAS (Statistical Analysis Software, SAS Institute Inc., Cary, NC, United States).
The primary analyses for immunogenicity were performed on the ATP cohort. It included subjects meeting all eligibility criteria, complying with the procedures and intervals defined in the protocol.
The GMC/geometric meant titer calculations were performed by taking the anti-log of the mean of the log transformations. Seropositivity rates against PT, FHA and PRN; seroprotection rates against HBs, DT, TT, PRP antigen and poliovirus types 1, 2 and 3 with 95% confidence intervals (CI) were calculated. Percentage of subjects with anti-diphtheria and anti-tetanus antibody concentrations ≥1.0 IU/ml; anti-HBs antibody concentrations ≥100 mIU/ml; anti-PRP antibody concentrations ≥1.0 μg/ml were calculated with 95% CI. GMC/Ts with 95% CI were tabulated for antibodies against each antigen. Vaccine response rates with exact 95% CI to PT, FHA and PRN were calculated one month after the third vaccine dose.
The analyses for safety and reactogenicity were performed on the TVCs, which included all subjects who had received at least one dose of the study vaccine. The percentage of subjects with at least solicited/unsolicited AE were tabulated with exact 95% CI after each vaccine dose and overall.
Trademark statement
Infanrix hexa is trademark of the GSK group of companies.
Centaur is a trademark of Siemens Healthcare.
Abbreviations
| Anti-HBs | = | antibodies to hepatitis B surface antigen |
| ATP | = | according-to-protocol |
| CI | = | confidence interval |
| DT | = | diphtheria toxoid |
| DTPa | = | diphtheria-tetanus-acellular pertussis vaccine |
| DTPa-HBV-IPV/Hib | = | combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus and Haemophilus influenzae type b vaccine |
| DTPw | = | diphtheria-tetanus-whole cell pertussis vaccine |
| EL.U | = | ELISA units |
| ELISA | = | enzyme-linked immunosorbent assay |
| FHA | = | filamentous haemagglutinin |
| GMC | = | geometric mean concentration |
| GMT | = | geometric mean titer |
| Hib | = | Haemophilus influenzae type b |
| IPV | = | inactivated poliovirus vaccine |
| IU | = | international unit(s) |
| mIU | = | milli-international units |
| OPV | = | oral poliovirus vaccine |
| PRN | = | pertactin |
| PRP | = | Hib capsular polysaccharide polyribosyl-ribitol phosphate |
| PT | = | pertussis toxoid |
| TT | = | tetanus toxoid |
| TVC | = | total vaccinated cohort |
| ED50 | = | median effective dose |
| VAPP | = | vaccine associated paralytic polio |
Disclosure of potential conflicts of interest
Htay Htay Han, Narcisa Mesaros, Olivier Van Der Meeren, Shailesh Mehta and Naveen Karkada are employees of GSK group of companies. Htay Htay Han, Narcisa Mesaros, Olivier Van Der Meeren and Shailesh Mehta also hold stock options or restricted shares from the sponsoring company. Sanjay K Lalwani, Balasubramanian Sundaram, Sharad Agarkhedkar, Shalaka Agarkhedkar, Niranjana S Mahantashetti and Nandini Malshe disclosed no conflicts of interest.
Contributorship
Shailesh Mehta, Htay Htay Han and Olivier Van Der Meeren were involved in study conception, planning or design. Shalaka Agarkhedkar, Shailesh Mehta, Sharad Agarkhedkar, Niranjana S Mahantashetti, Htay Htay Han, Sanjay K Lalwani and Nandini Malshe offered administrative, technical/logistic support; Shailesh Mehta and Olivier Van Der Meeren participated in the choice/recruitment of centers and investigators. Shalaka Agarkhedkar, Balasubramanian Sundaram, Shailesh Mehta, Sharad Agarkhedkar, Niranjana S Mahantashetti, Olivier Van Der Meeren, Sanjay K Lalwani, Nandini Malshe and Narcisa Mesaros participated in the acquisition and assembly of the data and supervised the study/research groups. Naveen Karkada was the statistician. Shailesh Mehta, Sharad Agarkhedkar, Naveen Karkada, Htay Htay Han, Sanjay K Lalwani and Narcisa Mesaros interpreted the results. All authors contributed to the development of this manuscript, had full access to the data, gave final approval before submission and are accountable for all aspects of the work.
Acknowledgments
The authors would like to thank the study participants and their families. The authors would also like to acknowledge Valérie Marichal (the study delivery Lead from GSK Vaccines), and Dipti Phatarpekar (former employee of GSK Vaccines) for their involvement in the study development. The authors also thank Anchal Sood (from GSK Vaccines) for medical writing, Julia Donnelly (freelance writer on behalf of GSK Vaccines) for language editing, and Angeles Ceregido (from XPE Pharma & Science on behalf of GSK Vaccines) for coordinating the publication development.
Funding
This study was sponsored and funded by GlaxoSmithKline Biologicals SA, Belgium. GlaxoSmithKline Biologicals SA was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with developing and publishing this manuscript.
References
- World Health Organization. Immunization coverage fact sheet. Available at http://www.who.int/mediacentre/factsheets/fs378/en/ (accessed September 20, 2016)
- World Health Organization. The top 10 causes of death fact sheet No 310. Available at http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed September 20, 2016)
- World Health Organization. Hepatitis B fact sheet. Available at http://www.who.int/mediacentre/factsheets/fs204/en/ (accessed September 20, 2016)
- Subaiya S, Dumolard L, Lydon P, Gacic-Dobo M, Eggers R, Conklin L. Global routine vaccination coverage, 2014. MMWR Morb Mortal Wkly Rep 2015; 64:1252-5; PMID:26562454; http://dx.doi.org/10.15585/mmwr.mm6444a5
- Zepp F, Schmitt HJ, Cleerbout J, Verstraeten T, Schuerman L, Jacquet JM. Review of 8 years of experience with Infanrix hexa (DTPa-HBV-IPV/Hib hexavalent vaccine). Expert Rev Vaccines 2009; 8:663-78; PMID:19485747; http://dx.doi.org/10.1586/erv.09.32
- Marshall GS, Happe LE, Lunacsek OE, Szymanski MD, Woods CR, Zahn M, Russell A. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J 2007; 26:496-500; PMID:17529866; http://dx.doi.org/10.1097/INF.0b013e31805d7f17
- Van Der Meeren O, Kuriyakose S, Kolhe D, Hardt K. Immunogenicity of Infanrix hexa administered at 3, 5 and 11 months of age. Vaccine 2012; 30:2710-4; PMID:22349525; http://dx.doi.org/10.1016/j.vaccine.2012.02.024
- Dhillon S. DTPa-HBV-IPV/Hib Vaccine (Infanrix hexa): A review of its use as primary and booster vaccination. Drugs 2010; 70:1021-58; PMID:20481658; http://dx.doi.org/10.2165/11204830-000000000-00000
- Silfverdal SA, Assudani D, Kuriyakose S, Van Der Meeren O. Immunological persistence in 5 y olds previously vaccinated with hexavalent DTPa-HBV-IPV/Hib at 3, 5, and 11 months of age. Hum Vaccin Immunother 2014; 10:2795-8; PMID:25483640; http://dx.doi.org/10.4161/21645515.2014.970494
- Avdicova M, Crasta PD, Hardt K, Kovac M. Lasting immune memory against hepatitis B following challenge 10–11 years after primary vaccination with either three doses of hexavalent DTPa-HBV-IPV/Hib or monovalent hepatitis B vaccine at 3, 5 and 11–12 months of age. Vaccine 2015; 33:2727-33; PMID:24962750; http://dx.doi.org/10.1016/j.vaccine.2014.06.070
- Gatchalian SBL, Cadrona-Carlos J, Espos R, Fortunato T, Hernandez-Tanueco V, Book-Montellano M, Reyes MA, Woo J, Sim D, Bock HL, et al. A hexavalent DTPa-HBV-IPV/Hib vaccine administered to Filipino infants at 6, 10 and 14 weeks and 12–15 months of age; importance of the birth dose of HBV. Philippine J Pediatr 2007; 56:153-61
- Government of India, Ministry of Health & Family Welfare. Universal Immunization Program in India. Available at http://mohfw.nic.in/WriteReadData/l892s/Immunization_UIP.pdf (accessed September 20, 2016)
- Singhal T, Amdekar YK, Agarwal RK, Thacker N, Choudhury P, Choudhury J, Aggarwal A, Mehta P, Chinappa J, Srirampur S, Kukreja S, Shah RC, Agrawal R, Sivananda SRK. Indian Academy of Pediatrics Committee on Immunization (IAPCOI) Consensus recommendations on immunization, 2008. Indian Pediatr 2008; 45:635-48; PMID:18723905
- World Health Organization. India: WHO and UNICEF estimates of immunization coverage. 2015 revision. 2016. Available at http://www.who.int/immunization/monitoring_surveillance/data/ind.pdf (accessed September 20, 2016)
- Bahl S, Kumar R, Menabde N, Thapa A, McFarland J, Swezy V, Tangermann RH, Jafari HS, Elsner L, Wassilak SG, et al. Polio-free certification and lessons learned–South-East Asia region, March 2014. MMWR Morb Mortal Wkly Rep 2014; 63:941-6; PMID:25340910
- Kohler KA, Banerjee K, Gary Hlady W, Andrus JK, Sutter RW. Vaccine-associated paralytic poliomyelitis in India during 1999: decreased risk despite massive use of oral polio vaccine. Bull World Health Organ 2002; 80:210-6; PMID:11984607
- Trimble R, Atkins J, Quigg TC, Burns CC, Wallace GS, Thomas M, Mangla AT, Infante AJ. Vaccine-associated paralytic poliomyelitis and BCG-osis in an immigrant child with severe combined immunodeficiency syndrome - Texas, 2013. MMWR Morb Mortal Wkly Rep 2014; 63:721-4; PMID:25144542
- World Health Organization. Polio vaccines: WHO position paper, January 2014. Wkly Epidemiol Rec 2014; 89:73-92; PMID:24707513
- Li R, Li CG, Li Y, Liu Y, Zhao H, Chen X, Kuriyakose S, Van Der Meeren O, Hardt K, Hezareh M, et al. Primary and booster vaccination with an inactivated poliovirus vaccine (IPV) is immunogenic and well-tolerated in infants and toddlers in China. Vaccine 2016; 34:1436-43; PMID:26873055; http://dx.doi.org/10.1016/j.vaccine.2016.02.010
- John TJ. Vaccine-associated paralytic polio in India. Bull World Health Organ 2002; 80:917; PMID:12481217
- John TJ, Vashishtha VM. Eradicating poliomyelitis: India's journey from hyperendemic to polio-free status. Indian J Med Res 2013; 137:881-94; PMID:23760372
- Meriste S, Lutsar I, Tamm E, Willems P. Safety and immunogenicity of a primary course and booster dose of a combined diphtheria, tetanus, acellular pertussis, hepatitis B and inactivated poliovirus vaccine. Scand J Infect Dis 2006; 38:350-6; PMID:16709537; http://dx.doi.org/10.1080/00365540500488857
- Lim FS, Phua KB, Lee BW, Quak SH, Teoh YL, Ramakrishnan G, Han HH, Van Der Meeren O, Jacquets JM, Bock HL. Safety and reactogenicity of DTPa-HBV-IPV/Hib and DTPa-IPV/I-Hib vaccines in a post-marketing surveillance setting. Southeast Asian J Trop Med Public Health 2011; 42:138-47; PMID:21323176
- Aristegui J, Dal-Re R, Diez-Delgado J, Mares J, Casanovas JM, Garcia-Corbeira P, De Frutos E, Van Esso D, Verdaguer J, De la Flor J, et al. Comparison of the reactogenicity and immunogenicity of a combined diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated polio (DTPa-HBV-IPV) vaccine, mixed with the Haemophilus influenzae type b (Hib) conjugate vaccine and administered as a single injection, with the DTPa-IPV/Hib and hepatitis B vaccines administered in two simultaneous injections to infants at 2, 4 and 6 months of age. Vaccine 2003; 21:3593-600; PMID:12922087; http://dx.doi.org/10.1016/S0264-410X(03)00420-1
- Tejedor JC, Moro M, Ruiz-Contreras J, Castro J, Gomez-Campdera JA, Navarro ML, Merino JM, Martin-Ancel A, Roca J, Garcia-del-Rio M, et al. Immunogenicity and reactogenicity of primary immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type B vaccine coadministered with two doses of a meningococcal C-tetanus toxoid conjugate vaccine. Pediatr Infect Dis J 2006; 25:713-20; PMID:16874171; http://dx.doi.org/10.1097/01.inf.0000227725.61495.c4
- Tichmann I, Grunert D, Habash S, Preidel H, Schult R, Pfletschinger U, Gildberg PK, Meurice F, Sanger R. Persistence of antibodies in children primed with two different hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type B vaccines and evaluation of booster vaccination. Hum Vaccin 2006; 2:249-54; PMID:17106268; http://dx.doi.org/10.4161/hv.2.6.3432
- Steiner M, Ramakrishnan G, Gartner B, Van Der Meeren O, Jacquet JM, Schuster V. Lasting immune memory against hepatitis B in children after primary immunization with 4 doses of DTPa-HBV-IPV/Hib in the first and 2nd year of life. BMC Infect Dis 2010; 10:9; PMID:20078876; http://dx.doi.org/10.1186/1471-2334-10-9
- GlaxoSmithKline Biologicals. Phase III b, open, randomized, multicenter study to evaluate the immunogenicity and safety of GlaxoSmithKline Biologicals' combined diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-conjugated Haemophilus influenzae type b vaccine (Infanrix hexa™) in Indian infants according to a 6–10–14 week schedule, when compared to Infanrix hexa™ given to Indian infants according to a 2–4–6 month schedule. Scientific Result Summary, Study ID 104005. Available at http://www.gsk-clinicalstudyregister.com/files2/19981.pdf (accessed September 20, 2016)
- Kalies H, Grote V, Verstraeten T, Hessel L, Schmitt HJ, von Kries R. The use of combination vaccines has improved timeliness of vaccination in children. Pediatr Infect Dis J 2006; 25:507-12; PMID:16732148; http://dx.doi.org/10.1097/01.inf.0000222413.47344.23
- Mittal S, Mathew J. Expanded program of immunization in India: time to rethink and revamp. J Pediat Sci 2010; 5:e44.
- UNICEF. Immunization Summary. Available at http://www.unicef.org/immunization/files/EN-ImmSumm-2013.pdf, accessed February 2016
- WHO SAGE pertussis working group. Background paper. SAGE April 2014. Available at http://www.who.int/immunization/sage/meetings/2014/april/1_Pertussis_background_FINAL4_web.pdf?ua=, accessed July 2016
- Gambhir M, Clark TA, Cauchemez S, Tartof SY, Swerdlow DL, Ferguson NM. A change in vaccine efficacy and duration of protection explains recent rises in pertussis incidence in the United States. PLoS Comput Biol 2015; 11:e1004138; PMID:25906150; http://dx.doi.org/10.1371/journal.pcbi.1004138
- World Health Organization. Pertussis vaccines: WHO position paper - August 2015. Wkly Epidemiol Rec 2015; 90:433-60; PMID:26320265