ABSTRACT
A cohort of 81 HIV-infected participants received seasonal trivalent inactivated influenza vaccine (TIV) and their humoral responses were monitored using hemagglutination inhibition (HAI) assay and enzyme-linked immunosorbent assay (ELISA). Three weeks after the vaccination, the percentage of the cohort that had an HAI titer of >1:40 was 35% (for H1N1), 43% (for H3N2) and 19% (for influenza B). An increase in HAI titer can be achieved by an increase in magnitude of the antibody responses, which can be measured by an increase in ELISA titer; as well as a quality improvement of the antibody responses through increased avidity to the virus. For some individuals, an increase in avidity alone is sufficient to reach the sero-protective titer. Notably, a number of volunteers showed an increase in ELISA titer without a rise in HAI titer. A total of 24 participants (30%) did not show any significant increase in both HAI and ELISA tests after vaccination. Apart from a lower peripheral CD4+ T cell count, the non responders' peripheral blood mononuclear cells (PBMC) also had a higher IL-10 mRNA expression after TIV vaccination ex vivo. Cytokine profiling demonstrated that, apart from a weaker MCP-1 expression in the non-responder group, PBMC from both groups responded comparably to lipopolysaccharide (LPS) stimulation in vitro. Since only 3 participants developed sero-protective titers against all 3 subtypes after vaccination, our study highlights a need to enhance the immunogenicity of the subunit vaccine for this population, potentially through harnessing the innate immunity with an external adjuvant.
KEYWORDS:
Introduction
Since 1985, a total of 4845 human immunodeficiency virus (HIV) infections have been notified to the Ministry of Health, Singapore; of those, 2319 have asymptomatic HIV infection and 1137 have Acquired Immune Deficiency Syndrome (AIDS) as of end 2010.Citation1 Annual vaccination with trivalent inactivated influenza vaccine (TIV) is recommended for this group due to their increased risk of developing influenza-related complications and mortality rates.Citation2 As memory CD4+ T cells are the targets of HIV-1,Citation3 the observation that CD4+ T cell count is an important determinant for vaccine response of HIV-infected individuals,Citation4-6 consistent with its important role in coordinating adaptive immune responses. Since a number of studies have shown that vaccine performance was blunted among different HIV cohorts,Citation7-10 this prospective study aims to evaluate the sero-conversion rate of a cohort of HIV-infected individuals in Singapore after TIV vaccination. In addition, increasing evidence suggest that the innate immune system also plays an important role in vaccination outcome through enhancing antigen capturing and presentation events of antigen presenting cells,Citation11 therefore, another aim of this study is to evaluate whether there is a significant difference between the innate immunity of non responders (NR) and responders (R) within this cohort, in term of their cytokine profile ex vivo or after in vitro stimulation.
In this study, 81 HIV-infected individuals were recruited. They received a single dose of TIV and a detailed analysis on the changes in the humoral responses against each subtype of influenza virus was performed. Various immunological parameters assessed include CD4+ T cell count; and changes in cytokine expression of peripheral blood mononuclear cells (PBMC) after vaccination, as well as after in vitro lipopolysaccharide (LPS) stimulation. The data revealed a sub-optimal performance of the TIV in this high risk group in Singapore, highlighting the need for a vaccine with stronger immunogenicity to better protect this cohort against seasonal influenza.
Results
The immunogenicity of the inactivated influenza vaccine was sub-optimal among the HIV-infected participants
Eighty-one participants were recruited in this study, consisting of 47 males and 34 females. The average age of the cohort was 43.5 y (range 23-69). Seventy-four percent of participants were on antiretroviral therapy with an average CD4+ T cell count of 328 per μl (). A blood sample was collected from every participant before vaccination to determine the baseline level. As shown in , ∼21% of them had humoral immunity against at least one of the viruses. Four individuals had pre-existing immunity against H1N1 (defined as >=1:40 of HAI titer). Another 8 and 3 individuals had immunity against H3N2 and influenza B, respectively. Two had immunity against both H1N1 and H3N2. For the remaining 64 individuals, their HAI titers against the 3 subtypes were below 1:40.
Figure 1. Number of participants that reached sero-protective titers pre- and post-vaccination. Paired serum samples were tested for the ability to inhibit hemagglutination activity of 4 HA units of the 3 viruses. A titer of 1:40 was considered to be positive.
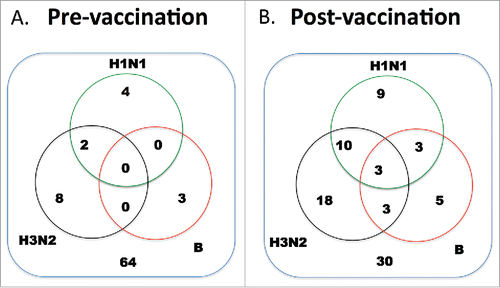
Table 1. Demographic characteristics of the cohort.
Serum samples of the cohort were tested again 21 d after vaccination. The number of individuals with protective humoral immunity against H1N1, H3N2 and influenza B was 25, 34 and 14 respectively (). For the 25 individuals with sero-protective titer against H1N1, 9 of them had immunity to the H1N1 virus alone, another 10 individuals had immunity against both H1N1 and H3N2 viruses. Three had immunity against H1N1 and influenza B. Three of them had immunity against all three viruses. For the 34 individuals with protective immunity to H3N2, 18 of them responded to H3N2 alone, 3 had immunity against both H3N2 and influenza B. Finally, for influenza B responders, 5 out of 14 of them responded to influenza B alone. Overall, 63% of participants had HAI titer of >=1:40 against at least one of the viruses after TIV vaccination. The remaining 30 individuals did not show significant increase in HAI titer after receiving the TIV.
Of the 9 volunteers that developed a protective titer against the H1N1 virus alone on D21, 7 of them (63%) had ≥ 4-fold increase in the HAI titer from the D0 sample (termed as sero-converted, ). The rest had an increase of less than 4-fold but with a final titer reaching the sero-protective titer of 1:40 (termed as sero-protected) on D21. For those that responded to the H3N2 virus alone, 13/18 (72%) sero-converted; as for influenza B virus, the sero-conversion rate was 60% (3/5 individuals). For the 10 volunteers that developed protective titer against both the H1N1 and H3N2 viruses, 7/10 (70%) sero-converted for both viruses. One volunteer (10%) sero-converted for the H1N1 virus and was sero-protected against H3N2. The remaining 2 participants were sero-protected against both subtypes. shows the detailed analysis of the data, including the mean HAI titer and fold change of HAI titers between D0 and D21 samples.
Table 2. Detailed analysis of the volunteers who attained a HAI titer of 1:40 or above 3 weeks after vaccination.
Some volunteers exhibited a rise in ELISA titer but not corresponding HAI titer
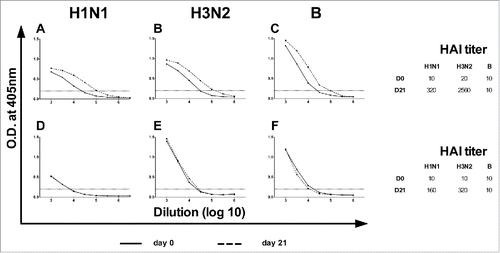
Apart from HA, TIV also contains other viral structural proteins, such as neuraminidase (NA) and nucleoproteins (NP),Citation12 induction of antibody responses against these targets cannot be detected by HAI test, therefore, we also performed ELISA with the paired sera. The subtype-specific ELISA titers of D0 and D21 samples for all the volunteers were determined using UV-inactivated intact virion as coating antigens. A typical result is shown in where there was a significant increase in the ELISA titers against the H1N1 and H3N2 viruses 21 d post vaccination. This increase correlated with the increase in HAI titers (side table of ). However, we also observed some deviation of this correlation in which the same individual showed a significant increase in ELISA titers without a corresponding rise in HAI titer for influenza B (). In total, there were 5, 5 and 3 individuals who showed similar discrepancies against H1N1, H3N2 and influenza B virus, respectively.
Figure 2. The profile of a responder and a non-responder in ELISA. Paired serum samples were tested for ELISA antibody titers prior to and after vaccination using UV-inactivated virions. (A-C) Profile of a responder with increase in ELISA titers. (D-F) Profile of a non-responder without increase in ELISA titers. (Inserts) The corresponding changes in HAI titers of the samples. The graphs are from 2 different volunteers.
An increase in antibody avidity to virion could result in an increase in HAI titer without a corresponding rise in ELISA titer
showed a particular individual with an increase in HAI titers (from 10 to 160 for H1N1 and 10 to 320 for H3N2) but no significant increase in corresponding ELISA titers. Of the 7 individuals who sero-converted against H1N1, 4 of them (57%) showed a significant increase in ELISA titers (). As for H3N2 and influenza B, most of the sero-converted individuals did not show significant increase in corresponding ELISA titers (9 out of 13, and 3 out of 3, respectively). None of those who were sero-protected had significant increase in ELISA titers ().
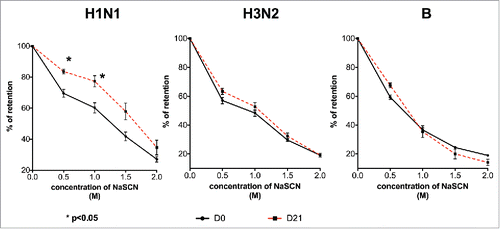
To evaluate whether improved avidity could account for this observation, the antibody avidity of volunteers with this particular profile (an increase in HAI titer without an increase in ELISA titer) was examined using a sodium thiocyanate-displacement ELISA. As shown in , for those with the profile against the H1N1 virus, their D21 serum samples showed a significantly higher retention levels than the D0 samples in the presence of sodium thiocyanate (p < 0.05). However, such an increase in avidity was not found in the H3N2 and influenza B samples.
Figure 3. Improved antibody avidity in atypical H1N1 responders. D0 and D21 serum samples were analyzed in a NaSCN-displacement ELISA. After incubating overnight at 4°C, different concentrations of NaSCN were added into wells to elute low avidity antibodies. The signal of the treated samples was expressed as a percentage of the control PBS-treated samples. The error bars represent the standard deviation of the group. *indicates p < 0.05.
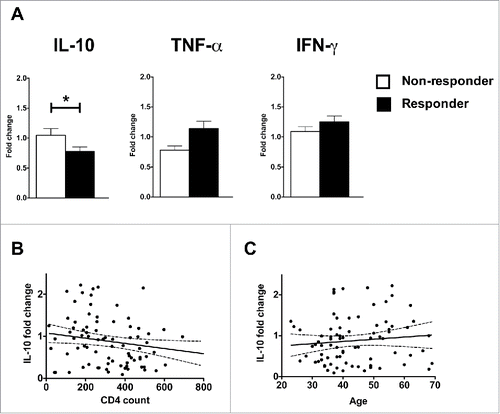
NR group is associated with lower peripheral CD4+ T count and higher IL-10 mRNA expression in PBMC
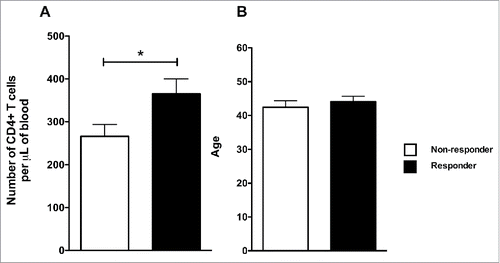
Since CD4+ T cell count and vaccinee's cytokine profile have been linked to vaccination outcome,Citation13 we were interested to determine whether these factors contributed to the sub-optimal responses observed among the NR group. Peripheral blood CD4+ T cell count for each volunteer was determined at the time of vaccination. Subsequently, the volunteers were stratified into 2 groups according to their HAI results. The NR group had the lower CD4+ count with a statistically significant difference compared to the R group (p = 0.0377, ) and the 2 groups had similar average in age (p = 0.6882, ).
Figure 4. Non-responders had fewer peripheral CD4+ T cells than responders. (A) The number of peripheral CD4+ T cells in blood for each volunteer was determined on D0. The non-responders were identified based on the results of . (B) The average age of each group. The bars and the error bars represent the mean and standard deviation of the group. *indicates p < 0.05.
We also examined the cytokine expression profile of each of our volunteers. PBMC were purified from peripheral blood of every volunteer on D0 and D2 after vaccination. Ex vivo mRNA expression levels of IL-10, TNF-α and IFN-γ were determined using real-time PCR assay. The D2 expression level was expressed in relation to D0 level. As shown in , the R group showed greater down-regulation of IL-10 mRNA expression than the NR population and the difference was statistically significant (p = 0.0281). The R group also had higher levels of TNF-α and IFN-γ mRNA expression than the NR group, but the differences did not reached statistical significance (p = 0.1332 and 0.3161). There was no strong correlation between IL-10 mRNA fold change and peripheral CD4+ T cell count (r = 0.05609, ) or the age of the volunteers (r = 0.01034, ).
Figure 5. Vaccine responders had lower IL-10 and higher TNF-α mRNA expression in their PBMC on Day 2 post vaccination than non-responders. mRNA was harvested from PBMC collected on D0 and D2 after vaccination and stored at −80°C till the end of the trial. (A) The mRNA samples from the volunteers were reverse-transcribed simultaneously and the levels of expression for IL-10, TNF-α and IFN-γ were determined by real-time PCR. The expression value of each cytokine was normalized by the β-actin expression. The fold change is calculated based on the normalized D2 value divided by the normalized D0 value. The bars and the error bars represent the mean and standard deviation of the group. *indicates p < 0.05. (B-C) Correlation between IL-10 mRNA fold change versus CD4+ T cell count (B) and age of the volunteers (C). The solid lines and dotted lines represent the best fitted lines and the 95% confidence intervals respectively.
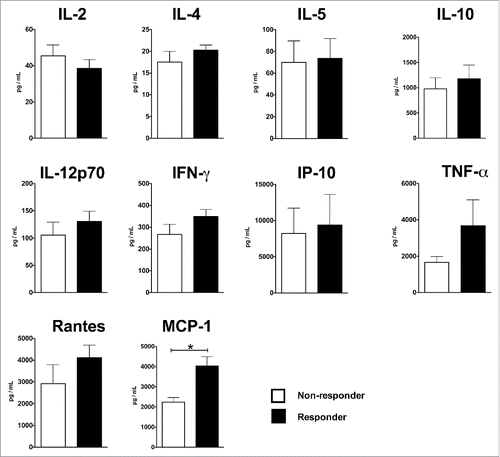
To determine if there is any inherent differences in the innate response to external stimulant between the PBMC derived from R and NR groups, PBMC from the 2 groups were stimulated with LPS in vitro, using an experimental setup similar to the one described in Corsini et al..Citation13. Forty-eight hours later, concentrations of various cytokines/chemokines in the culture supernatants were determined by the bioplex assay. As shown in , the NR group had weaker production of TNF-α, Rantes and MCP-1, and the difference for MCP-1 reached statistical significance (p = 0.0024). For the rest of the cytokines/chemokines examined, the 2 groups' responses to LPS stimulation were comparable.
Figure 6. Cytokine production profile of HIV+ R and HIV+ NR after 48 hrs of in vitro LPS stimulation. Five million of PBMC were stimulated with 1 μg per mL of LPS for 48 hours. Concentrations of various cytokines were determined by Bioplex assay. The bars and the error bars represent the mean and standard deviation of the groups. Note that, due to the differences in the expression levels of the various cytokines, the scales of the y axes are different. *indicates p < 0.05.
Discussion
As HIV-infected patients belong to a higher risk group in developing serious influenza-related complications, seasonal influenza vaccination is recommended.Citation2 However, a number of studies have shown that seasonal influenza vaccines are poorly immunogenic in the HIV-positive population.Citation7-10 In our study, only 3 volunteers had sero-protective titers against all 3 targeted viruses. Most responders responded to the H1N1 and/or the H3N2 viruses and only 14 volunteers had protective immunity against the influenza B virus. The poor sero-conversion rate in the HIV population could explain why influenza virus remain the primary cause of febrile respiratory illness for HIV-infected patients,Citation14 despite routine seasonal influenza vaccination. Clearly, there is a need to improve the immunogenicity of influenza vaccine for the HIV-infected population. While a number of trials have reported that live attenuated influenza vaccine (LAIV) is effective and well-tolerated in HIV-infected patients without prolonged shedding of the vaccine virus,Citation15-17 TIV remains the vaccine platform recommended for immunocompromised patients.Citation2 To improve the immunogenicity of TIV, strategies such as giving a booster or immunizing patients with higher dose of vaccine have been tested but the results showed that these strategies do not improve vaccine performance.Citation18,19
In our study, the PBMC from the R population showed a downregulation of IL-10 mRNA expression with a higher TNF-α expression relative to the non-responder (NR) population 2 d after vaccination (). Since TIV is well tolerated in humans without significant adverse side effects, therefore, the activation of the innate immunity by the vaccine would be expected to be moderate. Nevertheless, the ex vivo results () show a link between sero-conversion upon vaccination with cytokine responses of the PBMC of the HIV cohort—reduced mRNA expression of IL-10, with an increased expression of TNF-α and IFN-γ for responders.
Immunogenicity of vaccine preparations can be improved by incorporating various Toll-like receptor (TLR) ligands into the formulations.Citation20-24 The production of pro-inflammatory cytokines is thought to be one of the main mechanisms for the enhancement effect. Pro-inflammatory cytokines, such as IL-12, is able to enhance CD4+ T cell functionsCitation25,26 and bias toward Th1 responses.Citation27 The use of a TLR-ligand has been shown to potentiate Th1 response in mice and Rhesus macaques.Citation22,28 Restoring a strong Th1 response is important as an impaired Th1 response has been linked to age-related decline in vaccine efficacy.Citation29 Moreover, TLR-ligand, such as Poly I:C is capable of restoring age-related intrinsic declines in CD4+ T cell function in miceCitation30 and certain CD4+ T cells in mice are capable of interacting with Poly I:C through their TLR3.Citation31 This interaction can promote the survival of these CD4+ T cells directly. Furthermore, exogenous pro-inflammatory cytokines have been shown to be capable of overcoming the diminished IL-2 production and limited expansion capability of aged CD4+ T cells.Citation32 Our in vitro LPS stimulation experiment shows that the non-responders' PBMC can be activated by an external stimulant comparably to those observed with responders' PBMC. A number of clinical studies have reported improved sero-conversion using adjuvanted influenza vaccines with HIV-infected individuals.Citation33,34 In addition, it has been reported that HIV-infected individuals are hyporesponsive to hepatitis B virus vaccine as well and the inclusion of CPG7909 (an analog of CpG motifs that targets TLR9) in the vaccine enhanced the magnitude, as well as longevity of the protective humoral responses.Citation35,36 In short, due to a dysfunctional adaptive immune system, the use of immuno-modulators for more effective activation of the innate immune system could be the key to improve vaccine sero-conversion rate in the HIV-infected population.
Apart from HAI test, ELISA has also been used to detect HA-specific antibodies in serum.Citation37 In our study, there is generally a good correlation between the increments in ELISA and HAI titers among the responders after the vaccination. However, some discrepancies were observed between the results of the 2 assays. An increase in HAI titer without a rise in ELISA titer was observed in some H1N1 sero-converted volunteers. Increased avidity of the antibodies to the HA protein can lead to more effective agglutination of the virion, possibly accounting for this observation of the atypical responders. Apart from improved avidity, other mechanisms might also be involved, as a significant portion of the H3N2 and influenza B sero-converted volunteers showed an increase in HAI titers with neither a significant increase in ELISA titer nor avidity (69% and 100% of the sero-converted, respectively). As our volunteers were not immunologically naïve prior to vaccination, pre-existing B cells acquired from previous vaccinations or infections could have undergone further affinity maturation in response to the current vaccination. We have not been able to examine the changes of clonal expansion / distribution of memory B cells in this study, which warrant future studies. Finally, in our study, we identified responders that showed a significant increase in ELISA titer without an increase in HAI titer. Antibodies that target non-HAI regions of the HA proteins or other surface structural proteins, such as NA, could be a possible explanation. Anti-NA antibodies can reduce the severity of illness in humansCitation38 and animals.Citation39,40 Transient spike of anti-NP IgG shortly after vaccination has been reported in human and this rise could be attributed by the presence of NP found in TIV.Citation41 Although NP is an internal structural protein that is not found on the virion surface, the presence of detergent in our incubation buffer and wash buffers, might allow the anti-NP antibody to bind to the NP of the coating virion in the ELISA test. Nevertheless, the protective value of anti-NP antibody is in question as a high concentration of anti-NP antibody is required to protect mice against lethal influenza challenge.Citation12
One of the limitations of our study is the small sample size. Nevertheless, our results show that, looking at individual titer per se, many HIV patients did not have protective antibody responses following TIV immunization. The finding is consistent with observations reported in other trials.Citation7-10,42,43 Another limitation is that we only tested the serological responses at one time point post vaccination, assuming the activation and maturation of vaccine-induced B lymphocytes would be completed by Day 21. Future studies could include an additional time point. Finally, as a follow-up on the incidence of clinical influenza infection of the cohort would require a much larger sample size, another limitation for this study is the use of vaccine-induced antibody responses as a correlate of protection.
In summary, our results highlight the sub-optimal performance of the TIV in the HIV-infected population in Singapore and the need for a better vaccine. This issue can potentially be addressed by harnessing the innate immune system through modulating cytokine responses with an adjuvant.
Material and methods
Volunteers
Eighty-one participants were recruited from the National HIV Referral Center at Communicable Disease Center, Tan Tock Seng Hospital, Singapore. All study participants provided written informed consent. The protocols (2006/00328) were approved by Domain Specific Review Board, National Healthcare Group, Singapore.
Viruses
A/New Caledonia/20/99 (H1N1) and A/Winconsin/67/2005 (H3N2) were provided by Dr Kanta Subbarao at the National Institute of Allergy and Infectious Diseases. B/Malaysia/2506/2004 was obtained from WHO Collaborating Center for Reference and Research on Influenza at Melbourne, Australia. The viruses were amplified in Madin-Darby Canine Kidney (MDCK) cells in Eagle's minimal essential medium (EMEM, Lonza, Singapore) supplemented with L-glutamine, sodium pyruvate and 1 μg per mL of TPCK-treated trypsin (Worthington Biochemical Corporation, Lakewood, NJ, USA). The supernatants were harvested 72 hours later, aliquoted and stored at −80°C. For ultra-violet (UV) light inactivation, the virus-containing supernatants were exposed to UV light (Biolux, Atlantic ultraviolet corporation, Hauppauge, NY) for 2 hours on ice before storage.
Vaccination
All study participants received a commercial 2006-2007 trivalent influenza vaccine (TIV, Fluvax, CSL limited) containing 15 μg of hemagglutinin (HA) antigen from A/New Caledonia/20/99, A/Winconsin/67/2005 and B/Malaysia/2506/2004. The vaccine was given intramuscularly in the deltoid muscle region. The participants were observed for local and / or systemic reactogenicity. No serious adverse side effect was reported in the study.
Serum collection
Blood samples were collected just prior to vaccination (D0) and 21 d later (D21). For serum collection, the blood samples were collected in plain tubes and allowed to clot at room temperature (RT) for 1 hour. The tubes were spun at 3000 rpm for 10 min and serum samples were aliquoted and stored at −20°C.
Peripheral blood mononuclear cell (PBMC) isolation
Venous blood samples were collected at various time-points using sodium citrate tube (0.5M, BD Medical, NJ, USA). The blood samples were overlaid on 5 mL of Histopaque-1077 (Sigma-aldrich, St.Louis, MO) and spun at 3000 rpm at RT for 20 min without brake. Buffy coat was collected and washed with plain RPMI-1640 (Gibco, Invitrogen) twice. Red blood cells were lysed using commercial lysis buffer. Cells were re-suspended in T cell medium (TCM) which consists of RPMI-1640 supplemented with 10% heat-inactivated fetal calf serum (FCS); streptomycin; penicillin, sodium pyruvate, L-glutamine and 2-mercaptoethanol. All supplements were obtained from Gibco.
Enumeration of CD4+ T cells in blood
The number of CD4+ T cells in peripheral blood was determined by BD multi-test kit (BD Biosciences, CD3/CD8/CD45/CD4 with trucount tubes). The assay was performed according to the manufacturer's instructions. The samples were analyzed using a FACSCalibur (BD Biosciences, San Jose, CA).
In-vitro LPS stimulation
Five million PBMC were cultured in 5 mL of TCM and stimulated with or without LPS from E.coli (0111 strain, 1 μg per mL, Sigma-Aldrich). The flasks were incubated at 37°C with 5% CO2. PBMC were stimulated for 48 hours for cytokine / chemokine production. The supernatants were harvested and stored at −20°C till analysis. Supernatants were analyzed using the Bio-plex Pro Human Cytokine 27-plex assay and the Bio-Plex 200 reader (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions.
Receptor-destroying enzyme (RDE) treatment
Serum samples were incubated overnight at 37°C with RDE (Denka Seiken Co., LTD, Tokyo, Japan) in a ratio (vol/vol) of 1:3. Samples were then incubated at 56°C for 30 min to inactivate the RDE.
ELISA
Ninety–six-well microtiter plates (MaxiSorp, Nalge Nunc International, Rochester, NY) were coated with 50 μL of UV-inactivated influenza viruses at 1000 hemagglutinin (HA) unit per mL in PBS overnight at 4°C. The plates were washed with 0.1% Tween 20 in PBS (PBST) followed by blocking with 200 μL of 1% bovine serum albumin (BSA) in PBS for 2 hours. RDE-treated serum samples were diluted 1:1000 and subsequently serially diluted in a half-log fashion. The plates were incubated overnight at 4°C. The plates were washed 8 times and bound antibodies were detected with 50 μL of horseradish peroxidase-conjugated polyclonal rabbit anti-human immunoglobulin antibody (P0212, Dako Denmark, 1:1000 dilution). Three hours later, the plates were washed and 100 μl of ABTS (A3912, Sigma-Aldrich) was added. The reaction was stopped 15 mins later with 100 μL of 1% SDS. The optical density (OD) was measured by a microplate reader at 405 nm with a reference wavelength of 450 nm. The titers of antibody are expressed as the reciprocal of the highest dilution of serum to achieve an OD of 0.2. Changes in antibody titers > 0.3 between the paired samples (D0 and D21) is considered significant. All paired samples were tested on the same plate for consistency.
Avidity ELISA
The coating conditions of avidity ELISA were similar to ELISA. Based on the ELISA titers, the D0 and D21 samples of an individual were diluted to similar concentrations. After overnight incubation, the plates were washed 4 times with PBST. Different concentrations of sodium thiocyanate in PBS (Sigma-aldrich) were then added and incubated at room temperature for 15 mins before washing 8 times with PBST. Subsequent steps were performed similarly to ELISA. The percentage of retention was calculated by expressing the O.D. of the sodium thiocyanate-treated wells relative to those of PBS-treated wells.
Influenza-specific hemagglutinin inhibition (HAI) assay
RDE-treated serum samples were diluted 1:10 and subsequently in serial 2-fold dilutions with saline in U-bottom 96-well microtiter plates (Nalge Nunc). The samples were incubated with 4 HA unit of the viruses for 15 mins at RT followed by 1% guinea pig red blood cells in PBS. Plates were scored 1 hour later for hemagglutination. For each virus, all the samples were tested on the same day for consistency. Sero-conversion for influenza vaccination is defined as a 4-fold increase in HAI titers after vaccination.
RNA extraction and real-time PCR
RNA from PBMC was extracted using RNeasy kit (Qiagen Inc. Valenica, CA). The RNA was stored at −80°C till analysis. The RNA was reverse transcribed into cDNA using SuperScript III reverse transcriptase (Invitrogen, NY). The expression level of various cytokines was determined using a lightcycler (LC480, Roche Applied Science, IN) with LightCycler 480 probes Master mix. The primers, probes and running conditions are based on a publication from Giulietti et al.Citation44 Paired pre- and post-vaccination RNA samples from each individual were reverse transcribed and quantified in duplicates concurrently. The gene expression level of each cytokine gene was normalized with β-actin expression.
Statistical analysis
For sample size calculation, the α, β values were set at 0.05 and 0.2 respectively. Standard deviation of change was estimated as 1.0. The minimal sample size was 31. (http://www.sample-size.net/sample-size-study-paired-t-test/). Data are expressed in mean and standard deviation. Statistical analysis was assessed by the Mann-Whitney test using Prism 5 (GraphPad Software, San Diego, CA). p values of <0 .05 are considered significantly different.
Disclosure of potential conflicts of interest
All authors have no conflict of interest to declare.
Funding
This study was supported by DSO National Laboratories.
References
- Ministry of Health, Singapore; n.d.; available from https://www.moh.gov.sg/content/moh_web/home/diseases_and_conditions/h/hiv_aids.html
- Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recommendations Reports 2010; 59:1-62
- Hazenberg MD, Hamann D, Schuitemaker H, Miedema F. T cell depletion in HIV-1 infection: how CD4+ T cells go out of stock. Nat Immunol 2000; 1:285-9; PMID:11017098; http://dx.doi.org/10.1038/79724
- Yamanaka H, Teruya K, Tanaka M, Kikuchi Y, Takahashi T, Kimura S, Oka S, HIV/Influenza Vaccine Study Team. Efficacy and immunologic responses to influenza vaccine in HIV-1-infected patients. J Acquir Immune Defic Syndr 2005; 39:167-73; PMID:15905732
- Tebas P, Frank I, Lewis M, Quinn J, Zifchak L, Thomas A, Kenney T, Kappes R, Wagner W, Maffei K, et al. Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS 2010; 24:2187-92; PMID:20616698; http://dx.doi.org/10.1097/QAD.0b013e32833c6d5c
- Kroon FP, van Dissel JT, de Jong JC, van Furth R. Antibody response to influenza, tetanus and pneumococcal vaccines in HIV-seropositive individuals in relation to the number of CD4+ lymphocytes. AIDS 1994; 8:469-76; PMID:7912086; http://dx.doi.org/10.1097/00002030-199404000-00008
- Fuller JD, Craven DE, Steger KA, Cox N, Heeren TC, Chernoff D. Influenza vaccination of human immunodeficiency virus (HIV)-infected adults: impact on plasma levels of HIV type 1 RNA and determinants of antibody response. Clin Infect Dis 1999; 28:541-7; PMID:10194075; http://dx.doi.org/10.1086/515170
- Günthard HF, Wong JK, Spina CA, Ignacio C, Kwok S, Christopherson C, Hwang J, Haubrich R, Havlir D, Richman DD. Effect of influenza vaccination on viral replication and immune response in persons infected with human immunodeficiency virus receiving potent antiretroviral therapy. J Infect Dis 2000; 181:522-31; PMID:10669335; http://dx.doi.org/10.1086/315260
- Kroon FP, van Dissel JT, de Jong JC, Zwinderman K, van Furth R. Antibody response after influenza vaccination in HIV-infected individuals: a consecutive 3-year study. Vaccine 2000; 18:3040-9; PMID:10825608; http://dx.doi.org/10.1016/S0264-410X(00)00079-7
- Machado AA, Machado CM, Boas LSV, Lopes MC, Gouvêa A de FB, Succi RC de M, Mendoza TRT, Kanashiro TM, Machado DM. Short communication: immunogenicity of an inactivated influenza vaccine and postvaccination influenza surveillance in HIV-infected and noninfected children and adolescents. AIDS Res Hum Retroviruses 2011; 27:999-1003; PMID:21284525; http://dx.doi.org/10.1089/aid.2010.0306
- Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19:1597-608; PMID:24309663; http://dx.doi.org/10.1038/nm.3409
- Lamere MW, Moquin A, Lee FE-H, Misra RS, Blair PJ, Haynes L, Randall TD, Lund FE, Kaminski DA. Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance. J Virol 2011; 85:5027-35; PMID:21367900; http://dx.doi.org/10.1128/JVI.00150-11
- Corsini E, Vismara L, Lucchi L, Viviani B, Govoni S, Galli CL, Marinovich M, Racchi M. High interleukin-10 production is associated with low antibody response to influenza vaccination in the elderly. J Leukoc Biol 2006; 80:376-82; PMID:16707559; http://dx.doi.org/10.1189/jlb.0306190
- Klein MB, Lu Y, DelBalso L, Coté S, Boivin G. Influenzavirus infection is a primary cause of febrile respiratory illness in HIV-infected adults, despite vaccination. Clin Infect Dis 2007; 45:234-40; PMID:17578785; http://dx.doi.org/10.1086/518986
- King JC, Fast PE, Zangwill KM, Weinberg GA, Wolff M, Yan L, Newman F, Belshe RB, Kovacs A, Deville JG, et al. Safety, vaccine virus shedding and immunogenicity of trivalent, cold-adapted, live attenuated influenza vaccine administered to human immunodeficiency virus-infected and noninfected children. Pediatr Infect Dis J 2001; 20:1124-31; PMID:11740317; http://dx.doi.org/10.1097/00006454-200112000-00006
- King J, Treanor J, Fast P, Wolff M, Yan L, Iacuzio D, Readmond B, O'Brien D, Mallon K, Highsmith W, et al. Comparison of the safety, vaccine virus shedding, and immunogenicity of influenza virus vaccine, trivalent, types A and B, live cold-adapted, administered to human immunodeficiency virus (HIV)-infected and non-HIV-infected adults. J Infect Dis 2000; 181:725-8; PMID:10669363; http://dx.doi.org/10.1086/315246
- Levin MJ, Song L-Y, Fenton T, Nachman S, Patterson J, Walker R, Kemble G, Allende M, Hultquist M, Yi T, et al. Shedding of live vaccine virus, comparative safety, and influenza-specific antibody responses after administration of live attenuated and inactivated trivalent influenza vaccines to HIV-infected children. Vaccine 2008; 26:4210-7; PMID:18597900; http://dx.doi.org/10.1016/j.vaccine.2008.05.054
- Miotti PG, Nelson KE, Dallabetta GA, Farzadegan H, Margolick J, Clements ML. The influence of HIV infection on antibody responses to a two-dose regimen of influenza vaccine. JAMA 1989; 262:779-83; PMID:2787416; http://dx.doi.org/10.1001/jama.1989.03430060075029
- Cooper C, Thorne A, Klein M, Conway B, Boivin G, Haase D, Shafran S, Zubyk W, Singer J, Halperin S, et al. Immunogenicity is not improved by increased antigen dose or booster dosing of seasonal influenza vaccine in a randomized trial of HIV infected adults. PLoS One 2011; 6:e17758; PMID:21512577; http://dx.doi.org/10.1371/journal.pone.0017758
- Harrington DG, Crabbs CL, Hilmas DE, Brown JR, Higbee GA, Cole FE, Levy HB. Adjuvant effects of low doses of a nuclease-resistant derivative of polyinosinic acid. polycytidylic acid on antibody responses of monkeys to inactivated venezuelan equine encephalomyelitis virus vaccine. Infect Immun 1979; 24:160-6; PMID:110688
- Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol 2002; 169:4905-12; PMID:12391202; http://dx.doi.org/10.4049/jimmunol.169.9.4905
- Lau YF, Tang L-H, McCall AW, Ooi EE, Subbarao K. An adjuvant for the induction of potent, protective humoral responses to an H5N1 influenza virus vaccine with antigen-sparing effect in mice. J Virol 2010; 84:8639-49; PMID:20538850; http://dx.doi.org/10.1128/JVI.00596-10
- McCluskie MJ, Weeratna RD, Krieg AM, Davis HL. CpG DNA is an effective oral adjuvant to protein antigens in mice. Vaccine 2000; 19:950-7; PMID:11115721; http://dx.doi.org/10.1016/S0264-410X(00)00215-2
- Gallichan WS, Woolstencroft RN, Guarasci T, McCluskie MJ, Davis HL, Rosenthal KL. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J Immunol 2001; 166:3451-7; PMID:11207303; http://dx.doi.org/10.4049/jimmunol.166.5.3451
- Pape KA, Khoruts A, Mondino A, Jenkins MK. Inflammatory cytokines enhance the in vivo clonal expansion and differentiation of antigen-activated CD4+ T cells. J Immunol 1997; 159:591-8; PMID:9218573
- Curtsinger JM, Schmidt CS, Mondino A, Lins DC, Kedl RM, Jenkins MK, Mescher MF. Inflammatory cytokines provide a third signal for activation of naive CD4+ and CD8+ T cells. J Immunol 1999; 162:3256-62; PMID:10092777
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003; 3:133-46; PMID:12563297; http://dx.doi.org/10.1038/nri1001
- Stahl-Hennig C, Eisenblätter M, Jasny E, Rzehak T, Tenner-Racz K, Trumpfheller C, Salazar AM, Uberla K, Nieto K, Kleinschmidt J, et al. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog 2009; 5:e1000373; PMID:19360120; http://dx.doi.org/10.1371/journal.ppat.1000373
- Deng Y, Jing Y, Campbell AE, Gravenstein S. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol 2004; 172:3437-46; PMID:15004143; http://dx.doi.org/10.4049/jimmunol.172.6.3437
- Maue AC, Eaton SM, Lanthier PA, Sweet KB, Blumerman SL, Haynes L. Proinflammatory adjuvants enhance the cognate helper activity of aged CD4 T cells. J Immunol 2009; 182:6129-35; PMID:19414765; http://dx.doi.org/10.4049/jimmunol.0804226
- Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol 2004; 172:6065-73; PMID:15128790; http://dx.doi.org/10.4049/jimmunol.172.10.6065
- Haynes L, Eaton SM, Burns EM, Rincon M, Swain SL. Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo. J Immunol 2004; 172:5194-9; PMID:15100256; http://dx.doi.org/10.4049/jimmunol.172.9.5194
- Launay O, Desaint C, Durier C, Loulergue P, Duval X, Jacomet C, Pialoux G, Ghosn J, Raffi F, Rey D, et al. Safety and immunogenicity of a monovalent 2009 influenza A/H1N1v vaccine adjuvanted with AS03A or unadjuvanted in HIV-infected adults: a randomized, controlled trial. J Infect Dis 2011; 204:124-34; PMID:21628666; http://dx.doi.org/10.1093/infdis/jir211
- Fabbiani M, Di Giambenedetto S, Sali M, Farina S, Sansonetti P, Tamburrini E, Dal Verme LZ, Delogu G, De Luca A, Kelvin D, et al. Immune response to influenza A (H1N1)v monovalent MF59-adjuvanted vaccine in HIV-infected patients. Vaccine 2011; 29:2836-9; PMID:21349364; http://dx.doi.org/10.1016/j.vaccine.2011.02.020
- Cooper CL, Davis HL, Angel JB, Morris ML, Elfer SM, Seguin I, Krieg AM, Cameron DW. CPG 7909 adjuvant improves hepatitis B virus vaccine seroprotection in antiretroviral-treated HIV-infected adults. AIDS 2005; 19:1473-9; PMID:16135900; http://dx.doi.org/10.1097/01.aids.0000183514.37513.d2
- Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis 2008; 46:1310-4; PMID:18444872; http://dx.doi.org/10.1086/533467
- Murphy BR, Phelan MA, Nelson DL, Yarchoan R, Tierney EL, Alling DW, Chanock RM. Hemagglutinin-specific enzyme-linked immunosorbent assay for antibodies to influenza A and B viruses. J Clin Microbiol 1981; 13:554-60; PMID:7240388
- Murphy BR, Kasel JA, Chanock RM. Association of serum anti-neuraminidase antibody with resistance to influenza in man. N Engl J Med 1972; 286:1329-32; PMID:5027388; http://dx.doi.org/10.1056/NEJM197206222862502
- Schulman JL, Khakpour M, Kilbourne ED. Protective effects of specific immunity to viral neuraminidase on influenza virus infection of mice. J Virol 1968; 2:778-86; PMID:5701819
- Chen Z, Yoshikawa T, Kadowaki S, Hagiwara Y, Matsuo K, Asanuma H, Aizawa C, Kurata T, Tamura S. Protection and antibody responses in different strains of mouse immunized with plasmid DNAs encoding influenza virus haemagglutinin, neuraminidase and nucleoprotein. J Gen Virol 1999; 80(Pt 10):2559-64; PMID:10573147; http://dx.doi.org/10.1099/0022-1317-80-10-2559
- García-Cañas V, Lorbetskie B, Bertrand D, Cyr TD, Girard M. Selective and quantitative detection of influenza virus proteins in commercial vaccines using two-dimensional high-performance liquid chromatography and fluorescence detection. Anal Chem 2007; 79:3164-72; http://dx.doi.org/10.1021/ac0621120
- Hakim H, Allison KJ, Van De Velde LA, Li Y, Flynn P, McCullers JA. Immunogenicity and safety of inactivated monovalent 2009 H1N1 influenza A vaccine in immunocompromised children and young adults. Vaccine 2011; 30(5):885
- Zanetti AR, Amendola A, Besana S, Boschini A, Tanzi E. Safety and immunogenicity of influenza vaccination in individuals infected with HIV. Vaccine 2002; 20 Suppl 5:B29-32; http://dx.doi.org/10.1016/S0264-410X(02)00511-X
- Giulietti A, Overbergh L, Valckx D, Decallonne B, Bouillon R, Mathieu C. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 2001; 25:386-401; PMID:11846608; http://dx.doi.org/10.1006/meth.2001.1261
