ABSTRACT
No indigenous cases of poliomyelitis have occurred in the US since 1979; however the risk of importation persists until global eradication is achieved. The seropositivity rate for different age cohorts with exposures to different poliovirus vaccine types and wild virus in the US are not presently known.
A convenience sample was conducted in the Kansas City metropolitan area during 2012–2103 with approximately 100 participants enrolled for each of 5 age cohorts categorized based on vaccine policy changes over time in the US. Immunization records for poliovirus vaccination were required for participants <18 y of age. We evaluated the prevalence of serum antibodies to all 3 poliovirus serotypes. Seroprevalence was evaluated by demographics as well as between polio serotypes.
The overall seroprevalence to poliovirus was 90.7%, 94.4%, and 83.3%, for types 1, 2, and 3, respectively. Seroprevalence was high (88.6%–96.2%) for all 3 types of poliovirus for the 6–10 y old age group that was likely to have received a complete schedule of IPV-only vaccination. Children 2–3 y of age, who have not yet completed their full IPV series, had lower seroprevalence compared with all older age groups for types 1 and 2 (p-value <0. 05).
Seroprevalence was high for all 3 types of poliovirus in the population surveyed. Seroprevalence for subjects aged 2–3 y was lower than all other age groups for serotypes 1 and 2 highlighting the importance of completing the recommended poliovirus vaccine series with a booster dose at age 4–6 y.
Background
Pakistan and Afghanistan remain the only 2 countries where wild poliovirus (WPV) transmission has never been interrupted.Citation1-3 While the last cases of indigenously acquired WPV in the United States (US) occurred in 1979, the last WPV case in a US resident traveling abroad occurred in 1986, and the last WPV imported case occurred in 1993. Due to continued WPV transmission in a few remaining areas of the world, the Centers for Disease Control and Prevention (CDC) has provided interim vaccination guidance for travel to and from countries affected by wild poliovirus.Citation4
Additionally, circulating vaccine-derived poliovirus (cVDPV) must also be eliminated before polio eradication is achieved.Citation5 cVDPVs can occur from live vaccine virus in areas of low vaccine coverage. In 2015, cVDPVs represented 30% of the reported global polio cases.Citation6 From 1997 to 1999, the US implemented a sequential inactivated poliovirus vaccine (IPV) – oral poliovirus vaccine (OPV) schedule. Since 2000, the US has exclusively used IPV to prevent vaccine-associated paralytic poliovirus cases (VAPP), which averaged 8–10 cases per year in the US when OPV was routinely recommended.Citation7 Since that time, the recommended routine schedule is IPV at age 2, 4, and 6–18 months with a booster dose at age 4–6 y. No systematic serosurveys for poliovirus antibodies have been conducted in the US since the return to an all-IPV recommendation after the initial use of IPV in the 1950s and early 1960s.Citation8,9 In the past, population based polio serosurveys have not been used to monitor population immunity to polio in the US. This study describes the findings of a serosurvey conducted in the Kansas City metropolitan area during 2012–13.
Results
Study participants
504 persons aged 2–81 y were recruited through Children's Mercy Hospital and Turner Medical Center systems in the Kansas City Metropolitan area in 2012–2013. All participants were interviewed with a survey instrument and serum samples were obtained. Age and demographic characteristics are provided in .
Table 1. Characteristics of Serosurvey Participants, Kansas City Metropolitan Area, 2012–2013
Overall seroprevalence of poliovirus antibody for types 1, 2, and 3
During 2012–2013, among a regional population that was reasonably representative of the racial/ethnic makeup of the census of the Kansas City Metropolitan area and aged 2–81 y, overall poliovirus seroprevalence for types 1, 2, and 3 was 90.7% (95% CI: 88.1%-93.2%), 94.4% (95% CI: 92.4%-96.5%) and 83.3% (95% CI: 80.1%-86.6%), respectively. Seroprevalence was higher for type 2 compared with type 1 and type 3 (p < 0.05 and p < 0.001, respectively) and seroprevalence for type 1 was also higher than type 3 (p = 0.001). For males, seroprevalence to type 1 and type 2 was higher than type 3 (p < 0.05 and p < 0.001, respectively); for females, seroprevalence to type 2 was higher than type 3 (p = 0.001). For those US. Born, seroprevalence for type 2 was higher compared with type 1 and type 3 (p < 0.05 and p < 0.001, respectively) and seroprevalence for type 1 was also higher than type 3 (p = 0.001). Similar results were found for subjects with neither parent born abroad.
Seroprevalence of poliovirus type 1 antibody
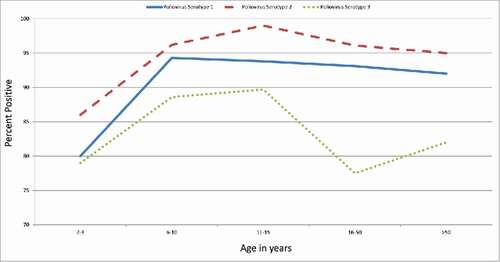
Poliovirus seroprevalence was lower among those aged 2–3 y compared with all other age groups (p < 0.05 for each comparison, and ). No other differences by age group were found. When stratified by gender, those aged 2–3 y had a lower seroprevalence than all other age groups for males (p < 0.05 for each comparison). No other age group differences were observed for males. No differences by age groups were observed for females. For those aged 2–3 years, males had a lower seroprevalence than females (p < 0.05).
Table 2. Percent positive by poliovirus serotype among serosurvey participants, Kansas City Metropolitan Area, 2012–2013
No overall differences were observed when comparing Black and White race. When stratified by age group, Blacks had higher seroprevalence than Whites for the age groups 6–10, 11–15, and >50 y (p < 0.05 for each comparison). The sample size was insufficient to evaluate differences between other race categories. No overall differences were observed comparing Hispanic to Non-Hispanic ethnicity. But when stratified by age group, Hispanics had a higher seroprevalence than Non-Hispanics for the 11–15 y age group (p < 0.05).
No overall differences were observed by US birth status, mother born abroad status, or father born abroad status. But when stratified by age group, Non-US born had a higher seroprevalence than US born for the >50 y age group (p < 0.05). No differences were observed for the 16–50 y age group; the sample size was insufficient to evaluate differences by the other age groups for US born status. Subjects with either parent born abroad had a higher seroprevalence for the 6–10 y age group and lower seroprevalence for the 16–50 y age group (p < 0.05 for each comparison). No other differences by age group and either parent born abroad status were observed.
No overall differences were observed for subject travel abroad or household member travel abroad. But when stratified by age group, for the 6–10 y age group, both the subject group that traveled abroad and the group with household members who traveled abroad had a higher seroprevalence than the group that did not travel abroad (p < 0.05 for each comparison). No other differences were observed by age groups for travel status.
Seroprevalence of poliovirus type 2 antibody
Poliovirus seroprevalence was lower among those aged 2–3 y compared with all other age groups (p < 0.001 for 11–15 years, p < 0.05 compared with the other age groups, ). No other differences by age group were found. When stratified by gender, those aged 2–3 y had a lower seroprevalence than all other age groups for males (p < 0.001 for 11–15 years, p < 0.05 compared with the other age groups). No other differences between other age groups were observed for males. No differences by age groups were observed for females. No differences were observed between males and females in each age group.
No overall differences were observed comparing Black and White race. But when stratified by age, Blacks had higher seroprevalence than Whites for the age groups 2–3 and 6–10 y (p < 0.05 for each comparison). The sample size was insufficient to evaluate differences between other race categories. No overall differences were observed comparing Hispanic to Non-Hispanic ethnicity. But when stratified by age, Hispanics had a higher seroprevalence than Non-Hispanics for the 6–10 y age group (p < 0.05).
No overall differences were observed by US born status, mother born abroad status, or father born abroad status. But when stratified by age, no differences were observed for the 16–50 and >50 y age group; the sample size was insufficient to evaluate differences by the other age groups for US born status. The group either parent born abroad had a higher seroprevalence for the 6–10 age group (p < 0.05). No other differences by age group and either parent born abroad status was observed.
No overall differences were observed for the group that traveled abroad or had a household member who traveled abroad. But when stratified by age, the 6–10 y age group, those who travel abroad had a higher seroprevalence than the 6–10 y age group that did not travel abroad (p < 0.05). For the 2–3 y age group, the group with a household member who traveled abroad had a lower seroprevalence than the same age group in which no family member traveled abroad (p < 0.05). No other differences were observed by age groups for travel status (sample size was insufficient to evaluate subject traveled abroad for the 2–3 y age group).
Seroprevalence of poliovirus type 3 antibody
Poliovirus seroprevalence was lower among those aged 2–3 y compared with those aged 11–15 years; those aged 16–50 y had lower seroprevalence than those aged 6–10 and 11–15 y (p < 0.05 for each comparison, ). When stratified by gender, males aged 2–3 y had a lower seroprevalence than the males aged 6–10 y (p < 0.05). No other differences between age groups were observed for males and no differences by age groups were observed for females. For those aged 2–3 years, males had a lower seroprevalence the females (p < 0.05).
Seroprevalence was higher overall for Black race compared with White (p < 0.001). The sample size was insufficient to evaluate differences between other race categories. When analyzing age groups by race, Blacks had higher seroprevalence than Whites for the age groups 2–3, 6–10, and 11–15 y (p < 0.001, p < 0.001, p < 0.05, respectively). No differences were observed for overall seroprevalence comparing Hispanic to Non-Hispanic ethnicity. But when analyzing age groups among Hispanics vs. Non-Hispanics, Hispanics had a higher seroprevalence than Non-Hispanics for the 6–10 and 11–15 y age group (p≤ 0.001).
No differences were observed for overall seroprevalence by US born status, mother born abroad status, or father born abroad status. Analysis by age group showed no differences for the 16–50 and >50 y age group; the sample size was insufficient to evaluate differences by the other age groups for US born status. Subjects with either parent born abroad had a higher seroprevalence for the 6–10 and 11–15 y age group (p < 0.001, <0.05, respectively). No other differences by age group and either parent born abroad status was observed.
No differences were observed for travel abroad or having a household member travel abroad. No differences were observed when comparing age groups for travel status of subjects or household members.
Comparison of seroprevalence of poliovirus antibody for types 1, 2, and 3
Seroprevalence for serotype 2 was higher than serotype 3 for age groups 6–10, 11–15, 16–50, and >50 y (p < 0.05, p < 0.05, p < 0.001, and p < 0.05, respectively, ). Seroprevalence for serotype 1 was higher than serotype 3 for age groups 16–50 and >50 y (p < 0.05 for each comparison). No other differences between serotypes by age group were observed.
Seroprevalence for serotype 3 was lower than serotype 1 and 2 for Whites (p < 0.001). There were no significant differences for Blacks. Seroprevalence for non-Hispanics was higher for serotype 1 compared with serotype 3, and for serotype 2 compared with serotype 1 and 3 (p = 0.001, p < 0.05, p < 0.001, respectively).
Seroprevalence for non-U.S born was higher for serotype 1 compared with serotype 3, and for serotype 2 compared with serotype 1 and 3 (p = 0.001, p < 0.05, p < 0.001, respectively). Seroprevalence for the group with mother not born abroad was higher compared with the group with a mother born abroad for serotype 1 compared with serotype 3, and for serotype 2 compared with serotype 1 and 3 (p < 0.001, p < 0.05, p < 0.001, respectively). Seroprevalence for father not born abroad was higher compared with the group with father born abroad for serotype 1 compared with serotype 3, and for serotype 2 compared with serotypes 1 and 3 (p < 0.001, p < 0.05, p < 0.001, respectively).
Seroprevalence by vaccine status
All subjects aged <18 y with documentation of no poliovirus vaccine were negative for all 3 serotypes (N = 5). Seven of 100 subjects aged 2–3 y were negative to all 3 serotypes (2 unvaccinated, 1 with one poliovirus vaccine dose, 1 with 2 doses, and 3 with 3 doses). Five of 209 6–17 y old subjects, who were eligible for a complete poliovirus vaccine schedule, were negative to all 3 serotypes (3 unvaccinated and 2 with a complete vaccine schedule of 4 or 5 vaccine doses). Two of 195 subjects aged ≥ 18 y were negative to all 3 serotypes (vaccine records unavailable).
Discussion
Overall seroprevalence for antibodies against poliovirus serotypes 1–3 was high in the study population aged 2–81 y during 2012–2013 consistent with high vaccine coverage in the study and in the overall United States. Of note, seroprevalence for subjects aged 2–3 y was lower than all other age groups for serotypes 1 and 2, highlighting the importance of completing the recommended poliovirus vaccine series with a booster dose at age 4–6 y. The seroprevalence levels in this age group were lower than what was found during the transition for OPV to IPV among subject that were first eligible for an all-IPV schedule.Citation9 This may be due to children no longer being exposed to live-vaccine virus either through vaccine or environmentally. Differences by age group for serotype 3 were not consistent (e.g. 2–3 y were lower than 11–15 years; 16–50 y were lower than 6–10 and 11–15 y olds) although the lower seroimmunity of type 3 is well known and consistent with previous findings and the known immunogenicity differences among serotypes in the vaccine. In some subcategories, males had a lower seroprevalence than females; however, this was not a universal finding and did not fit a consistent pattern. Similarly, Blacks had higher seroprevalence than Whites, and Hispanics had higher seroprevalence than non-Hispanics in some subcategories. Birthplace of subjects and parents and travel history of subjects and household members showed differences in seroprevalence in a small number of subcategories. Lower seroprevalence among some subgroups may indicate certain subpopulations may be at increased risk for transmission and outbreaks if poliovirus was introduced; however, the seroprevalence among all subgroups evaluated was near or above the expected crude herd immunity threshold in a developed country, which has been estimated at a range of 80–86%.Citation10 On an individual level, seronegativity to one or more serotypes may still be protective if individuals were primed because they were previously vaccinated or exposed to poliovirus. Nevertheless, given that overall seronegativity rates were 9.3%, 5.6%, and 16.7% for serotypes 1, 2, and 3, respectively, there remains some low level of susceptibility. As long as poliovirus circulation continues anywhere in the world, importations remain a risk and consequently, there remains a limited risk of possible outbreaks among unvaccinated subpopulations.
This study has some limitations. The analyses relied on data from a sampling of participants in the Kansas City metropolitan area and it is unknown if these results are generalizable to the current status of poliovirus immunity in the US population as a whole. A larger, national based serosurvey was recently published, but did not cover the youngest age groups and did not have vaccine records available.Citation11 In addition, vaccination history was obtained only for those aged <18 years, so we were unable to distinguish between immunity due to infection versus vaccination for the older ages. Among US born persons, natural infection would be expected to be very rare since circulation of WPV has been at an extremely low level (or zero) in the US during the lifetime of all but the oldest of study subjects. Acquisition of infection by a US-born person while traveling abroad is a possibility, but is also likely to be relatively rare. Finally, the proportion of individuals that were surveyed and tested varied by age group, race/ethnicity, and US birth status, and the study may not identify small subgroups with increased susceptibility to polio due to the small sample size.
Results from this study indicate high seroprevalence to poliovirus in the subjects surveyed in the Kansas City metropolitan area aged 2–81 y. These results provide evidence to support prevention of sustained disease transmission if poliovirus is imported into the US. The lower seroprevalence in younger children also highlight the importance of completing the recommended poliovirus vaccine series, including a booster dose at 4–6 y. This result may influence global polio vaccine policy with the switch to an IPV only schedule. WPV remains endemic in only 3 countries, limiting the risk of importation to the US, but not eliminating it. Monitoring seroprevalence is an important component of understanding risks for polio in the US. Continued high vaccine coverage remains paramount in the near future while the world completes the global elimination of polio.
Materials and methods
A convenience sample of participants was enrolled from patients and volunteers (employees and employee referrals) from Children's Mercy Hospital and Clinics (CMH) and Truman Medical Center-Lakewood (TMC) serving the Kansas City metropolitan area. Informed consent was obtained from all participants or their legal guardians, and the Institutional Review Board of CDC, CMH, and TMC approved the protocol. The sampling was designed within each cohort to represent a reasonable distribution similar to the racial/ethnic diversity of the Kansas City metropolitan area as determined by the census and be split equally between genders. From the 2005 census, the Kansas City metropolitan area was 74% white, 16% black, 6% Hispanic, 1% Asian, and 3% other compared with 66%, 17%, 7%, 1%, and 9%, respectively in the study group. Additional information was gathered regarding country of birth (US born vs. not US born) for subjects and each of their parents as well as travel abroad in the previous 10 y for the subject and household members. To be eligible, documentation of vaccine status was required for subjects <18 y of age. Written documentation of immunization by provider record, parental record, or registries was acceptable. Parental recall of immunization was not accepted. Those with medical conditions that may impact immune status were excluded, specifically persons who are on cancer chemotherapy, who have active HIV/AIDS, have received a stem cell or organ transplant, received blood products including IVIG within the past 6 months, or have taken high doses of corticosteroids (≥ 2 mg/kg/day) on a daily basis within the past 3 months.
Seroprevalence for antibodies against poliovirus serotypes 1–3 were compared by demographic characteristics for 504 individuals aged 2 to 81 y who participated in the 2012–2013 study. The following age cohorts were recruited with the goal of ∼100 subjects per cohort:
Age 2–3 y representing children recommended for an all-IPV primary series before their 4–6 y old booster;
Age 6–10 y representing children recommended for an all-IPV series including the 4–6 y old booster;
Age 11–15 y which would include individuals during the transition from an all-OPV recommendation to an all-IPV recommendation;
Age 16–50 y which would include individuals born before the transition from an all-OPV recommendation to an all-IPV recommendation;
Age >50 y which would include individuals born when vaccine was being introduced (IPV and OPV) and pre-vaccine era.
Serum samples from participants were tested for poliovirus-specific antibodies. Samples were tested using a standard microneutralization assay for antibodies to poliovirus types 1, 2, and 3, according to established protocols at the Polio Global Specialized Laboratory, CDC.Citation12 Briefly, 80–100 CCID50 (50% cell culture infectious dose) of each poliovirus serotype and 2-fold serial dilutions of serum were separately combined and pre-incubated at 35°C for 3 hours before addition of HEp-2(C) cells (human cervix carcinoma cell line). After incubation for 5 d at 35°C and 5% CO2, plates were stained with crystal violet and cell viability measured by optical density in a plate spectrophotometer. Each specimen was run in triplicate, with parallel specimens from one study subject tested in the same assay run, and the neutralization titers estimated by the Spearman-Kärber method and reported as the reciprocal of the calculated 50% end point.Citation13 Each run contained multiple replicates of a reference antiserum pool to monitor performance variation. A serum sample was considered positive if the 50% end point titer was ≥ 1:8 dilution.
The data were analyzed using SUDAAN 11.0 (Research Triangle Institute, Research Triangle Park, NC) and SAS 9.3 (SAS Institute, Cary NC): t-tests were used to evaluate differences in seroprevalence by demographic characteristics and serotype. P-values <0.05 were considered statistically significant and were not adjusted for multiple comparisons. Results based on <5 individuals were excluded from the analysis and not reported.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or Children's Mercy Hospital and Clinics.
Abbreviations
| CCID50 | = | 50% cell culture infectious dose |
| CDC | = | US Centers for Disease Control and Prevention |
| cVDPV | = | circulating vaccine-derived poliovirus |
| DVD | = | Division of Viral Diseases |
| = | CDC | |
| HEp-2(C) cells | = | human cervix carcinoma cell line |
| IPV | = | inactivated poliovirus vaccine |
| OPV | = | oral poliovirus vaccine |
| US | = | United States of America |
| WPV | = | wild poliovirus |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author's contributions
All authors contributed to the design, interpretation of data, and writing of the manuscript. Barbara Pahud and Christopher Harrison led the execution of the study and acquisition of data and samples, and contributed to interpretation of the results. William Weldon and Steven Oberste were responsible for laboratory evaluation, analysis, and interpretation of the data. Aaron Curns performed data analysis and interpretation of the data and Gregory Wallace was the lead for the conception of the study.
Acknowledgments
The authors would like to acknowledge the laboratory staff, Will Hendley, Sharla McDonald, Patricia Mitchell, Deborah Moore, and Yiting Zhang, of the Polio and Picornavirus Laboratory Branch, Division of Viral Diseases (DVD), US. Centers for Disease Control and Prevention (CDC), for performing serology testing, and Daoling Bi of the Epidemiology Branch, DVD, CDC for assisting with the statistical analyses. The authors would also like to thank Cindy Olson-Burgess, Nancy Neilan, Shannon Clark, Joanne Thurber and the staff at Children's Mercy Hospital and Truman Medical center for their help with this study.
Funding
The Centers for Disease Control and Prevention provided funding to Children's Mercy Hospital and Clinics to recruit subjects, conduct surveys, and obtain serum samples for analysis.
References
- Farag NH, Wadood MZ, Safdar, RM, Ahmed N, Hamdi S, Tangermann RH, Ehrhardt D. Progress toward poliomyelitis eradication – Pakistan, January 2014-September 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1271-5; PMID:26584026; https://doi.org/http://dx.doi.org/10.15585/mmwr.mm6445a4
- Mbaeyi C, Saatcioglu A, Tangermann RH, Hadler S, Ehrhardt D. Progress toward poliomyelitis eradication – Afghanistan, January 2014-August 2015. MMWR Morb Mortal Wkly Rep 2015; 64:1166-70; PMID:26492280; https://doi.org/http://dx.doi.org/10.15585/mmwr.mm6441a2
- Etsano A, Gunnala R, Shuaib F, Damisa E, Mkanda P, Ticha JM, Banda R, Korir C, Chevez AE, Enemaku O, et al. Progress toward poliomyelitis eradication – Nigeria, January 2014-July 2015. MMWR Morb Mortal Wkly Rep 2015; 64:878-82; PMID:26292207; https://doi.org/http://dx.doi.org/10.15585/mmwr.mm6432a5
- Wallace GS, Seward JF, Pallansch MA. Interim CDC guidance for polio vaccination for travel to and from countries affected by wild poliovirus. MMWR Morb Mortal Wkly Rep 2014; 63:591-4; PMID:25006826
- Immunization Systems Management Group of the Global Polio Eradication Initiative. Introduction of inactivated poliovirus vaccine and switch from trivalent to bivalent oral poliovirus vaccine – Worldwide, 2013–2016. MMWR Morb Mortal Wkly Rep 2015; 64:699-702; PMID:26135591
- Global Polio Eradication Initiative – Data and Monitoring – Polio this week. Available from http://www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx (accessed on September 14, 2016)
- Prevots DR, Burr RK, Sutter RW, Murphy TV. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2000; 49(No. RR-5):1-22; PMID:10993565
- Chen RT, Hausinger S, Dajani A, Hanfling M, Baughman AL, Pallansch MA, Patriarca PA. Seroprevalence of antibody against poliovirus in inner-city preschool children. Implications for vaccination policy in the United States. JAMA 1996; 275:1639-45; PMID:8637136; https://doi.org/http://dx.doi.org/10.1001/jama.1996.03530450029028
- Prevots DR, Pascual FB, Angellili ML, Brayden R, Irigoyen M, Larussa P, Sawyer M, Baughman AL, Pallansch MA. Population immunity to polioviruses among preschool children from 4 urban underserved low income communities, United States, 1997–2001. PIDJ 2004; 23:1130-6
- Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev 1993; 15:265-302; PMID:8174658
- Wallace GS, Curns AT, Weldon WC, Oberste MS. Seroprevalence of poliovirus antibodies in the United States population, 2009–2010. BMC Public Health 2016; 16:721; PMID:27492318; https://doi.org/http://dx.doi.org/10.1186/s12889-016-3386-1
- Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol 2016; 1387:145-76; PMID:26983734; https://doi.org/http://dx.doi.org/10.1007/978-1-4939-3292-4_8
- Karber G. Beitrag zur kollektiven behandlung pharmakologischer reihenversuche. Archiv Fuer Experimentelle Pathologie und Pharmakologie 1931; 162:480-3