ABSTRACT
The WHO recommends integration of universal mass vaccination (UMV) against hepatitis A virus (HAV) in national immunization schedules for children aged ≥1 year, if justified on the basis of acute HAV incidence, declining endemicity from high to intermediate and cost-effectiveness. This recommendation has been implemented in several countries. Our aim was to assess the impact of UMV using monovalent inactivated hepatitis A vaccines on incidence and persistence of anti-HAV (IgG) antibodies in pediatric populations. We conducted a systematic review of literature published between 2000 and 2015 in PubMed, Cochrane Library, LILACS, IBECS identifying a total of 27 studies (Argentina, Belgium, China, Greece, Israel, Panama, the United States and Uruguay). All except one study showed a marked decline in the incidence of hepatitis A post introduction of UMV. The incidence in non-vaccinated age groups decreased as well, suggesting herd immunity but also rising susceptibility. Long-term anti-HAV antibody persistence was documented up to 17 y after a 2-dose primary vaccination. In conclusion, introduction of UMV in countries with intermediate endemicity for HAV infection led to a considerable decrease in the incidence of hepatitis A in vaccinated and in non-vaccinated age groups alike.
Introduction
Most data on the incidence of acute HAV infection and prevalence of immunity cited in the literature are relatively old. According to World Health Organization (WHO) estimates, there were 126 million cases of acute hepatitis A in 2005.Citation1,2 Acute hepatitis A-related morbidity and mortality increase with age. In children aged <6 years, ∼70% of infections are asymptomatic; if illness does occur, it is typically anicteric. In contrast, in older children, adolescents and adults, infection often leads to clinically overt acute hepatitis.Citation3,4 Acute hepatitis A in adults may lead to prolonged incapacitation and rarely also to acute liver failure in previously healthy individuals and in patients with chronic liver disease.Citation5 There is no specific treatment for acute hepatitis A except for supportive care and liver transplantation in the rare cases with liver failure.Citation6
The virus is transmitted through the fecal-oral route, either through person-to-person contact or through contaminated food or water.Citation6 The highest rates of infection are found in areas with poor sanitary conditions and hygienic practices and lack of access to clean water.Citation7,8 Other risk factors for acquiring HAV include intravenous drug abuse and men having sex with men (MSM).Citation9 Improvements in sanitation and access to clean water reduce viral circulation and infection and therefore the risk of waterborne HAV transmission and the overall rates of transmission. This reduction can be observed in the absence of vaccination as well as when vaccination programs are in place.
The first commercially produced hepatitis A vaccine was launched in 1992.Citation10 Both inactivated and live attenuated vaccines against hepatitis A are currently available.Citation11 A live attenuated vaccine is mainly used in China; most other countries use inactivated vaccines.Citation12 Several monovalent inactivated hepatitis A vaccines are available, which are licensed for children aged one year or older (Table S1).Citation11-14 The WHO considers that HAV vaccines of different brand names are interchangeable.Citation11 The antigen content differs between vaccines,Citation14,15 however, all are considered safe and immunogenic.Citation13,16-20 Long-term persistence of antibodies has been shown with 2-dose vaccination schedules in adults.Citation21,22
Areas with high viral transmission rates have a lower rate of severe morbidity and mortality than areas with lower viral transmission rates, as there are few susceptible adults in areas with high transmission rates.Citation2,23 However, epidemiologic shifts from high to intermediate levels of HAV circulation, resulting from improvements in sanitation and hygiene, are paradoxically associated with an increase in susceptibility to infection due to decreasing immunity in the population as well as to more symptomatic disease due to older age at first infection.Citation7,10 The impact of vaccination can therefore be confirmed by a decline of reported symptomatic cases, of fulminant hepatitis cases and of liver transplants.Citation24 In these settings, the WHO recommends the integration of HAV vaccination into the national immunisation schedule for children aged one year and above, if indicated on the basis of incidence of acute hepatitis and consideration of cost-effectiveness.Citation1 Most countries that have introduced hepatitis A vaccination in their immunisation programs use the available monovalent vaccines. Combined vaccines that include hepatitis A and B or hepatitis A and typhoid have also been developed. However, with the exception of Quebec in CanadaCitation25 and Catalonia in SpainCitation26 where the combined hepatitis A and B vaccine is used in the, pediatric immunisation programmes, these are mainly intended for use in adult travelers or patients with specific risks like chronic liver diseases.Citation27 Furthermore, hepatitis B vaccination has been introduced as a birth dose, monovalent or combined with other antigens, since the late 1990s or early 2000s in most countries. This review is therefore focused on the use of monovalent hepatitis A vaccine in the universal mass vaccination (UMV) setting.
Single-dose inactivated hepatitis A vaccines have been introduced in the national immunisation program in Argentina and additional countries in Latin America are considering adopting a similar protocol. This option seems to be comparable in terms of short and intermediate-term effectiveness, and is less expensive and easier to implement than the classical 2-dose schedule.Citation1,24 However, until further long-term experience has been obtained with a single-dose schedule, in individuals at substantial risk of contracting hepatitis A, and in immunocompromised individuals, a 2-dose schedule may be preferable.Citation1 Following an increase in the number of HAV outbreaks in the 1990s, Israel was the first country to introduce nationwide UMV for 18 months old toddlers using 2 doses of Havrix™.Citation28 Additional countries that introduced UMV programs for hepatitis A include among others Argentina,Citation24 Bahrain,Citation29 Brazil,Citation30 China,Citation31 Greece,Citation32,33 Panama,Citation34 the USCitation35 and UruguayCitation36; as well as regions of Belarus (Minsk City),Citation37 Canada (Quebec),Citation25 Italy (Puglia)Citation38 and Spain (Catalonia).Citation39
The objectives of this systematic review were to: (1) summarize data on the impact of monovalent inactivated hepatitis A vaccines in the context of UMV on the incidence of acute hepatitis A; (2) assess the impact of UMV on other parameters than incidence (e.g. indirect effects such as herd immunity); (3) summarize data on the long-term persistence of anti-HAV (IgG) antibodies in pediatric populations.
Methods
Search strategy
The PubMed, Cochrane, LILACS and IBECS databases were searched for literature in English, Spanish and Portuguese published between January 1st 2000 and July 25th 2016 (date of search). LILACS and IBECS are bibliographic databases with health science literature from Latin America, the Caribbean (LILACS) and Spain (IBECS). A search string combining terms on hepatitis A, vaccines and antibodies was built and adapted for use in each database. The search string used in PubMed was: “Hepatitis A Antibodies”[Mesh] OR “Hepatitis A Vaccines”[Mesh] OR “Hepatitis A/prevention and control”[Mesh] OR ((“Hepatitis A”[Mesh] OR “Hepatitis A virus”[Mesh] OR hepatitis A[tiab]) AND (“Vaccination”[Mesh] OR “Antibodies”[Mesh] OR vaccin*[tiab] OR immuniz*[tiab] OR immunis*[tiab] OR immune[tiab] OR immunity[tiab] OR immunology[tiab] OR antibod*[tiab])) OR Anti-HAV[tiab].
Inclusion and exclusion criteria
In this systematic review only peer-reviewed primary research articles were included; review articles were excluded, but the reference lists of systematic reviews were screened to identify additional relevant primary articles. Review of the gray literature was not included. For review objectives 1 and 2, only observational studies conducted in a setting with UMV with monovalent, inactivated hepatitis A vaccines were included (Table S1). Studies from settings where hepatitis A vaccination was only implemented at the regional level (for example in Puglia, ItalyCitation38 or Minsk City, BelarusCitation37), or from settings in which live attenuated hepatitis A vaccines or only combined hepatitis A vaccines were used in the UMV programs were excluded. Furthermore, studies in at risk populations, outbreak studies, modeling studies and economic evaluations were excluded; as were studies that did not present incidence or prevalence baseline data (i.e. data from the era prior to the introduction of UMV). For review objective 3, studies were only included if they were conducted with monovalent, inactivated hepatitis A vaccines in children (at time of primary vaccination) and provided follow-up data for a minimum of 5 y.
Selection process
Articles were selected in 3 steps. Firstly, titles and abstracts identified through the search strategy were screened to identify potentially relevant articles. All titles and abstracts were screened in duplicate by 2 independent researchers. Any disagreements were resolved by the 2 reviewers by discussing the title and abstract; in case any doubts remained, the full-text was screened to ascertain if the article answered one of the research questions. Secondly, the full-text of the selected articles was screened, keeping in mind the inclusion and exclusion criteria described above, to determine whether it answered one of the review questions. If any aspects of the methodology were unclear, a comment was placed in the results table. Thirdly, for articles that presented duplicate data, the article that presented the most complete data (e.g., longer follow-up) was included.
Results
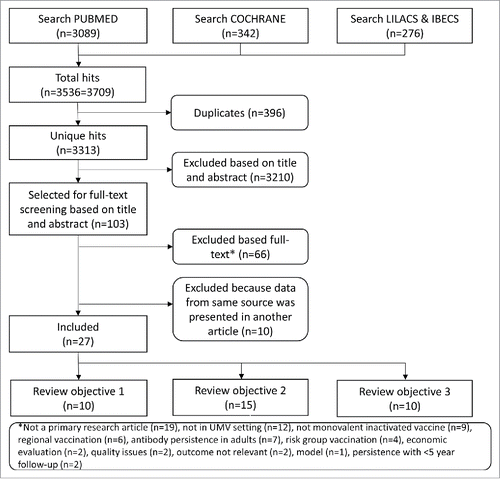
The search resulted in 3313 unique hits, of which 27 were included in this systematic review (). In total, 10 articles were included for review objective 1, 15 for review objective 2 and 10 for review objective 3. Some articles presented data for more than one review objective.
Objective 1: Impact of UMV on HA incidence
Reduction in incidence
Ten studies provided data on incidence of acute hepatitis A before and after the introduction of hepatitis A UMV programs; all but one study (in Greece) found a marked decrease in acute hepatitis A incidence after UMV was implemented (). Declines were independent of the brand of the hepatitis A vaccine used in the programs; the number of doses that was given; the target age at first vaccination, which ranged from 12 to 24 months; or the attained vaccination coverage (range 25%–96.8%). After the introduction of UMV, the percent reduction in the incidence of acute hepatitis A was 88% in Argentina, >95% in Israel, 93% in Panama and 96% in Uruguay going form incidence rates ranging 6.0 to 142.4 per 100,000 population before vaccine introduction to a range of 0.4 to 7.9 per 100,000 population.
Table 1. Effect of universal mass vaccination programs for hepatitis A on incidence of acute hepatitis A.
In Greece, a UMV program was initiated in 2008 however due the low endemcity level (<3.0 per 100,000 population) registered since the late 1980s, the program has not had significant impact on the notification rate of acute hepatitis A cases.Citation40
Objective 2: Impact of UMV on other measures and indirect effects
Impact on other outcomes
In Argentina between 2000–2003 approximately 17 cases of fulminant hepatitis A were reported while between 2008–2011, 2 y after the introduction of UMV, no more cases were reported ().Citation24 A study that looked at hepatitis A outbreaks in day care centers in the Southern District of Israel showed that no more outbreak-related acute hepatitis A cases were reported.Citation41 Hepatitis A vaccination was implemented is some US States as of 1999, the rate of hepatitis A-related ambulatory healthcare visits among enrollees going from 20.9 in 1996–1997 to 8.7 in 2004.Citation42 The age-adjusted hepatitis A-mortality rate decreased significantly from 0.51 in 1999–1995 to 0.28 in 2000–2004.Citation43 The 2011 hepatitis A incidence rate was the lowest ever recorded for the United States, data form the National Inpatient Survey have shown a reduction in the HA hospitalization rates from 0.64 in 2004–2005 vs. 0.29 in 2010–2011,Citation44 however the relative rates of hospitalized hepatitis A cases among overall acute hepatitis A cases increased. In Greece, the number of HA-related hospital admission per 1000 hospital admissions among children dropped from 77.3 (95% CI 58.7–95.9) in 1999 (year of introduction of vaccine in private market) to 18.5 (95% CI 8.2–28.9) in 2013.Citation45 Furthermore the outbreaks in 2013 among Roma populations did not spread to the general population.Citation40
Indirect effects
A decline in acute hepatitis A incidence was seen in all age groups after the introduction of UMV in Israel in 1999Citation28,46,47 as well as in the US, where vaccination was introduced in 1999 in some States,Citation35,48,49 Such a drop was also recorded in Argentina in 2005Citation24 and in Panama in 2007Citation50 ().
Table 2. Effect of universal mass vaccination programs for hepatitis A on other outcome measures.
Table 3. Decline (%) in hepatitis A incidence by age group (years) before and after the introduction of universal vaccination.a
Declines in incidence were generally highest in the age groups that contained the most vaccinated children.Citation24,28,35,51 Incidence rates also dropped among children too young to be vaccinated in the programs.Citation28,35,50 In most studies, the smallest declines in acute hepatitis A incidence were noted in the oldest investigated age groups.Citation24,28,35,50 Similarly, a drop in hepatitis A-associated hospitalization rates was observed in non-vaccinated age groups in the US.Citation44 In settings where many adults are likely to have natural immunity from prior infection, the drop in incidence in age groups not targeted by the UMV programs suggest a remarkable degree of herd immunity.
Objective 3: Long-term persistence of anti-HAV antibodies
Of the 10 included studies that reported on persistence of anti-HAV (IgG) antibodies more than 5 y after vaccination of a pediatric population, 2 studies were performed in Argentina, one in Belgium, 2 in China, one in Israel and 4 in the US (). In six studies, authors reported that children who received booster vaccinations after the primary immunisation schedule were excluded from the follow-up analyses. Follow-up among the included studies ranged from 5 to 17 y. In the study with the longest follow-up, 87 to 100% (depending on the vaccination schedule) of the children whose antibody levels were measured at follow-up were found to be seroprotected up to 17 y after vaccination.Citation52
Table 4. Long-term persistence of anti-hepatitis A antibodies.
The vaccination schedule, the number of doses, the antibody-status of the mother and age at vaccination were all found to influence the height of the geometric mean concentration (GMC) of anti-HAV antibodies. In the study with the longest follow-up, children who received the third dose of hepatitis A vaccine at month 12 compared to month 2 of the vaccination schedule had a higher GMC after 17 y (354 mIU/mL [95%CI 142–880] vs.129 mIU/mL [95%CI 61–270]).Citation52 In another study, the GMC at 5 y follow-up was significantly higher among those who had received 2 doses of hepatitis A vaccine compared to those who had received only one dose (592 mIU/mL [95%CI 480–729] vs. 123 mIU/mL [95%CI 111–135]).Citation53 In 2 studies, the presence of maternal antibodies was significantly associated with lower GMCs at 6 and 15 y follow-up among infants vaccinated at aged 2 and 6 months, respectively.Citation54,55 One of these studies also showed that the GMC was higher among children vaccinated at age 12 or 18 months compared to those aged 6 months.Citation55
Discussion
In 2012, Ott et al. reviewed the literature on the long-term protective effect of hepatitis A vaccines,Citation56 and new studies have been published since then.Citation52,53,55,57-59 To our knowledge, the present communication is the first systematic review that examines the overall impact of universal mass vaccination with inactivated hepatitis A vaccines.
Impact of UMV programs
The goal of the hepatitis A UMV programs in countries with intermediate endemicity for HAV is to protect individuals from infection and disease and reducing the virus circulation. Most UMV programs are aimed at very young children, as they represent the reservoir of the infection representing an important vehicle in the transmission of HAV.Citation28,60-63 UMV programs are generally based on 2-dose vaccination, however Argentina and Brazil have decided to introduce a one dose only program.Citation24,30 This review focused only on countrywide UMV with monovalent inactivated vaccines. In China, hepatitis A vaccination was introduced into the routine childhood program in 2008. However, as 92% of the 16 million children aged 18 months are vaccinated annually with a single dose of live attenuated vaccines, data from China was beyond the scope of this review.Citation64,65 Likewise, some areas implemented vaccination only in a particular region of a country (e.g. Puglia, ItalyCitation38; or Catalonia, Spain where a combined hepatitis A and B vaccine was usedCitation39) or only in one city (e.g., Minsk, BelarusCitation37), and were not included.
All but one of the reviewed studies that looked at incidence of acute hepatitis A showed a marked decline in the incidence after the introduction of hepatitis A UMV programs in countries with intermediate levels of endemicity defined elsewhere.Citation66 However reductions in the rate of transmission are also attributable to the improved hygiene resulting from cleaned water access. It has been in fact shown that Hepatitis A virus (HAV) is associated with inadequate water and sanitation, the increases in clean water access lead to reduced risk of waterborne HAV transmission.Citation67 The incidence in non-vaccinated age groups was also found to decrease, likely indicating a strong impact of vaccination programs on herd immunity.Citation24,28,35,46,48,49 However an increase in the proportion of acute hepatitis A cases hospitalized in the United States was reported and this could be explained by the increase in age of the susceptible population which is predominantly adults more prone to clinically overt and severe disease.Citation44,51
Greece is the only country where the introduction of UMV for hepatitis A did not show a strong impact on the incidence of hepatitis A, as reported in other countries. Differently than in other countries the UMV in Greece was implemented when already at least a third of children on a national level had been vaccinate in the private market and specifically data from Athens metropolitan area showed a vaccine uptake >50% prior to the UMV introduction. The low impact of UMV showed in Greece is likely due to the fact that Greece was already a country with low endemicity at the time UMV was introduced,Citation66 and that children in the main high risk group, the Roma population, are not vaccinated at the recommended level to prevent transmission.Citation40
The evaluation of the impact of hepatitis A vaccination has been carried out mostly using national or state-wide passive surveillance systems, based primarily on laboratory-confirmed or epidemiologically-linked cases of acute hepatitis A,Citation24,35,36,40, 47-50 and 2 Israeli studies used health insurance organization data.Citation46,68 None of the systems ascertained asymptomatic infections or those with acute hepatitis A disease that did not seek medical care. None of the authors reported any changes in underreporting over time. If these factors had any effect on the outcome, it is likely they led to an underestimation of the reduction in acute hepatitis A, rather than an overestimation.
Outbreaks have often been the direct trigger for the introduction of UMV programs, such as the 2003–2004 outbreaks in Argentina.Citation24 In some areas, the overall incidence of acute hepatitis A was already declining in the decade prior to the introduction of routine vaccination programs, but maintained its cyclic pattern.Citation28,35,48 For example, Singleton et al. state that increasing rates of in-home running water perhaps contributed to somewhat lower HAV incidence in the later pre-licensure period in Alaska.Citation48 However, the decline in incidence after the introduction of UMV was unprecedented in magnitude. No major changes in water or sanitation infrastructure were reported that coincided with the introduction of UMV and to which the decrease could be attributed.Citation24,28,48 Furthermore the decline can't be entirely explained by the cyclical nature of the disease, as the decline was accompanied by shifts in the relative age distribution of acute hepatitis A to older age groupsCitation24,35,44,46,68 and declines were larger in vaccinating than non-vaccinating states of the US.Citation35 Finally, epidemic peaks have been disappearing. The decline in incidence was sustained over time; studies in this review included up to 8 y of data post-UMV introduction,Citation48,49,68 during which no increase in acute hepatitis A incidence was observed.
Vaccination coverage varied widely among geographical areas; reaching over 95% of young children in Argentina, but only 25% in US states where vaccination was “considered” from 1999. Efforts to target children at highest risk in certain areas of the US might explain the high impact of UMV despite this low vaccination coverage.Citation35 The impact of UMV on acute hepatitis A incidence was evident despite limited vaccine coverage rates in some countries.
Due to the heterogeneity of the data, meta-analysis was not performed. The periods compared in the before vs. after comparison differ too much between the studies, especially in terms of years since the introduction of UMV. Additionally, Vizzotti et alCitation24 describes a 1-dose vaccination program while the other publications refer to 2-dose vaccination programs. As with any systematic literature review, this review is subject to the limitations of the included articles.
Almost all included studies showed a decline in the incidence following the introduction of hepatitis A UMV programs. As these studies were conducted in settings with intermediate endemicity, the results should be interpreted within this context and differences in the surveillance systems should also been considered when interpreting the data. In the study from Greece, a country with low endemicity, no such decline was seen. Improved hygiene over the past century has led to low endemicity in much of the developed world. The resulting high susceptibility may increase the risk of outbreaks when exposure does occur, and this has been the cause of recent large outbreaks through food contamination, e.g. through frozen pomegranate arils in the United StatesCitation69 and frozen berries in Europe.Citation70 However, this does not necessarily indicate that mass immunization programs with hepatitis A vaccine should be introduced in low endemic countries.
Long-term persistence
In this review, evidence of long-term persistence of anti-HAV (IgG) antibodies in children for up to 17 y following vaccination with monovalent inactivated vaccines was found.Citation52 However, these data have been generated following vaccination with 3 doses of an inactivated Hepatitis A vaccine containing 360 EU per dose (HAVRIX˜ 360 EU), not used anymore. Long-term immune memory is important so that children vaccinated under childhood vaccination programs will still be protected by the time they reach the age at which disease is likely to be symptomatic.Citation12,71 Infants vaccinated before the age of one year appear to have a lower antibody response.Citation54,55 This supports the target age of one year or older for children immunized in UMV programs. A recent model-based assessment of vaccine induced immune memory against HAV in adults suggests that anti-HAV antibodies will persist for at least 20 y in >95% of vaccines.Citation22
A limitation in interpretation of most long-term persistence studies is that a fraction of the children that received the primary vaccination series also received an additional booster dose before the last follow-up. Indeed, boosters complicate the interpretation of follow-up data. For instance, 5 of the included studies reported that children who received booster doses were excludedCitation52-55,72; this results in an overestimation in the GMCs and the percentage of children who were seropositive years after the primary vaccination series, as boosters were likely given to precisely those children whose antibodies levels dropped below a certain threshold. The interpretation is further hampered by the fact that most studies did not report how many children were excluded for this reason. For these studies, it can be concluded that anti-HAV-antibodies can persist up to the time of last follow-up (6 to 14–15 years).Citation54,58,59,72-74 For the 2 studies that did report how many children were excluded due to the receipt of a booster dose, it can be concluded that antibodies persist to the time of last follow-up (5 and 17 y) in the majority of children who were not lost to follow-up.Citation52,53,55,57
A limitation of all studies on long-term persistence of antibodies and immune memory is the large number of participants that were lost to follow-up over time. Additionally, the possibility that the children received a ‘natural booster’ due to exposure to circulating HAV cannot be excluded, especially in the early years of the vaccination programs. However, there is also no proof that the long-term persistence of antibodies was the result of natural boosting.Citation54 Furthermore, seroprotection against HAV was defined as a GMC of at least 5, 10, 20, or 33 mIU/mL depending on individual assays and vaccines used in the included studies. However, the true lowest limit of anti-HAV that confers protection is unknown and might be even lower than the detection limit of a particular assay.Citation71,75
Information on long-term persistence after administration of a single dose of vaccine in children is limited, but has been documented in adults.Citation76-78 This information would suggest that protective anti-HAV antibody levels after a single dose of inactivated hepatitis A vaccine can persist for up to 11 y. A recent publication also suggests that antibody titers are lower and antibodies decay faster in younger children (aged 1–7 years).Citation74 Long-term persistence data after one dose in children would therefore be valuable, especially as not all children are vaccinated twice, either because the second dose is missed or because only one dose is recommended, e.g., in Argentina and Brazil.
The impact on the disease incidence and other related health outcomes as well as the long term antibody persistence provided by the vaccination are all critical considerations of vaccine program impact, however every country must also assess the cost-effectiveness of the program in deciding for or against the implementation of hepatitis A UMV. The data reported present certain heterogeneity in terms of epidemiology and reporting systems and therefore the data should not be read as a comparison of the impact of immunization programs in the different country but solely as a descriptive assessment of the country by country outcomes.
Conclusion
Introduction of UMV with monovalent inactivated hepatitis A vaccines in countries with intermediate endemicity for HAV infection led to a considerable decrease in the incidence of hepatitis A in vaccinated and in non-vaccinated age groups alike.
Abbreviations
| UMV | = | universal mass vaccination |
| HAV | = | hepatitis A virus |
| WHO | = | world health organization |
| MSM | = | men having sex with men |
| GMC | = | geometric mean concentration |
Disclosure of potential conflicts of interest
LDM and CM are employees of the GSK group of companies and hold stock options or restricted shares. EB and ALS are/were employees of Pallas Health Research and Consultancy BV company and they declare that Pallas Health Research and Consultancy BV company received a grant for the research from the GSK group of companies during the conduct of the study and also outside the submitted work. DS does not have anything to declare.
Author contributions
The manuscript was written by Anke L. Stuurman. All authors have contributed toward the conception of this research, development of this manuscript and critical review and approval of the final content.
KHVI_A_1242539_Supplementary_material.zip
Download Zip (27.8 KB)Acknowledgments
The authors would like to thank Lakshmi Hariharan (employee of the GSK group of companies) for providing editorial assistance and coordinated the development of this publication.
Funding
This systematic review was sponsored and funded by GlaxoSmithKline Biologicals S.A., Belgium. GlaxoSmithKline Biologicals S.A. was involved in all stages of the study conduct and analysis; and also took charge of all costs associated with the development and the publishing of the manuscript.
References
- WHO position paper on hepatitis A vaccines - June 2012. Wkly Epidemiol Rec 2012; 87:261-76; PMID:22905367
- Mohd Hanafiah K, Jacobsen KH, Wiersma ST. Challenges to mapping the health risk of hepatitis A virus infection. Int J Health Geogr 2011; 10:57; PMID:22008459; http://dx.doi.org/10.1186/1476-072X-10-57
- Centers for Disease Control Prevention. Chapter 3 infectious diseases related to travel - Hepatitis A. In: Nelson NP, Murphy TV, eds. CDC Health Information for International Travel 2016. New York: Oxford University Press, 2016.
- Ciocca M. Clinical course and consequences of hepatitis A infection. Vaccine 2000; 18 Suppl 1:S71-4; PMID:10683554; http://dx.doi.org/10.1016/S0264-410X(99)00470-3
- Prevention of hepatitis A through active or passive immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 1996; 45:1-30; PMID:9005304
- Burroughs AK, Westaby D. Chapter 7 - Liver, biliary tract and pancreatic disease. In: Kumar P, Clark M, eds. Clinical Medicine London: Elsevier Saunders, 2005:362-4.
- Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol 2005; 34:600-9; PMID:15831565; http://dx.doi.org/10.1093/ije/dyi062
- Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect 2004; 132:1005-22; PMID:15635957; http://dx.doi.org/10.1017/S0950268804002857
- Klevens RM, Miller JT, Iqbal K, Thomas A, Rizzo EM, Hanson H, Sweet K, Phan Q, Cronquist A, Khudyakov Y, et al. The evolving epidemiology of hepatitis a in the United States: incidence and molecular epidemiology from population-based surveillance, 2005–2007. Arch Intern Med 2010; 170:1811-8; PMID:21059974; http://dx.doi.org/10.1001/archinternmed.2010.401
- Van Herck K, Van Damme P. Prevention of hepatitis A by Havrix: a review. Expert Rev Vaccines 2005; 4:459-71; PMID:16117704; http://dx.doi.org/10.1586/14760584.4.4.459
- Shouval D. The immunological basis for immunization series. Immunization vaccines and biologicals Geneva: World Health Organization, 2011:1-39.
- Van Damme P, Van Herck K. Effect of hepatitis A vaccination programs. JAMA 2005; 294:246-8; PMID:16014600; http://dx.doi.org/10.1001/jama.294.2.246
- Wu JY, Liu Y, Chen JT, Xia M, Zhang XM. Review of 10 years of marketing experience with Chinese domestic inactivated hepatitis A vaccine Healive(R). Hum Vaccin Immunother 2012; 8:1836-44; PMID:23032165; http://dx.doi.org/10.4161/hv.21909
- Irving GJ, Holden J, Yang R, Pope D. Hepatitis A immunisation in persons not previously exposed to hepatitis A. Cochrane Database Syst Rev 2012; 7:CD009051; PMID:22786522; http://dx.doi.org/10.1002/14651858
- Franco E, Giambi C, Ialacci R, Maurici M. Prevention of hepatitis A by vaccination. Expert Opin Biol Ther 2003; 3:965-74; PMID:12943455; http://dx.doi.org/10.1517/14712598.3.6.965
- Ambrosch F, Wiedermann G, Jonas S, Althaus B, Finkel B, Gluck R, Herzog C. Immunogenicity and protectivity of a new liposomal hepatitis A vaccine. Vaccine 1997; 15:1209-13; PMID:9286045; http://dx.doi.org/10.1016/S0264-410X(97)00015-7
- Innis BL, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik CA, Suntayakorn S, Suknuntapong T, Safary A, Tang DB. Protection against hepatitis A by an inactivated vaccine. JAMA 1994; 271:1328-34; PMID:8158817; http://dx.doi.org/10.1001/jama.1994.03510410040030
- Lopez EL, Del Carmen Xifro M, Torrado LE, De Rosa MF, Gomez R, Dumas R, Wood SC, Contrini MM. Safety and immunogenicity of a pediatric formulation of inactivated hepatitis A vaccine in Argentinean children. Pediatr Infect Dis J 2001; 20:48-52; PMID:11176566; http://dx.doi.org/10.1097/00006454-200101000-00009
- Ren YH, Chen JT, Wu WT, Gong XJ, Zhang YC, Xue WH, Ren YF, Han LJ, Kang WX, Li SP, et al. [The study on the 0, 12 month vaccination schedule' of Healive inactivated hepatitis A vaccine in children]. Zhonghua Liu Xing Bing Xue Za Zhi 2003; 24:1013-5; PMID:14687502
- Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med 1992; 327:453-7; PMID:1320740; http://dx.doi.org/10.1056/NEJM199208133270702
- Van Herck K, Van Damme P. Inactivated hepatitis A vaccine-induced antibodies: follow-up and estimates of long-term persistence. J Med Virol 2001; 63:1-7; PMID:11130881; http://dx.doi.org/10.1002/1096-9071(200101)63:1%3c1::AID-JMV1000%3e3.0.CO;2-U
- Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine 2014; 32:1507-13; PMID:24508042; http://dx.doi.org/10.1016/j.vaccine.2013.10.088
- Jacobsen KH, Wiersma ST. Hepatitis A virus seroprevalence by age and world region, 1990 and 2005. Vaccine 2010; 28:6653-7; PMID:20723630; http://dx.doi.org/10.1016/j.vaccine.2010.08.037
- Vizzotti C, Gonzalez J, Gentile A, Rearte A, Ramonet M, Canero-Velasco MC, Pérez Carrega ME, Urueña A, Diosque M. Impact of the Single-dose Immunization Strategy Against Hepatitis A in Argentina. Pediatr Infect Dis J 2014; 33:84-8; PMID:24352191; http://dx.doi.org/10.1097/INF.0000000000000042
- Gouvernement du Québec. Programme québécois d'immunisation. Portail santé mieux-être 15 October 2015; Available from: http://sante.gouv.qc.ca/programmes-et-mesures-daide/programme-quebecois-d-immunisation/ [Accessed on 17 November 2015].
- Domínguez A, Oviedo M, Carmona G, Jansá J, Borrás E, Salleras L, Plasència A. Epidemiology of hepatitis A before and after the introduction of a universal vaccination programme in Catalonia, Spain. Journal of viral hepatitis 2008; 15:51-6; http://dx.doi.org/10.1111/j.1365-2893.2008.01030.x
- Van Damme P, Van Herck K. A review of the efficacy, immunogenicity and tolerability of a combined hepatitis A and B vaccine. Expert Rev Vaccines 2004; 3:249-67; PMID:15176942; http://dx.doi.org/10.1586/14760584.3.3.249
- Dagan R, Leventhal A, Anis E, Slater P, Ashur Y, Shouval D. Incidence of hepatitis A in Israel following universal immunization of toddlers. JAMA 2005; 294:202-10; PMID:16014594; http://dx.doi.org/10.1001/jama.294.2.202
- Ministry of Health - Kingdom of Bahrain. Recommended Immunization Schedule for the Expanded Program on Immunization, Bahrain. Available from: http://www.moh.gov.bh/en/healthinformation/Immunizations.aspx?print=true [Accessed on 16 January 2015].
- Ministério da Saúde - Brasil. SUS passa a oferecer vacina contra hepatite A para crianças. Blog da Saúde July 29, 2014; Available from: http://www.blog.saude.gov.br/index.php/570-destaques/34211-sus-passa-a-oferecer-vacina-contra-hepatite-a-para-criancas [Accessed on 9 June 2015].
- Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W, Yuansheng H, Gong X, Chen Y, Liang X. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol 2009; 19:189-95; PMID:19561383; http://dx.doi.org/10.2188/jea.JE20080087
- Viral Hepatitis Prevention Board. Prevention and control of viral hepatitis in Greece: Lessons learnt and the way forward. Viral Hepatitis 2008; 16.
- Kyrka A, Tragiannidis A, Cassimos D, Pantelaki K, Tzoufi M, Mavrokosta M, Pedeli X, Athanassiadou F, Hatzimichael A, Konstantopoulos A, et al. Seroepidemiology of hepatitis A among Greek children indicates that the virus is still prevalent: Implications for universal vaccination. Journal of medical virology 2009; 81:582-7; PMID:19235841; http://dx.doi.org/10.1002/jmv.21434
- Ministerio de Salud - Panama. Esquema Nacional de Vacunación. 2013; Available from: http://www.minsa.gob.pa/sites/default/files/programas/esquema_de_vacunacion_revisado_marzo_2013.pdf [Accessed on 16 January 2015].
- Wasley A, Samandari T, Bell BP. Incidence of hepatitis A in the United States in the era of vaccination. JAMA 2005; 294:194-201; PMID:16014593; http://dx.doi.org/10.1001/jama.294.2.194
- Romero C, Perdomo V, Chamorro F, Assandri E, Pírez MC, Montano A. Prevención de hepatitis A mediante vacunación en Uruguay (2005–2010). Rev Méd Urug 2012; 28:115-22
- Fisenka EG, Germanovich FA, Glinskaya IN, Lyabis OI, Rasuli AM. Effectiveness of universal hepatitis A immunization of children in Minsk City, Belarus: four-year follow-up. J Viral Hepat 2008; 15 Suppl 2:57-61; PMID:18837836; http://dx.doi.org/10.1111/j.1365-2893.2008.01031.x
- Martinelli D, Bitetto I, Tafuri S, Lopalco PL, Mininni RM, Prato R. Control of hepatitis A by universal vaccination of children and adolescents: an achieved goal or a deferred appointment? Vaccine 2010; 28:6783-8; PMID:20688041; http://dx.doi.org/10.1016/j.vaccine.2010.07.069
- Martinez A, Broner S, Sala MR, Manzanares-Laya S, Godoy P, Planas C, Minguell S, Torner N, Jané M, Domínguez A, et al. Changes in the epidemiology of hepatitis A outbreaks 13 years after the introduction of a mass vaccination program. Hum Vaccin Immunother 2014; 11; PMID:25483535; PMCID:PMC4514321; http://dx.doi.org/10.4161/hv.35861
- Mellou K, Sideroglou T, Papaevangelou V, Katsiaflaka A, Bitsolas N, Verykouki E, Triantafillou E, Baka A, Georgakopoulou T, Hadjichristodoulou C. Considerations on the current universal vaccination policy against hepatitis A in Greece after recent outbreaks. PLoS One 2015; 10:e0116939; PMID:25590132; http://dx.doi.org/10.1371/journal.pone.0116939
- Belmaker I, Dukhan L, Yosef Y, Leventhal A, Dagan R. Elimination of hepatitis a infection outbreaks in day care and school settings in southern Israel after introduction of the national universal toddler hepatitis a immunization program. Pediatr Infect Dis J 2007; 26:36-40; PMID:17195703; http://dx.doi.org/10.1097/01.inf.0000247105.45185.13
- Zhou F, Shefer A, Weinbaum C, McCauley M, Kong Y. Impact of hepatitis A vaccination on health care utilization in the United States, 1996–2004. Vaccine 2007; 25:3581-7; PMID:17306908; http://dx.doi.org/10.1016/j.vaccine.2007.01.081
- Vogt TM, Wise ME, Bell BP, Finelli L. Declining hepatitis A mortality in the United States during the era of hepatitis A vaccination. J Infect Dis 2008; 197:1282-8; PMID:18422440; http://dx.doi.org/10.1086/586899
- Collier MG, Tong X, Xu F. Hepatitis a hospitalizations in the United States, 2002 – 2011. Hepatology 2015; 61:481-5; PMID:25266085; http://dx.doi.org/10.1002/hep.27537
- Papaevangelou V, Alexopoulou Z, Hadjichristodoulou C, Kourlamba G, Katsioulis A, Theodoridou K, Spoulou V, Theodoridou M. Time trends in pediatric hospitalizations for hepatitis A in Greece (1999–2013): Assessment of the impact of universal infant immunization in 2008. Hum Vaccin Immunother 2016; 12:1852-6; PMID:27141813; PMCID:PMC4964822; http://dx.doi.org/10.1080/21645515.2016.1151589
- Chodick G, Green MS, Heymann AD, Rosenmann L, Shalev V. The shifting epidemiology of hepatitis A following routine childhood immunization program in Israel. Prev Med 2007; 45:386-91; PMID:17599401; http://dx.doi.org/10.1016/j.ypmed.2007.05.011
- Levine H, Kopel E, Anis E, Givon-Lavi N, Dagan R. The impact of a national routine immunisation programme initiated in 1999 on Hepatitis A incidence in Israel, 1993 to 2012. Euro Surveill 2015; 20:3-10; PMID:25719962; http://dx.doi.org/10.2807/1560-7917.ES2015.20.7.21040
- Singleton RJ, Hess S, Bulkow LR, Castrodale L, Provo G, McMahon BJ. Impact of a statewide childhood vaccine program in controlling hepatitis A virus infections in Alaska. Vaccine 2010; 28:6298-304; PMID:20637769; http://dx.doi.org/10.1016/j.vaccine.2010.06.113
- Erhart LM, Ernst KC. The changing epidemiology of hepatitis A in Arizona following intensive immunization programs (1988–2007). Vaccine 2012; 30:6103-10; PMID:22835739; http://dx.doi.org/10.1016/j.vaccine.2012.07.029
- Estripeaut D, Contreras R, Tinajeros O, Castrejon MM, Shafi F, Ortega-Barria E, DeAntonio R. Impact of Hepatitis A vaccination with a two-dose schedule in Panama: Results of epidemiological surveillance and time trend analysis. Vaccine 2015; 33:3200-7; PMID:25981490; http://dx.doi.org/10.1016/j.vaccine.2015.04.100
- Ly KN, Klevens RM. Trends in Disease and Complications of Hepatitis A Virus Infection in the United States, 1999–2011: A New Concern for Adults. J Infect Dis 2015; 212:176-82; PMID:25637352; http://dx.doi.org/10.1093/infdis/jiu834
- Raczniak GA, Bulkow LR, Bruce MG, Zanis CL, Baum RL, Snowball MM, Byrd KK, Sharapov UM, Hennessy TW, McMahon BJ. Long-term immunogenicity of hepatitis A virus vaccine in Alaska 17 years after initial childhood series. J Infect Dis 2013; 207:493-6; PMID:23204169; http://dx.doi.org/10.1093/infdis/jis710
- Espul C, Benedetti L, Linares M, Cuello H, Rasuli A. Five-year follow-up of immune response after one or two doses of inactivated hepatitis A vaccine given at 1 year of age in the Mendoza Province of Argentina. J Viral Hepat 2014; PMID:25262590; http://dx.doi.org/10.1111/jvh.12317
- Fiore AE, Shapiro CN, Sabin K, Labonte K, Darling K, Culver D, Bell BP, Margolis HS. Hepatitis A vaccination of infants: effect of maternal antibody status on antibody persistence and response to a booster dose. Pediatr Infect Dis J 2003; 22:354-9; PMID:12690277; http://dx.doi.org/10.1097/01.inf.0000059446.52063.b9
- Spradling PR, Bulkow LR, Negus SE, Homan C, Bruce MG, McMahon BJ. Persistence of seropositivity among persons vaccinated for hepatitis A during infancy by maternal antibody status: 15-year follow-up. Hepatology 2016; 63:703-11; PMID:26637987; http://dx.doi.org/10.1002/hep.28375
- Ott JJ, Irving G, Wiersma ST. Long-term protective effects of hepatitis A vaccines. A systematic review. Vaccine 2012; 31:3-11; PMID:22609026; http://dx.doi.org/10.1016/j.vaccine.2012.04.104
- Dagan R, Ashkenazi S, Livni G, Go O, Bagchi P, Sarnecki M. Long-term serologic follow-up of children vaccinated with a pediatric formulation of virosomal hepatitis a vaccine administered with routine childhood vaccines at 12–15 months of age. Pediatr Infect Dis J 2016; 35:e220–8; PMID:27093164; http://dx.doi.org/10.1097/INF.0000000000001176
- Raczniak GA, Thomas TK, Bulkow LR, Negus SE, Zanis CL, Bruce MG, Spradling PR, Teshale EH, McMahon BJ. Duration of protection against hepatitis A for the current two-dose vaccine compared to a three-dose vaccine schedule in children. Vaccine 2013; 31:2152-5; PMID:23470239; http://dx.doi.org/10.1016/j.vaccine.2013.02.048
- Yu C, Song Y, Qi Y, Li C, Jiang Z, Li C, Zhang W, Wang L, Xia J. Comparison of Immunogenicity and Persistence between Inactivated Hepatitis A Vaccine Healive(R) and Havrix(R) among Children: A 5-Year Follow-up Study. Hum Vaccin Immunother 2016; 12(10):2595-602; PMID:27385349; http://dx.doi.org/10.1080/21645515.2016.1197450
- Bell BP, Shapiro CN, Alter MJ, Moyer LA, Judson FN, Mottram K, Fleenor M, Ryder PL, Margolis HS. The diverse patterns of hepatitis A epidemiology in the United States-implications for vaccination strategies. J Infect Dis 1998; 178:1579-84; PMID:9815207; http://dx.doi.org/10.1086/314518
- Hadler SC, Webster HM, Erben JJ, Swanson JE, Maynard JE. Hepatitis A in day-care centers. A community-wide assessment. N Engl J Med 1980; 302:1222-7; PMID:6245363; http://dx.doi.org/10.1056/NEJM198005293022203
- Smith PF, Grabau JC, Werzberger A, Gunn RA, Rolka HR, Kondracki SF, Gallo RJ, Morse DL. The role of young children in a community-wide outbreak of hepatitis A. Epidemiol Infect 1997; 118:243-52; PMID:9207735; http://dx.doi.org/10.1017/S0950268897007462
- Staes CJ, Schlenker TL, Risk I, Cannon KG, Harris H, Pavia AT, Shapiro CN, Bell BP. Sources of infection among persons with acute hepatitis A and no identified risk factors during a sustained community-wide outbreak. Pediatrics 2000; 106:E54; PMID:11015549; http://dx.doi.org/10.1542/peds.106.4.e54
- Xu Z-Y, Wang X-Y. Live attenuated hepatitis A vaccines developed in China. Hum Vaccin Immunother 2014; 10:659-66; PMID:24280971; http://dx.doi.org/10.4161/hv.27124
- Cui F, Hadler SC, Zheng H, Wang F, Zhenhua W, Yuansheng H, Gong X, Chen Y, Liang X. Hepatitis A surveillance and vaccine use in China from 1990 through 2007. J Epidemiol 2009; 19:189-95; PMID:19561383; http://dx.doi.org/10.2188/jea.JE20080087
- Jacobsen KH. The global prevalence of hepatitis A virus infection and susceptibility: a systematic review. World Health Organization 2009; 1-413. Available from: http://whqlibdoc.who.int/hq/2010/WHO_IVB_10.01_eng.pdf [Accessed on 24 October 2015]
- Franco E, Meleleo C, Serino L, Sorbara D, Zaratti L. Hepatitis A: Epidemiology and prevention in developing countries. World J Hepatol 2012; 4:68-73; PMID:22489258; http://dx.doi.org/10.4254/wjh.v4.i3.68
- Chodick G, Heymann AD, Ashkenazi S, Kokia E, Shalev V. Long-term trends in hepatitis A incidence following the inclusion of Hepatitis A vaccine in the routine nationwide immunization program. J Viral Hepat 2008; 15 Suppl 2:62-5; PMID:18837837; http://dx.doi.org/10.1111/j.1365-2893.2008.01032.x
- Collier MG, Khudyakov YE, Selvage D, Adams-Cameron M, Epson E, Cronquist A, Jervis RH, Lamba K, Kimura AC, Sowadsky R, et al. Outbreak of hepatitis A in the USA associated with frozen pomegranate arils imported from Turkey: an epidemiological case study. The Lancet Infectious Diseases 2014; 14:976-81; PMID:25195178; http://dx.doi.org/10.1016/S1473-3099(14)70883-7
- Severi E, Verhoef L, Thornton L, Guzman-Herrador BR, Faber M, Sundqvist L, Rimhanen-Finne R, Roque-Afonso AM, Ngui SL, Allerberger F, et al. Large and prolonged food-borne multistate hepatitis A outbreak in Europe associated with consumption of frozen berries, 2013 to 2014. Euro Surveill 2015; 20:21192; PMID:26227370; http://dx.doi.org/10.2807/1560-7917.ES2015.20.29.21192
- Sharapov UM, Bulkow LR, Negus SE, Spradling PR, Homan C, Drobeniuc J, Bruce M, Kamili S, Hu DJ, McMahon BJ. Persistence of hepatitis A vaccine induced seropositivity in infants and young children by maternal antibody status: 10-year follow-up. Hepatology 2012; 56:516-22; PMID:22371069; http://dx.doi.org/10.1002/hep.25687
- Lopez EL, Contrini MM, Mistchenko A, Kieffer A, Baggaley RF, Di Tanna GL, Desai K, Rasuli A, Armoni J. Modeling the long-term persistence of hepatitis A antibody after a two-dose vaccination schedule in Argentinean children. Pediatr Infect Dis J 2015; 34:417-25; PMID:25764099; http://dx.doi.org/10.1097/INF.0000000000000605
- Bian GL, Ma R, Dong HJ, Ni HX, Hu FJ, Chen YR, Chen JQ, Zhou SY, Lin YX, Xu GZ. Long-term clinical observation of the immunogenicity of inactivated hepatitis A vaccine in children. Vaccine 2010; 28:4798-801; PMID:20471440; http://dx.doi.org/10.1016/j.vaccine.2010.04.096
- Van Herck K, Hens A, De Coster I, Vertruyen A, Tolboom J, Sarnecki M, Van Damme P. Long-term antibody persistence in children after vaccination with the pediatric formulation of an aluminum-free virosomal hepatitis A vaccine. Pediatr Infect Dis J 2015; 34:e85–91; PMID:25389920; http://dx.doi.org/10.1097/INF.0000000000000616
- Fiore AE, Wasley A, Bell BP. Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2006; 55:1-23; PMID:16708058
- Hatz C, Van der Ploeg R, Beck BR, Frösner G, Hunt M, Herzog C. Successful memory response following a booster dose with a virosome-formulated hepatitis A vaccine delayed up to 11 years. Clin Vaccine Immunol 2011; 18:885-7; PMID:21411599; http://dx.doi.org/10.1128/CVI.00358-10
- Iwarson S, Lindh M, Widerstrom L. Excellent booster response 4 to 8 years after a single primary dose of an inactivated hepatitis A vaccine. J Travel Med 2004; 11:120-1; PMID:15109480; http://dx.doi.org/10.2310/7060.2004.17079
- Landry P, Tremblay S, Darioli R, Genton B. Inactivated hepatitis A vaccine booster given≥ 24 months after the primary dose. Vaccine 2000; 19:399-402; PMID:11027799; http://dx.doi.org/10.1016/S0264-410X(00)00188-2