ABSTRACT
Study aim: to assess the incidence, risk factors and outcome of invasive pneumococcal disease (IPD) among the Israeli HIV population. A matched case-control study nested in a nationwide, prospective, population-based, cohort of adult IPD was performed. In addition, the HIV-IPD patients were compared to the general adult HIV population in Israel. Study period: from the introduction of PCV into the national immunization program (NIP) in July 2009 to June 2014. Each HIV patient within the IPD cohort was matched to 4 non-HIV controls. Serotyping was performed by a central laboratory using the Quellung reaction. Thirty-five IPD episodes in 33 HIV patients were identified, with a median annual incidence of 128/100,000 HIV+ persons compared to 5.1/100,000 in the age-matched, non-HIV population. Compared to the general HIV population, HIV-IPD patients practiced intravenous drug use more frequently and originated from a country with generalized epidemic (OGE), mainly non-citizens lacking medical insurance. The proportion of men who have sex with men (MSM) was lower than in the general HIV population. Pneumonia was the most common clinical presentation (81%), while meningitis occurred in only one patient. Outcomes were similar to those of the IPD non-HIV population. Nineteen serotypes were identified, of which only 42% were covered by PCV13 vaccine. By 2014, none of the HIV-IPD cases belonged to serotypes covered by PCV13. In conclusion, most HIV IPD cases were from marginalized populations with poor access to health services. A decrease in IPD cases covered by PCV 13 was observed.
Introduction
HIV is a known risk factor for IPD, even in the anti-retroviral treatment (ART) era. Previous studies found that HIV patients have a 60–100-fold greater risk than that of the aged-matched, HIV-negative, general population. However, the incidence of IPD in HIV patients varies greatly among populations. Some of the differences are related to the prevalence of risk factors for IPD in the HIV-specific community, and some to the level of medical services available in the HIV population.Citation1-3
Pneumococcal conjugated vaccines (sequential PCV7/PCV13) were added to the Pediatric NIP in Israel in 2009, since then there is an ongoing nationwide prospective population-based active surveillance for IPD. Data on risk factors, including HIV status are being collected for all patients.Citation4
All citizens of Israel are covered with a universal health insurance that includes all costs of HIV care. Treatment and follow up are carried in dedicated HIV clinics throughout the country. Thus, most of the diagnosed HIV population is routinely followed and treated.Citation5
The aim of this study was to assess the incidence, risk factors and outcomes of IPD in the HIV patient population in Israel.
Results
From July 2009 to June 2014, 2,197 IPD episodes were diagnosed in Israeli adults (18 y and older), of whom 35 were diagnosed in 33 HIV patients (one patient had 3 episodes). HIV diagnosis date was known in 28 patients, Six (21%) of whom were diagnosed with HIV during the IPD episode. HIV patients were a mean age of 43.9 y (SD 13.3, range 18–75) and 24 (73%) were male.
The most common risk factor for HIV acquisition (15/33, 45%) was immigration or relocation from a country with a generalized HIV epidemic (OGE), 5 from Ethiopia, and the remaining were illegal immigrants or refugees from Africa. The other common HIV risk group was IVDU (11/33, 33%). MSM comprised only 10% of this group. Mode of HIV acquisition risk was unknown in 4 patients. The risk factors for HIV among the patients with IPD were compared to the general HIV population in Israel (). More IVDU (p = 0.01) and fewer MSM (p = 0.04) were found in the IPD patient population. Of note, in the HIV-IPD OGE patient population, 5/15 (30%) were Israeli citizens of Ethiopian origin, while 70% were illegal immigrants. This is in contrast to the general HIV population, where 73% of the general OGE-HIV population (1,852 out of 2,553) is Israeli citizens. CD4 count at the time of IPD diagnosis was known in 19 patients. Although CD4 ranged from 6 to 879, the median value was 109; 15/19 (79%) had CD4 below 200. The annual incidence of IPD in the HIV population ranged from 44 to 152 per 100,000 patient years, with a median incidence in these years of 128 per 100,000 patients. The incidence among adults ages 35–50 in the general Israeli population ranged from 3.7 to 6.7 per 100,000 patient years, with a median of 5.1/100,000 patient years.
Table 1. HIV transmission risk group.
The clinical syndromes included bacteremic pneumonia in 27 patients (81%), 5 cases of bacteremia with no source (15%) and only one case of meningitis. Nine (27%) patients were hospitalized in the intensive care unit, 7 (21%) were ventilated, and 4 (12%) died.
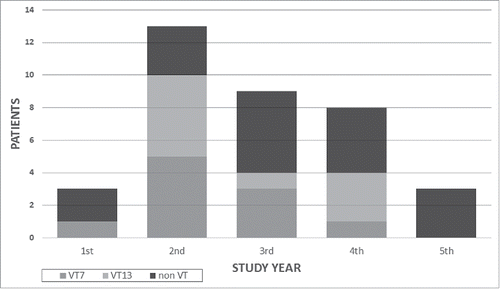
Serotypes were determined in isolates from 31 patients (94%); 19 different serotypes were found in this group; the most common was 19A (in 4 patients). The serotypes for the 4 fatal cases were 15A, 15B, 19A, and 23B. Only 42% of the serotypes identified were covered by PCV13. In the first year, only 3 HIV-IPD patients were detected, 2 of which were caused by non-VT strains. Yet, in the second year, 13 HIV-IPD cases were detected, of which 77% were VT13 strains. From the second to the fifth years (2010–2014), VT13 strains gradually decreased. They were not detected at all among the 3 cases in the last year. Due to the small numbers, the study was not powered to detect statistically significant changes (). Most of the isolates were fully sensitive to penicillin (81%); one was resistant and 5 were intermediate.
We compared the IPD HIV cohort to the IPD non-HIV cohort (). Patients were matched for age and IPD risk factors (other than HIV). There were more men, drug users and smokers among the HIV group. Pneumonia was a more frequent source of pneumococcal infection among HIV patients and meningitis was less common, but this did not reach statistical significance. Several outcomes, including length of stay, ICU stay and mortality were very similar to those of the general population. As for bacterial characteristics, similar antimicrobial susceptibility patterns were observed. In the HIV population, more cases were due to serotypes not covered by PCV13 (non-VT13), but this did not reach statistical significance.
Table 2. Comparison of IPD patients with and without HIV.
Discussion
This is the first study to assess the incidence and characteristics of IPD in the HIV patient population in Israel. Although the observed incidence of IPD was 25 times higher than that of the general population (128/100,000), it was considerably lower than the incidence found in the pre-ART era; 822/100,000 in the USA,Citation1 2410/100,000 in Spain,Citation6 and in the ART era 342/100,000 in Alberta, Canada,Citation2 820/100,000 in Spain,Citation6 and 245/100/000 in the UK.Citation3 A possible explanation for this difference is the high proportion of HIV patients who are treated and followed, resulting in a higher rate of preserved CD4 counts. The low incidence of IPD among MSM and among Israelis of Ethiopian origin in the cohort support this explanation. Both populations receive good medical care. In contrast, most HIV patients with IPD were IVDU and patients from OGE other than Ethiopia, the majority of which were undocumented immigrants with no health insurance. Moreover, CD4 was lower than 200 in 79% of cases with IPD and known CD4. This suggests no treatment among the majority of patients in Israel with IPD as a complication of HIV.
Of the patients with known HIV diagnosis date, 21% were diagnosed during the hospitalization for IPD. Similar numbers were found in other studies (18%).Citation3 The correlation between HIV and pneumococcal infection is well known and should serve as a reminder to physicians to test for HIV in young adults with IPD.
Although previous vaccination data were not available, 87% had pneumococcal infection with serotypes not covered by the PCV7, and almost 60% of the co-infected population had infection with serotypes not covered by PCV13. Due to the small numbers, it is unclear whether the decrease in VT-infections was due to the vaccine. Thus, while PCV vaccination should be recommended to all HIV patients as a tool to decrease IPD, it seems that the role of early HIV diagnosis and enrollment into care as a strategy to reduce IPD cannot be overemphasized.
As mentioned, our HIV population was immunosuppressed, with many patients with CD4<200, even though length of stay, ICU stay and mortality were similar to those of the age-adjusted IPD general population. Similar observations were noted in several studies in the pre-ART era,Citation7,8 as well as in the ART era.Citation9 Moreover, Jordano et al.Citation10 did not find a correlation between mortality and CD4 or virus levels. An exception was a recent study from South Africa that included 2,309 HIV-IPD patients.Citation11 In this study, with clearly increased power compared to our study or the previously mentioned studies, HIV was a risk factor for mortality with an odds ratio of 1.7. However, even in that cohort, the increased mortality in HIV patients was seen only in patients with meningitis, and not among patients with bacteremia.
The current study had several limitations. Due to the small number of HIV-IPD patients, the study was not powered to detect significant changes in clinical and bacterial characteristics within the 5 y after PCV was introduced. Yet, this nationwide surveillance study enabled accurate estimations of incidence rates and comparisons to non-HIV-IPD patients and to the general HIV population. Another limitation was the lack of personal vaccination information.
To conclude, we present a lower than previously described incidence of IPD in an HIV population in Israel; the majority of which have serotypes not included in the PCV13. Moreover, morbidity is seen mainly in marginalized populations with poor access to health services.
Methods
Study design: Matched case control study nested in a nationwide, prospective, population-based, cohort of adult IPD. In addition, the HIV IPD patients were compared to the general HIV population in Israel to determine predictors of IPD.
Study period: Five consecutive years, from July 1, 2009, when PCV 7 was introduced into the NIP, until June 30, 2014.
PCV7 was approved in 2007 and introduced to the pediatric national immunization plan (NIP) on July 2009. Starting in November 2010, PCV7 was gradually replaced by PCV13, with no catch-up plan.
Nationwide surveillance included all 27 medical centers and their laboratories that routinely tested blood and cerebrospinal fluid cultures. This covered all culture-confirmed IPD cases in the Israeli adult population. In 2009, the Israeli adult population 18 y and over was 5,029,600 and by 2014 it had increased to 5,504,900 (http://www.cbs.gov.il/shnaton66/st02_05x.pdf). The diagnosed adult Israeli HIV population was 6,701 by 2014. Data on HIV prevalence and modes of transmission were made available by The Department of Tuberculosis and AIDS in the Israel Ministry of Health (http://www.health.gov.il/PublicationsFiles/HIV1981_2014.pdf).
Case definition: An IPD episode was defined by isolation of Streptococcus pneumoniae from blood or CSF. HIV diagnoses were cross-referenced with the national virology laboratory. A case was defined as a patient with IPD who was also diagnosed as HIV-positive (before or during the IPD episode). A control was a patient with IPD and without HIV. Four IPD controls were matched to each HIV-IPD patient (case). Matching was according to the year of IPD diagnosis, age, and risk factors for IPD other than HIV.
Data Collection: Data regarding the IPD infection were collected from hospital medical files within 2–3 months of hospitalization. They included demographics, medical history, outcome, substance abuse, and smoking history. More detailed information on HIV status (date of diagnosis, mode of transmission, CD4 on diagnosis, CD4 close to the IPD diagnosis, and antiretroviral treatment) was collected from HIV treatment centers caring for the specific patients.
Laboratory testing: Susceptibility testing of the S. pneumoniae isolate was done at the local laboratories of each medical center. Serotyping was performed on all viable isolates by the main laboratory (The Pediatric Infectious Diseases Laboratory at Soroka Medical Center) using the Quellung reaction (Staten Serum Institute, Copenhagen, Denmark).
Statistical analysis: Incidence of HIV-IPD was calculated as the number of cases each year, divided by the adult Israeli HIV population in the same year. The incidence of all adult IPD was calculated as the number of IPD cases divided by the Israeli adult population using data from the Israel Central Bureau of Statistics recorded in the population registry (http://www.cbs.gov.il/). When applicable, incidence in a particular age group was calculated accordingly. To compare outcomes of HIV-infected IPD patients to those without HIV, we matched 4 non-HIV to each HIV case by age, year of IPD episode and comorbidities (other than HIV). Continuous factors between HIV-infected and non-HIV-infected IPD patients were compared using 2-sample t-tests or one-way analysis of variance (ANOVA), as applicable. Categorical variables were compared using Chi-square or Fisher's exact test, as applicable. Statistical analyses were performed using SAS 9.4 software.
Abbreviations
| ART | = | anti-retroviral treatment |
| HIV | = | Human Immunodeficiency virus |
| IPD | = | Invasive pneumococcal disease |
| IVDU | = | intravenous drug use |
| MSM | = | men who have sex with men |
| NIP | = | national immunization program |
| OGE | = | originating from a country with generalized epidemic |
| PCV | = | pneumococcal conjugated vaccine |
| VT | = | vaccine type |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ronit Trefler for serotyping, Etti Kreiff and Efrat Steinberger for assisting with data management and Shirley Khakshoor for assisting with the statistical analysis.
The IAIPD is part of the IsraNIP project. The IAIPD study investigators (2014): Study PI: Gili Regev-Yochay, Sheba Medical Center, Ramat Gan, IsraNip Project PI: Ron Dagan (Ben Gurion University of the Negev, Beer Sheva), Co-investigators: Marc Assous (Shaarei Tzedek Medical Center, Jerusalem), Rita Bardenstein (Kaplan Medical Center, Rehovot), Haim Ben-Zvi (Rabin Medical Center, Petach Tikva), Jihad Bishara (Rabin Medical Center, Petach Tikva), Larisa Brik (Asaf Harofe Medical Center, Zrifin), Bibiana Chazan (Haemek Medical Center, Afula), Alicia Embon (Barzilai Medical Center, Ashkelon), Sarit Freidman (Hillel Yaffe Medical Center, Hadera), Yuval Geffen (Rambam Medical Center, Haifa), Daniel Glikman (Western Galilee Hospital, Nahariyah), Zeev Gordonitzky (Ministry of Health, Jerusalem), Kamillia Houri-Aassi (French Hospital, Nazareth), Valery Istomin (Hillel Yaffe Medical Center, Hadera), Michal Katzir (Meir Medical Center, Kfar Saba), Yoram Kenes (Haemek Medical Center, Afula) Yasmin Maor (Wolfson Medical Center, Holon) Dan Miron (Ziv Medical Center, Safed), Sari Mendelbaum (Laniado Medical Center, Netanya), Ilana Oren (Rambam Medical Center, Haifa), Yossi Paitan (Meir Medical Center, Kfar Saba), Nehama Peled (Soroka University Medical Center, Beer Sheva), Avi Peretz (Poriah Medical Center, Tiberius), Israel Potasman (Bnei Zion Medical Center, Haifa), Galia Rahav (Sheba Medical Center, Ramat Gan), Hagai Rechnitzer (Ziv Medical Center, Safed), Klaris Reisenberg (University Medical Center, Beer Sheva), David Schwartz (Tel Aviv Medical Center, Tel Aviv), Orna Schwartz (Wolfson Medical Center, Holon), Pninit Shaked-Mishan (Carmel Medical Center, Haifa), Yehudith Sheindler (Maayanei Hayeshua Hospital, Bnei Brak), Gill Smollan (Sheba Medical Center, Ramat Gan), Itzhak Srugo (Bnei Zion Medical Center, Haifa), Michal Stein (Wolfson Medical Center, Holon), Jacob Strahilevitz (Hadassah-Hebrew University Medical Center, Jerusalem), Olga Sverdlob (Maccabi Healthcare Services, Rehovot), Violetta Temper (Hadassah-Hebrew University Medical Center, Jerusalem), Gabriel Weber (Carmel Medical Center, Haifa), Miriam Weinberger (Assaf Harofeh Medical Center, Tzrifin), Yonit Viener-Well (Shaare Zedek Medical Center, Jerusalem), Yevgenia Tziba (Barzilai Medical Center, Ashkelon).
References
- Dworkin MS, Ward JW, Hanson DL, Jones JL, Kaplan JE. Pneumococcal disease among human immunodeficiency virus-infected persons: incidence, risk factors, and impact of vaccination. Clin Infect Dis 2001; 32:794-800; PMID:11229848; http://dx.doi.org/10.1086/319218
- Siemieniuk RA, Gregson DB, Gill MJ. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis 2011; 11:314; PMID:22078162; http://dx.doi.org/10.1186/1471-2334-11-314
- Yin Z, Rice BD, Waight P, Miller E, George R, Brown AE, Smith RD, Slack M, Delpech VC. Invasive pneumococcal disease among HIV-positive individuals, 2000–2009. AIDS 2012; 26:87-94; PMID:22008657; http://dx.doi.org/10.1097/QAD.0b013e32834dcf27
- Regev-Yochay G, Paran Y, Bishara J, Oren I, Chowers M, Tziba Y, Istomin V, Weinberger M, Miron D, Temper V, et al. Early impact of PCV7/PCV13 sequential introduction to the national pediatric immunization plan, on adult invasive pneumococcal disease: A nationwide surveillance study. Vaccine 2015; 33:1135-42; PMID:25613717; http://dx.doi.org/10.1016/j.vaccine.2015.01.030
- Mor Z, Weinstein R, Grotto I, Levin Y, Chemtob D. Thirty years of HIV in Israel: current epidemiology and future challenges. BMJ Open 2013; 3(7); PMID:23833144; http://dx.doi.org/10.1136/bmjopen-2013-003078
- Grau I, Pallares R, Tubau F, Schulze MH, Llopis F, Podzamczer D, Linares J, Gudiol F. Epidemiologic changes in bacteremic pneumococcal disease in patients with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Intern Med 2005; 165:1533-40; PMID:16009870; http://dx.doi.org/10.1001/archinte.165.13.1533
- Campo RE, Campo CE, Peter G, Zuleta J, Wahlay NA, Cleary T, Kolber MA, Dickinson GM. Differences in presentation and outcome of invasive pneumococcal disease among patients with and without HIV infection in the pre-HAART era. AIDS Patient Care STDs 2005; 19:141-9; PMID:15798381; http://dx.doi.org/10.1089/apc.2005.19.141
- Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med 2000; 132:182-90; PMID:10651598; http://dx.doi.org/10.7326/0003-4819-132-3-200002010-00003
- Grau I, Ardanuy C, Linares J, Podzamczer D, Schulze MH, Pallares R. Trends in mortality and antibiotic resistance among HIV-infected patients with invasive pneumococcal disease. HIV Med 2009; 10:488-95; PMID:19459987; http://dx.doi.org/10.1111/j.1468-1293.2009.00717.x
- Jordano Q, Falco V, Almirante B, Planes AM, del Valle O, Ribera E, Len O, Pigrau C, Pahissa A. Invasive pneumococcal disease in patients infected with HIV: still a threat in the era of highly active antiretroviral therapy. Clin Infect Dis 2004; 38:1623-8; PMID:15156452; http://dx.doi.org/10.1086/420933
- Cohen C, Naidoo N, Meiring S, de Gouveia L, von Mollendorf C, Walaza S, Naicker P, Madhi SA, Feldman C, Klugman KP, et al. Streptococcus pneumoniae serotypes and mortality in adults and adolescents in South Africa: Analysis of national surveillance data, 2003 – 2008. PloS One 2015; 10:e0140185; PMID:26460800; http://dx.doi.org/10.1371/journal.pone.0140185