ABSTRACT
The effectiveness of passive immunisation post-exposure to measles appears subject to a dose-response effect. New Zealand and the United Kingdom have increased the recommended dose of polyclonal human immunoglobulin for post-exposure prophylaxis within the last decade in response to concerns about decreasing levels of measles antibodies in these products. This study used the plaque-reduction neutralization test (PRNT) to measure the titer of measles-specific antibodies in Australian immunoglobulin products for post-exposure prophylaxis and compared the utility of an enzyme-linked immunosorbent assay (ELISA) to the PRNT in available Australian and international samples: Australian intramuscular (n = 10), Australian intravenous (n = 28), New Zealand intramuscular (n = 2), Hizentra (subcutaneous)(USA) (n = 3), and Privigen (intravenous)(USA) (n = 2). Measles titres in Australian IM and IV immunoglobulins ranged from 51 to 76 IU/mL and 6 to 24 IU/mL respectively, as measured by PRNT calibrated to the WHO 3rd international standard. ELISA titres were variable but higher than PRNT titres in all tested samples. Measles antibody titres in Australian immunoglobulin products meet consensus-prescribed international thresholds. Development of a convenient, standardized, readily accessible assay for determination of measles titres in immunoglobulin products would be useful for future studies and facilitate international comparisons.
Measles has been targeted for elimination by the World Health Organisation (WHO).Citation1 However, even in countries with high vaccination coverage where elimination has been declared, outbreaks still occur, usually as a result of imported cases.Citation2-5 In recent years, the global burden of measles has increased rather than decreased and elimination targets are under threat.Citation6
In high-income countries, post-exposure prophylaxis for measles typically consists of either active immunisation within 3 d of exposure, or passive immunisation within 6 d of exposure.Citation7-10 Hence, passive immunisation plays an important role in measles control.Citation11 A recent systematic review confirmed that passive immunisation is effective up to 7 d after exposure to measles.Citation12 The review noted that included studies were mostly conducted in the pre-vaccine era, when the concentration of measles antibodies in the blood products tested were the result of immunity following infection rather than immunisation. In fact, the final meta-analysis included only one study from the post-vaccine era. It has been shown that immunisation results in lower antibody titres when compared to measles infection.Citation13 Further, the review supported a likely dose response effect with respect to post-exposure passive immunisation.Citation12 Thus, the concentration of measles antibodies in current immunoglobulin products may impact on their effectiveness for preventing measles.
Levels of measles-specific antibodies in the intramuscular (IM) immunoglobulin products that are used for passive immunisation post-exposure to measles in New Zealand and the United Kingdom have been published.Citation10,14 Within the last decade, these countries have increased the recommended volume of immunoglobulin to be administered for post-exposure prophylaxis based on those reported levels.Citation10,14,15 New Zealand increased the recommended dose from 0.2mL/kg to 0.6mL/kg.14 Because the recommended volume, dependent on an individual's weight, may then be considerable, New Zealand have also recommended that intravenous (IV) rather than IM immunoglobulin be considered in certain cases.Citation14,15
In the United States of America (US) immunoglobulins must meet a specified measles antibody level.Citation16 Due to the decreasing titer in donor plasma, the Food and Drug Administration, with advice from the Blood Products Advisory Committee, lowered the required concentration of measles antibodies in US IV and subcutaneous immunoglobulin products in 2007, though not in IM products.Citation17 However, an increase in the dose of IM immunoglobulin was recommended for immunocompetent people and, because of the large volume then required, IV immunoglobulin was recommended for immunocompromised people and pregnant women for post-exposure prophylaxis.Citation7
Australia does not require the routine measurement of the concentration of measles antibodies in immunoglobulin products. The volume currently recommended for immunocompetent individuals for post-exposure prophylaxis for measles in Australia is 0.2 mL/kg9; lower than that used in the United Kingdom (0.6 mL/kg for infants under 9 months)Citation18, US (0.5 mL/kg)7 or New Zealand (0.6 mL/kg).Citation8
This study aimed to establish the current titer of measles-specific antibodies in the IM and IV immunoglobulin products produced in Australia and available for post-exposure prophylaxis against measles. Antibody titer was established by the pharmacopoeia prescribed plaque reduction neutralization test (PRNT).Citation19 Although PRNT is a clinically relevant assay, measuring biologically active neutralising antibodies, it is more labor-intensive and less readily available than ELISA. Thus, a further aim was to establish the utility of an ELISA for quantitating measles antibody titres in immunoglobulin products by comparing the results of the PRNT with those obtained by ELISA using immunoglobulin products from Australia, New Zealand and the US.
Results
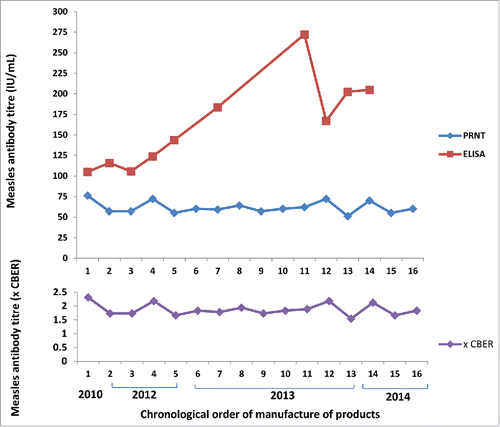
Measles titres in the Australian IM immunoglobulins ranged from 51 to 76 IU/mL as measured by PRNT calibrated to the WHO 3rd international standard (). When standardised to protein concentration, values were 0.32 to 0.48 IU/mg of IgG. The GMT ± GMSD was 61 ± 1.12 IU/mL (0.38 ± 1.12 IU/mg) for all 16 samples, and 62 ± 1.15 IU/mL (0.39 ± 1.15 IU/mg) for the 10 samples also tested by ELISA.
Figure 1. Measles antibody concentrations in Australian intramuscular immunoglobulin products by ELISA and PRNT and expressed as times CBER units.
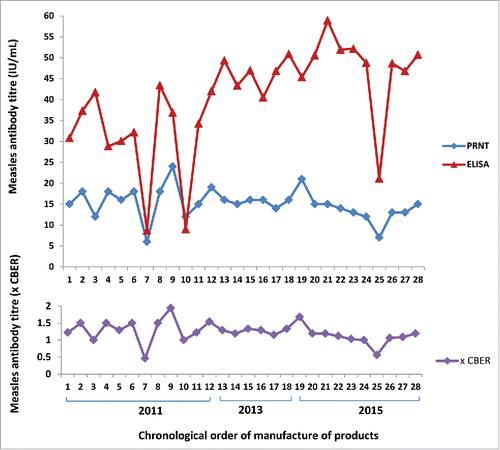
Measles titres in the Australian IV immunoglobulins ranged from 6 to 24 IU/mL as measured by PRNT calibrated to the WHO 3rd international standard (). When standardised to protein concentration, values were 0.10 to 0.40 IU/mg of IgG. The GMT± GMSD was 14 ± 1.34 IU/mL (0.24 ±1.34 IU/mg).
Figure 2. Measles antibody concentrations in Australian intravenous immunoglobulin products by ELISA, PRNT and expressed as times CBER units.
There was a statistically significant difference between the geometric mean measles titres of Australian IM immunoglobulin and Australian IV immunoglobulin obtained by PRNT (p<0.001).
When titres were expressed relative to Lot 176 CBER standard, Australian IM and IV immunoglobulin values ranged from 1.54 to 2.31, and 0.46 to 1.94 times the standard respectively (). One CBER unit equated to 0.2 IU/mg of IgG.
Pearson's correlation co-efficients (r) for Australian IM and IV products respectively were −0.156 (p = 0.666) and 0.317 (p = 0.1). There was a statistically significant difference between the geometric mean measles titres obtained by PRNT compared to those obtained for the same product by ELISA for Australian IM and IV products ().
Table 1. ELISA compared to PRNT measles-specific antibody results for Australian immunoglobulin products.
Measles titres in New Zealand and US products were also lower when measured by PRNT than ELISA ().
Table 2. ELISA compared to PRNT measles-specific antibody results for New Zealand and United States of America immunoglobulin products.
Discussion
Measles vaccination results in lower titres of measles antibodies compared to natural disease.Citation13 As the plasma pools for immunoglobulin products become increasingly sourced from donor populations with predominantly vaccine-induced immunity to measles, there is a concern that the measles titer in these immunoglobulin products may be declining. This study aimed to establish the current titer of measles-specific antibodies in IM and IV immunoglobulin products produced in Australia. The current recognized test for measles titer quantitation, the PRNT, is complex and not readily available. Therefore, the opportunity was taken to also test samples with an ELISA to investigate the utility of this alternative assay across a range of available Australian and international products.
The range of titres of measles-specific antibodies in Australian IM immunoglobulin was 51 – 76 IU/mL when measured by PRNT calibrated to the WHO 3rd international standard, and 1.5–2.3 times the CBER standard (lot 176). The US minimum requirement for measles antibodies in immunoglobulins is 0.6 times the CBER standard for IM products and 0.48 times the CBER standard for IV products.Citation17 Thus the results for Australian IM immunoglobulin would exceed US specifications.
The Australian IV immunoglobulin values ranged from 6 – 24 IU/mL when measured by PRNT calibrated to the WHO 3rd international standard and 0.5 – 1.9 times the CBER standard (lot 176). The PRNT results for Australian IV immunoglobulin as compared to the CBER standard exceeded US specifications for all but one sample that was manufactured in 2011.
The measles titer in Australian IM immunoglobulin was statistically significantly higher than that in Australian IV immunoglobulin. The difference in Australian products is likely due to the manufacturing process as both products are derived from the same plasma pool.
The titer of measles-specific antibodies in Australian IM and IV immunoglobulin was higher when measured by ELISA, at 105–272 IU/mL and 9–59 IU/mL respectively, than by PRNT. The differences noted between results for the same product according to the method of testing did not seem unique to Australian immunoglobulin products. Measles antibodies were between 1.48 and 3.56 times higher when measured using the Enzygnost anti measles virus/IgG ELISA (Seimens, Germany) compared to PRNT for New Zealand, and US samples, however, given the small sample sizes, these results were not statistically compared.
Others have previously reported higher ELISA results as compared to PRNT.Citation20,21 Siennicka et al found a ratio of 3.18 : 1 using the same commercial ELISA kit compared to PRNT when testing samples of the WHO 3rd international standard anti-measles preparation.Citation20 Terletskaia-Ladwig et al found ELISA results were 4.76 and 2.28 times higher using the same commercial ELISA kit compared to PRNT when testing samples of pooled human sera and an immunoglobulin product respectively.Citation21
ELISA and PRNT results for Australian IM and IV products did not significantly correlate. Indeed, ELISA results in this study exhibited considerable variability that did not seem to be mirrored in the PRNT results. This is likely due in part to the inherent differences between the tests, in that PRNT measures biologically active neutralising antibodies, where ELISA measures total antibodies and thus the ratio between the 2 measures is not consistent across batches of IG. While it requires further investigation, it is also possible this reflects differing sensitivity and signal response ratios between the 2 assays.
A limitation of this study is the unknown effect that long-term storage of immunoglobulin samples may have had on quantitation of antibody levels. However, PRNT results were consistent across the chronological order of manufacture of the product batches and the ratio of ELISA:PRNT did not appear to increase with the age of the samples. It should be noted that the oldest samples available were manufactured in 2010. A lack of historical product available to test does limit conclusions about time trends in antibody titres to the period of available samples. Similarly, the lack of availability of international product samples restricts international comparisons.
The results of this study do allow estimation of the dose of measles-specific antibodies offered for post exposure prophylaxis under current national recommendations. Australian guidelines recommend 0.2mL/kg of intramuscular immunoglobulins to immunocompetent people and 0.5mL/kg to immunocompromised people to a maximum of 15 mL.9 Considering the lowest PRNT result for Australian IM immunoglobulin (51 IU/mL), this is equivalent to at least 10.2IU/kg measles antibodies for immunocompentent individuals and 25.5IU/kg for immunocompromised individuals.
New Zealand guidelines recommend 0.6mL/kg intramuscular immunoglobulins (to a maximum of 5 mL for infants and a maximum of 15 mL for pregnant women and immunocompromised people).Citation8 Best et al reported the measles titer range for New Zealand intramuscular immunoglobulin as 14–16 IU/mL.14 The 2 NZ batches tested by PRNT in this study had titers of 39 and 47 IU/mL. The reason for the discrepancy between the measured values and those reported by Best et al is not known. Possible reasons include a rise in measles-specific antibody titer in New Zealand plasma pools following large outbreaks of measles in New ZealandCitation22,23, the small number of New Zealand samples tested in this study, and differences in test methodology. Best et al did not indicate the methodology employed to obtain the reported results. At a dose of 0.6mL/kg, the lower concentration quoted by Best et al equates to 8.4IU/kg measles antibodiesCitation14, whereas, the lower result as measured in this study equates to 23.4 IU/kg.
The US recommends 0.5mL/kg intramuscular immunoglobulins to a maximum of 15mL.Citation7 IM products in the US must have a minimum titer of 0.6 CBER.Citation17 Based on data from this study, 0.6 CBER equates to 0.12IU/mg. Considering a 15%–18% solution as is available for measles IM post-exposure prophylaxis in the USCitation24,25, this equates to 9–10.8 IU/kg measles antibodies.
There is a lack of evidence for what constitutes a protective dose of measles antibody when administered as post exposure prophylaxis. A single study undertaken by Endo et alCitation25 in 1999 suggested 10.9 IU/kg as an optimal dose. However, it is noteworthy that Endo et al quantified the measles antibody concentration in the immunoglobulin used in their study by haemagglutination inhibition rather than the PRNT. Further, it does not appear that participants were allocated randomly to receive the various doses of measles antibodies administered and it is unclear whether any of the participants were immunocompromised. Further studies addressing this topic are required. Though randomized clinical studies of post exposure prophylaxis are ethically and logistically difficult, pharmacokinetic simulation studies using published data may assist to quantify the confidence in the results of Endo et al.
In the absence of other studies, United Kingdom guidelines cite 11 IU/kg as an optimal dose of measles antibodies for post exposure prophylaxis.Citation10 The results of the current study suggest Australian guidelines typically meet this suggested target, as do US and New Zealand guidelines.
Unlike other countriesCitation10,17, measles antibody titers in Australian immunoglobulin products do not appear to have decreased over the timespan of the samples available to this study (2010–2015). However, given the significant decreases in these other countries that have led to revision of policy around immunoglobulins for post-exposure prophylaxis, it would be pertinent to regularly measure measles antibodies in Australian immunoglobulin products in the future, at least once per generation.
This study and associated literature clearly shows that ELISA cannot be immediately substituted for the PRNT assay for determination of measles titer in immunoglobulin products. The development of a convenient, standardized, readily accessible assay for determination of measles titer in immunoglobulin products would be valuable for future studies and facilitate international comparisons.
Methods
Samples from 16 batches of IM and 28 batches of IV Australian immunoglobulin products, manufactured between 2010 and 2015 were obtained from CSL Behring (Australia) Pty Ltd. The IM product was manufactured by the Cohn-Oncley ethanol precipitation procedure, while the IV product was manufactured using a chromatographic-based process. The formulated products differ with respect to protein concentration, pH and excipient [IM (16% w/v; pH 6.6; glycine); IV (6% w/v; pH 4.25; Maltose)].
PRNT was performed as described by Cohen et al.Citation26 The proportion of infectious foci within a well of a Vero cell culture was calculated to generate a quantitative result. Results were expressed calibrated to the WHO 3rd international reference standard and Lot 176 CBER standard.
The geometric mean titer (GMT) and geometric standard deviation (GSD) for each product was calculated. The measles titres of the Australian IM and IV products obtained by PRNT were compared using the Mann-Whitney U test. A nonparametric test was chosen because of the small sample sizes.
Six of the 16 samples of Australian IM immunoglobulin were not tested by ELISA because of insufficient sample volume. The remainder were tested using the Enzygnost anti measles virus/IgG ELISA kit (Siemens, Germany) according to the manufacturer's instructions. The solid phase antigen in the Enzygnost kit is permanent simian kidney cells infected with measles virus. Testing was performed by the Victorian Infectious Disease Research Laboratory (VIDRL). Initial results demonstrated the need for dilution to minimise the matrix effects of the samples. Dilution was performed with the diluent provided with the kit. In accordance with the results of the dilution study, testing of samples was performed in duplicate at 1:20 and 1:40 for IM immunoglobulin products, and at 1:16 and 1:32 for IV immunoglobulin products. Results were expressed in international units (IU) using the WHO 3rd international reference standard. The titer of the sample was the average of the 2 results. Inter-assay precision was 10.5%.
ELISA values were plotted against PRNT values to ensure the assumptions of Pearson's correlation co-efficient were met before this test was carried out. The geometric mean titer (GMT) and geometric standard deviation (GSD) for each product was calculated. Geometric mean measles titres obtained by ELISA were compared to those obtained by PRNT using the Wilcoxon signed-rank test because of the small sample sizes.
Available samples of the following immunoglobulin products were also tested with both PRNT and the Enzygnost anti measles virus/IgG ELISA kit (Siemens, Germany): New Zealand IM immunoglobulin (16% w/v) (CSL Behring (Australia) Pty Ltd), Hizentra (20% w/v) (CSL Behring AG), and Privigen (10% w/v) (CSL Behring AG). While small sample numbers prevented statistical hypothesis testing, individual sample measles titer results and GMT ± GMSD are presented for qualitative comparison.
Ethical approval was not required for this study.
Disclosure of potential conflicts of interest
Megan Young is a PhD student examining the effectiveness and efficiency of normal human immunoglobulin for the public health management of communicable diseases. She is also a public health physician practising in Queensland.
Joseph Bertolini, Pushpa Kotharu and Darryl Maher are employees of CSL Behring (Australia) Pty Ltd and provided in-kind support for this study. Joseph Bertolini and Daryl Maher own shares in CSL Limited. All the immunoglobulin products investigated were manufactured by the CSL Behring group of companies.
Allan Cripps is the supervisor of Megan Young's PhD.
Funding
This study was not grant funded. In kind support was provided by CSL Behring (Australia) Pty Ltd and Griffith University.
References
- World Health Organisation. Global measles and rubella strategic plan 2012–2020 2012. http://www.unicef.org/immunization/files/Measles_Rubella_StrategicPlan_2012_2020.pdf
- De Serres G, Gay N, Farrington P. Epidemiology of transmissible diseases after elimination. Am J Epidemiol 2000; 151(11):1039-48; PMID:10873127; http://dx.doi.org/10.1093/oxfordjournals.aje.a010145
- Giddings G, Sibbald B. Imported measles outbreaks prompt call for parents to vaccinate their children. CMAJ 2014; 186(7):E205-6; http://dx.doi.org/10.1503/cmaj.109-4759
- Pegorie M, Shankar K, Welfare W, Wilson R, Khiroya C, Munslow G, Fiefield D, Bothra V, McCann R. Measles outbreak in Greater Manchester, England, October 2012 to September 2013: epidemiology and control. Euro Surveill 2014; 19(49); PMID:25523970
- Zipprich J, Winter K, Hacker J, Xia D, Watt J, Harriman K. Measles outbreak - California, December 2014-February 2015. MMWR - Morb Mortal Wkly Rep 2015; 64(6):153-4; PMID:25695321
- World Health Organisation. Progress in global measles control, 2000–2010. Wkly Epidemiol Rec 2012; 87(5):45-52; PMID:22308581
- Centers for Disease Control and Prevention. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mort Wkly Rep 2013; 62(RR04):1-34; PMID:23302815
- Ministry of Health. Immunisation Handbook 2014. Wellington: Ministry of Health; 2014. http://immunisation.book.health.govt.nz Accessed 21 June 2016
- CDNA. Measles: National guidelines for public health units. Canberra: Australian Government Department of Health; 2015
- Ramsay M, Manikkavasagan G, Brown K, Craig L. Post exposure prophylaxis for measles: revised guidance May 2009. UK: Health Protection Agency; 2009
- Young M, Cripps A. Passive immunization for the public health control of communicable diseases: current status in four high-income countries and where to next. Hum Vaccin Immunother 2013; 9(9):1885-93; PMID:23783220; http://dx.doi.org/10.4161/hv.25311
- Young M, Nimmo G, Cripps A, Jones M. Post-exposure passive immunisation for preventing measles. Cochrane Database Syst Rev 2014; (4):CD010056
- Viana P, Ono E, Miyamoto M, Salomao R, Costa-Carvalho B, Weckx L, et al. Humoral and cellular immune responses to measles and tetanus: the importance of elapsed time since last exposure and the nature of the antigen. J Clin Immunol 2010; 30:574-82; PMID:20405177; http://dx.doi.org/10.1007/s10875-010-9420-7
- Best, Voss, Roberts, Freeman. Measles - Infection Control Definitions & Guidelines Auckland, New Zealand: Auckland District Health Board; 2011 [updated October 2011]. http://www.adhb.govt.nz/starshipclinicalguidelines/_Documents/Measles.pdf
- Sinden J. Post exposure prophylaxis for measles New Zealand: NZBlood; 2012. http://www.nzblood.co.nz/assets/Transfusion-Medicine/PDFs/POST-EXPOSURE-PROPHYLAXIS-FOR-MEASLES-111G001.pdf
- CFR - Code of Federal Regulations Title 21, Food and Drugs, Section 640.104. Sect Volume 7 2011
- US Food and Drug Administration, Blood Products Advisory Committee. Meeting minutes August 16, 2007. Measles antibody levels in US immune globulin products. http://www.fda.gov/ohrms/dockets/ac/07/minutes/2007-4317M.htm Accessed September 28, 2015
- Immunisation Department. Measles. 2009. In: Immunoglobulin Handbook [Internet]. UK: Health Protection Agency. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1242198450982 (accessed October 9, 2012)
- European Pharmacopoeia. 8th ed. Strasbourg, France: European Department for the Quality of Medicines, Council of Europe; 2014
- Siennicka J, Czescik A, Trzcinska A. The significance for epidemiological studies anti-measles antibody detection examined by enzyme immunoassay (EIA) and plaque reduction neutralisation test (PRNT). Przegl Epidemiol 2014; 68:417-20; PMID:25394302
- Terletskaia-Ladwig E, Enders G, Meier S, Dietz K, Enders M. Development and evaluation of an automatable focus reduction neutralisation test for the detection of measles virus antibodies using imaging analysis. J Virol Methods 2011; 178:124-8; PMID:21939689; http://dx.doi.org/10.1016/j.jviromet.2011.08.026
- Reynolds G, Dias C, Thornley S, King R, Morrison A, Matson A, Hoskins R. Analysis of the Auckland 2014 measles outbreak indicates that adolescents and young adults could benefit from catch-up vaccination. N Z Med J 2015; 128(1422):53-62
- Minister for Health Tony Ryall. Measles outbreak prompts vaccination push [Media Statement]: New Zealand Governement; August 6 2009. https://www.beehive.govt.nz/release/measles-outbreak-prompts-vaccination-push (accessed June 1, 2016)
- Grifols Therapeutics Inc. Immune Globulin (Human): GamaSTAN S/D 2013. http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM371376.pdf
- Endo A, Izumi H, Miyashita M, Taniguchi K, Okubo O, Harada K. Current efficacy of postexposure prophylaxis against measles with immunoglobulin. J Pediatr 2001; 138:926-8; PMID:11391343; http://dx.doi.org/10.1067/mpd.2001.113710
- Cohen B, Audet S, Andrews N, Beeler J. Plaque neutralization test for measles antibodies: description of a standardised laboratory method for use in immunogenicity studies of aerosol vaccination. Vaccine 2007; 21(1):59-66; http://dx.doi.org/10.1016/j.vaccine.2007.10.046
