ABSTRACT
Dengue has become a major global public health threat with almost half of the world's population living in at-risk areas. Vaccination would likely represent an effective strategy for the management of dengue disease in endemic regions, however to date there is only one licensed preventative vaccine for dengue infection. The development of a vaccine against dengue virus (DENV) has been hampered by an incomplete understanding of protective immune responses against DENV. The most clinically advanced dengue vaccine is the chimeric yellow fever-dengue vaccine (CYD) that employs the yellow fever virus 17D strain as the replication backbone (Chimerivax-DEN; CYD-TDV). This vaccine had an overall pooled protective efficacy of 65.6% but was substantially more effective against severe dengue and dengue hemorrhagic fever. Several other vaccine approaches have been developed including live attenuated chimeric dengue vaccines (DENVax and LAV Delta 30), DEN protein subunit V180 vaccine (DEN1–80E) and DENV DNA vaccines. These vaccines have been shown to be immunogenic in animals and also safe and immunogenic in humans. However, these vaccines are yet to progress to phase III trials to determine their protective efficacy against dengue. This review will summarize the details of vaccines that have progressed to clinical trials in humans.
Introduction
Dengue virus (DENV) has become one of the most important global public health threats in recent decades.Citation1-3 It is estimated that almost half of the worlds population live in at-risk areas, with the majority residing in the Asia-Pacific region.Citation2 Consequently, dengue has the propensity to produce large epidemics with a significant disease and economic burden in endemic regions. The incidence of dengue has increased signficantly in recent years.Citation4-10 A recent modeling study reported that in 2010 globally there were an estimated 96 million apparent dengue infections, a further 294 million subclinical infections, 500,000 hospitalizations and 25,000 deaths, primarily among children.Citation10 These conservative estimates for endemic disease might substantially increase pending climate changes that will expand the at risk areas, regions and territories.
Dengue infection also poses a significant health risk for travelers from non-endemic countries visiting endemic regions.Citation11-13 Current dengue control measures rely on targeting mosquito vectors, but many dengue endemic countries do not have preventative vector control measures in place and the methods used are often of questionable effectiveness in reducing vector population density.Citation3,Citation14 Although vaccination would likely represent an effective strategy for the management of dengue disease in endemic regions, there is currently only one licensed dengue vaccine. A major fundamental obstacle in progressing vaccine clinical trials is our poor understanding of surrogate clinical endpoints that could be used to predict “in the field” vaccine efficacy. A better understanding of protective immune responses to dengue would greatly facilitate further vaccine development. This review will summarize the key elements of what is understood about protective immune responses to dengue and describe in detail vaccines that have progressed to clinical trials.
Subversion of DENV immune responses
Our understanding of protective immunity to DENV is incomplete and this is the result of a number of viral and host factors. First, DENV exists as four genetically and antigenically distinct DENV serotypes with approximately 40% divergence between the amino acid sequences of the serotypes and up to 9% mismatch within a serotype (reviewed inCitation15). Another limiting factor has been the association between secondary infection with heterotypic DENV and the development of severe dengue. Although the mechanism underlying this is likely to be multifactorial this phenomonen has been associated with the presence of low affinity suboptimal neutralizing antibodies (NAb) against heterotypic DENV that enhances viral uptake and replication in macrophages and monocytes (Antibody Dependent Enhancement, ADE). Additionally, dysfunctional CD8+ and CD4+ T cell responses associated with the development of an inflammatory cytokine response are also thought to impact on adverse clinical outcomesCitation16 (reviewed in ref. Citation17). In addition, DENV has developed numerous ways to subvert immunological responses responsible for protection.Citation18,Citation19
A further compounding factor is that secondary infection is associated with the reactivation of cross-reactive memory B and T cells that are specific for the previous rather than the current DENV infection. The result is that the acute T cell response is mostly directed toward the previous infecting serotype leading to the expansion of low avidity cross-reactive memory T cells, rather than higher avidity T cells, an ineffective response to the new infecting DENV,Citation20,16 and increased production of inflammatory cytokines including TNFα.Citation21,Citation22 Over time, however, T cells against conserved, cross-reactive epitopes are preferentially expanded, eventually resulting in a broad protective DENV response.
Similarly, for B cells a predominant monotypic high avidity response against the infecting serotype is observed in the first week after DENV infection. However, over the next 6 months, a broad cross-reactive B cell repertoire develops although the resulting cross-reactive antibodies may be of low avidity and this has also been postulated to contribute to enhanced severity of secondary dengue disease. Eventually after a secondary heterotypic infection, stable populations of DENV-broad cross-reactive B cells that produce high-avidity antibodies develop thereby providing effective cross-protective immunity.
These observations suggests that for a safe and effective vaccine needs to induce long-term protective immune responses and high titer neutralising antibody responses to all four DENV serotypes simultaneously. Understanding how DENV is able to modulate immunological responses would be a pivotal advance in our understanding of the mechanisms underlying the nature of protective immune responses following DENV infection and the administration of a DENV vaccine.
Protective immune responses to DENV
The development of a protective immune response against dengue involves a combination of innate,Citation18,Citation23 neutralizing antibody (NAb), CD4+ and CD8+ T cell responses. Both B and CD4+ T cell responses are predominantly directed against the E protein while CD8+ T cell responses are directed toward the NS3 and NS5 proteins.Citation24,Citation25 Broad NAb responses provide serotype specific protection against DENV.Citation26,Citation27 Effective antibody mediated neutralization of DENV involves a “multi-hit” phenomenon that requires the binding of multiple antibodies in order to neutralize the virus.Citation26 However, defining the critical neutralizing epitopes recognized by humans has been challenging. Early studies of NAb responses in mice demonstrated that the majority of DEN-NAb targeted domain III (EDIII) of the E protein. However, studies involving the depletion of human immune sera with recombinant EDIII have shown a minimal impact on DENV neutralisation and other studies using human monoclonal antibodies have shown that NAb in humans are directed to regions outside EDIII including domain I (DI), and the DI-DII hinge of the E protein only on whole virus particles.Citation27-31 In fact the antibody response in humans appears to consist of a minor population of strongly neutralizing antibodies and a major population of non-neutralizing cross-reactive antibodies that have the potential to enhance the severity of dengue infection.Citation32 In addition, NAb in human sera that target epitopes in EDIII only make up a very small proportion of NAbs that develop following DENV infection.Citation33 Finally, the plasticity of the viral envelope also results in the masking of neutralising epitopes, adding to the challenge of identifying critical neutralisation domains.Citation34,Citation35
Recent studies have also shown that the maturation state of flaviviruses influences both the range of host target cells that they can infect and the interaction of the virion with particular antibodies, thus impacting on the results of neutralization assays.Citation36 Another important conceptual problem is that DENV virions are not static but rather dynamic structures, thus enabling antibodies with “cryptic” epitopes to bind and also exposing the membrane underneath the layer of viral E and M proteins.Citation26,Citation34,Citation35,Citation37 As a result, these factors can dramatically alter the measurement of NAb titers. The value of measuring NAb alone has also come in to question as shown in recent study using an alum- and ODN-adjuvanted EDIIIC-2 subunit DENV vaccine in conditional IFNAR knock out mice. This study, like the CYD-TDV trials, demonstrated that measuring NAb alone may be insufficient in predicting the ability of a vaccine to provide protection from viral challenge in mice.Citation38
A vigorous multifunctional CD8+ T cell response has also been associated with protection from DENV,Citation24,Citation25,Citation39 and may play an impottant role in vaccine induced protective responses.Citation40 However, the exact correlates of protective cell mediated immunity are not known and this has hampered vaccine development. Understanding the nature of T cell responses to DENV has also been hampered by the lack of a robust translatable animal model that completely recapitulates the pathogenic chain of events that occurs in primary and secondary infection or that results in the production of a ‘protective’ immune responses. CD4+ T cell responses have an important role in the clearance of DENV.Citation24,Citation41 Once such subset is germinal center T follicular helper (GCTfh) cells and their circulating counterparts Tfh cells which represent a circulating compartment of GCTfh lineage cells (reviewed inCitation42). Tfh cells are strongly correlated with the development of protective antibody responses against influenza infection and following influenza vaccination,Citation43Citation44 and have a critical role in the development of HBeAg seroconversion and clearance of viraemia in patients with chronic hepatitis B infection.Citation45 Recently it has been shown that Tfh cells are also involved in the control of HCV viraemia and in developing HCV specific memory B cell responses.Citation46 Tfh cells have been shown to have an important role in interacting with DENV specific B cells to promote protective humoral immune responses and play a direct role in viral clearance through the production of IFN-γ 24. Finally, macrophages also play a critical role in controlling dengue virus infection and IFN signaling in macrophages is important in the development of effective adaptive immune responses to dengue.Citation38
Preventative DENV vaccines
CYD tetravalent dengue vaccine (Sanofi Pasteur)
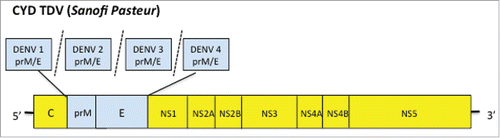
The only vaccine currently approved for use in endemic populations for the prevention of dengue is the recombinant live-attenuated chimeric yellow-fever-dengue virus tetravalent vaccine (Dengvaxia®; CYD-TDV) (). The CYD-TDV vaccine is composed of four recombinant live attenuated chimeric viral vaccines based on the 17D yellow fever vaccine (YFV 17D) backbone. Each chimeric viral vaccine expresses the prM and envelope genes of one of the four dengue virus serotypes.Citation47 Pre-clinical studies with CYD-TDV demonstrated that, like the YFV 17D vaccine virus, the CYD-TDV vaccine viruses are also genetically and phenotypically stable. In addition CYD-TDV is non-hepatotropic, less neurovirulent than YFV 17D and does not infect mosquitoes.Citation47 Although the vaccine contains the YFV 17D non-structural genes it lacks the YF envelope protein gene and consequently does not induce neutralising antibody resposes to YFV. Furthermore, preexisiting immunity to YFV does not interefere with immune responses to CYD.Citation48,Citation47 The vaccine subsequently underwent extensive preclinical development in murine and primate modelsCitation49-54 before progressing to large multicenter Phase 3 clinical trials. Only the results of the Phase 2b and 3 clincial trials will be discussed in this review as these focus on vaccine efficacy and will reflect its clinical impact. The vaccine is comprised of the prM/E proteins of the four DENV serotypes (DENV1, DENV2, DENV3 and DENV 4) and the non-structural (NS) and capsid proteins of the attenuated yellow fever (YF) 17D vaccine virus.Citation51 The efficacy of CYD-TDV was first studied in a proof-of-concept Phase 2b trial in Thailand (CYD23) Citation55 followed by two large Phase 3 studies in SE Asia Citation56 (CYD14) and latin AmericaCitation9 (CYD15). In addition CYD-TDV has also been shown to be safe and immunogenic in dengue naïve adults (CYD17).Citation57
Figure 1. CYD TDV is recombinant live-attenuated chimeric yellow-fever-dengue virus tetravalent vaccine that was produced by replacing the prM and E of the 17D yellow fever vaccine virus with the prM and E proteins of DEN-1, -2, -3 or -4 viruses.
In CYD23, participants were assigned to receive vaccine (2669 participants) or placebo (1333 participants).Citation55 The primary endpoint of the study was to assess the protective efficacy against virologically-confirmed and symptomatic dengue occurring at least 1 month after the third injection. Overall, 3673 participants were included in the primary analysis (2452 in the vaccine group and 1221 in the control group). There were 134 cases of virologically confirmed dengue throughout the study and the overall efficacy of the vaccine was 30.2%. However, vaccine efficacy varied according to DENV seroptype. The efficacy for DENV-1 was 61.2%, 81.9% for DENV-3 and 90.0% for DENV-4 but for DENV-2 the efficacy was only 3.5% ().Citation55 Inspite of the variation in efficacy, the vaccine demonstrated strong neutralising antibody responses to all four serotypes at 28 d after the third dose. The geometric mean titers (GMT) for DENV-1 neutralising antibody was 146; 310 for DENV-2; 405 for DENV-3 and 155 for DENV-4 (). The vaccine was well tolerated with no major safety conerns identified. The discrepany between in-vitro neutralization markers and clinical efficacy of the vaccine warrants further consideration and may indicate a problem with the quality of the antibody response elicited in vivo, the isotype of antibodies, or alternatively the inadequacey of NAb alone to protect against disease. Antibodies may also have functions beyond their simple ability to neutralize virus including participating in antibody dependent cell mediated cytotoxicity.Citation58,Citation59
Table 1. Summary of seroconversion rates, neutralising antibody titres and vaccine efficacy of CYD TDV in phase 2b and III clinical trials.
This study was followed by two phase III efficacy trials conducted in South East Asia (CYD14)Citation56 and Latin America (CYD15).Citation9 Both trials examined the efficacy of a three-dose schedule (0, 6, and 12 months) of CYD-TDV to reduce symptomatic and virologically-confirmed dengue during a period of 12 months starting 28 d after the third dose. Vaccine recipients in both trials received 5.0 log10 median cell-culture infectious doses (CCID50) per serotype, the same dose as the licensed vaccine, Dengvaxia®. The Asian study involved 11 sites and recruited 10,275 children between the ages of 2 and 14 y. The efficacy of the vaccine against virologically-confirmed dengue was 56.5%. Like the CYD23 trial the vaccine efficacy varied with the different dengue seroptypes. The efficacy was highest for serotypes 3 (78.4%) and 4 (75.3%), lowest with serotype 2 (35.0%) and intermediate against serotype 1 (50%). The efficacy was also higher in children aged 12 to 14 y (74.4%) compared to children aged 6 to 11 y (59.5%) and 2 to 5 y (33.7%). Seropositivity for dengue at baseline was also associated with a higher efficacy (74.3%) compared to individuals who were seronegative at baseline (35.5%) ().Citation56
Despite the variation in protective efficacy the vaccine produced strong neutralising antibody responses against all four seroptypes. The GMTs for DENV-1 neutralising antibody was 166, 355 for DENV-2, 207 for DENV-3 and 151 for DENV-4. One of the major benefits however, was that the vaccine showed good efficacy in preventing severe dengue disease (80.8%), dengue hemorrhagic fever (DHF) (88.5%) and in reducing hospitalization (67.2%) ().Citation56
The Latin American study, CYD15, involved 22 sites in Central and South America and recruited 20,869 children aged between 9 and 16 years.Citation9 In this trial the overall efficacy against virologically-confirmed dengue was 60.8%. The vaccine efficacy was highest for serotype 4 (77.7%) followed by 3 (74.0%) and then serotype 1 (50.3%). Once again the efficacy was lowest for serotype 2 (42.3%). Neutralising antibody responses were highest for serotype 2 (574) and 3 (508) and lowest for serotype 1 (395) followed by seroptype 4 (241) (). Like CYD14, vaccine efficacy was high against severe dengue disease (95.5%), DHF (95.0%) and in reducing hospitalisation (80.3%) ().Citation9
In both trials almost 80% of the children tested at baseline were seropositive for one or more dengue serotypes and the efficacy of CYD-TDV among these children was approximately twice as high as the seronegative individuals (83.7% vs. 43.2%), suggesting that, as with T-cell responses, protection was largely mediated by the vaccine boosted pre-existing immune responses rather than de novo vaccine-mediated immunity.Citation9,Citation56
A subsequent pooled analysis of efficacy and long-term (4 year) follow up of CYD23, 14 and 15 Citation60 demonstrated an efficacy against virologically confirmed dengue of any severity for all serotypes in participants aged 9 y or over of 65.6%. The protective efficacy was also higher in children who were DENV seropositive at baseline than in those who were DENV seronegative. In children aged 9 y and older and seropositive at baseline the efficacy was 81.9% compared with 52.5% for those who were seronegative at baseline. Similarly, in children less than 9 y of age the efficacy was 70.1% in those seropositive compared with 14.4% who were seronegative at baseline. The pooled analyses in children aged 9 y and over also demonstrated that vaccine efficacy against hospitalization for virologically confirmed dengue was 80.8%, 93.2% against severe dengue fever and 92.9% against any grade of DHF. However, for children under 9 y the efficacy in reducing hospitalization was 56.1%, 44.5% against severe dengue and 66.7% against DHF.Citation60
In a subsequent pooled analysis of safety data from 18 clinical trials it was shown that vaccine reactogenicity did not increase with successive doses of CYD-TDV. In addition the frequency and nature of serious adverse events occurring within 28 d of any dose were similar in the CYD-TDV and placebo groups. Importantly there were no vaccine-related neurotropic or viscerotropic events reported.Citation61 Another important finding was that the increase in dengue related hospitalization, and severe dengue observed in participants aged < 9 y in the CYD-TDV group compared with the placebo group was less marked in the fourth year of follow up. From year 4 in the phase IIb (CYD23) and the phase III (CYD14) trials that enrolled participants aged < 9 y the relative risk for hospitalization fell from 1.57 in Year 3 to 0.54 in the phase IIb trial and from 1.58 to 1.19 in the phase III trial.Citation61
Finally, a pooled analysis of data for 3,736 individuals who received either CYD-TDV or placebo in the immunogenicity subsets of two phase-III trials showed that both symptomatic and asymptomatic dengue infections in healthy children and adolescents aged 2–16 y were reduced.Citation62
DENVax (Takeda)
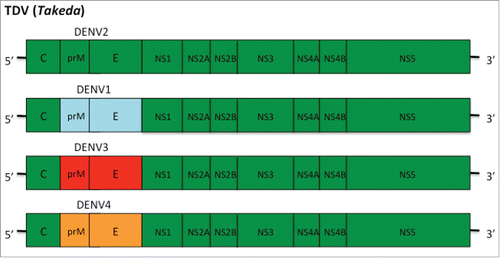
A second vaccine (DENVax or TDV) now progressing through clinical trials has been developed by Takeda Vaccines Inc. The vaccine consists of a live attenuated DENV-2 strain (TDV-2) and three chimeric viruses that contain the prM and E proteins of DENV-1, 3 and 4 on the attenuated DENV-2 genome backbone (TDV-1, -3 and -4) ().Citation63 The manufacturing seeds for the vaccine have been genetically and phenotypically characterized.Citation64 The vaccine has been shown to induce both humoral and NAb responses to DENV.Citation65,Citation66 In addition, as the vaccine contains the non-structural genome of DEN-2 it is also able to produce cellular immune responses against dengue viruses,Citation65,Citation66 and anti-NS1 antibody responses which have been show to be protective against dengue infection.Citation67
Figure 2. DENVax TDV consists of a live attenuated DENV-2 strain (TDV-2) and three chimeric viruses that contain the prM and E proteins of DENV-1, 3 and 4 on the attenuated DENV-2 genome backbone (TDV-1, -3 and -4).
In AG129 mice the monovalent vaccine constructs have been shown to be immunogenic, producing high levels of type-specific neutralizing antibodies.Citation65 In addition, the vaccine induces low levels of cross-reactive NAb and is protective against challenge with mouse adapted DENV-1 and -2 viruses.Citation66 In macaques, inoculation with tetravalent DENVax resulted in low level viremia of only DENV-2 and was well tolerated.Citation68 The vaccine induced NAb responses against all 4 serotypes after one or two injections,Citation66,Citation69 and dengue specific CD4+ and CD8+ T cell responses.Citation69
In a randomized double blind Phase 1 dose escalation trial conducted in Columbia, 96 participants between the ages of 18 and 45 y were assigned to receive two injections 90 d apart of either a low or high dose of DENVax by subcutaneous or intradermal injection.Citation70 The first cohort of 40 participants received a low-dose vaccine containing 8 × 103, 5 × 103, 1 × 104 and 2 × 105 plaque forming units (PFU) of DEN-1, -2, -3 and -4 respectively. The second cohort of 36 participants received a high-dose vaccine containing 2 × 104, 5 × 104, 1 × 105 and 3 × 105 PFU of DEN-1, -2, -3 and -4 respectively. The vaccine was well tolerated with no significant systemic adverse events reported. Of the vaccine recipients solicited systemic adverse events were recorded in 86% of participants compared with 76% in the placebo group. By contrast local systemic reactions were significantly more common in the vaccine recipients (85%) compared with the placebo group (29%).Citation70 The vaccine was immunogenic with 62% of participants seroconverting to all four serotypes and 96% to three or more serotypes regardless of the route of administration and dose of vaccine. The vaccine also produced strong NAb responses with comparable titres between the high and low dose formaulations at 30 d after the second dose. The NAb titres were highest against DENV-2 and lowest for DENV-4 in all groups ().Citation70
Table 2. Summary of seroconversion rates and neutralising antibody titres of DENVax and LAV Delta 30 in Phase 1 clinical trials.
In a second study, 72 flavivirus-naive healthy adults between the ages of 18 and 45 y were enrolled in to a Phase 1 double-blinded, randomized, placebo controlled dose-escalation trial again comparing the low and high dose DENVax formulations given by subcutaneous or intradermal route in 2 doses 90 d apart.Citation71 Once again the vaccine was well tolerated with no major systemic adverse events reported and as with the study in Columbia injection site pain and erythema were more frequent in vaccine recipients than the placebo group (52% vs 17% and 73% vs 25% respectively).Citation71 The vaccine achieved high seroconversion rates of 84.2% for DENV-1, 92.1% DENV-2, 86.8% DENV-3 and lowest 71.1% for DENV-4. The geometric mean neutralization titers after 2 doses were 54.1 for DENV-1, 292.8 for DENV-2, 32.3 for DENV-3 and 15.0 for DENV-4. More than 90% of high dose recipients developed broad trivalent neutralizing antibody responses ().Citation71
The results of the two phase 1 studies paved the way for a multicenter, double-blind, phase 2 study in Puerto Rico, Colombia, Singapore, and Thailand.Citation72 This study investigated the safety and immunogenicity of TDV in children and adults and was conducted in two stages. In the first stage participants between the ages of 1.5 and 45 y were enrolled sequentially into 4 groups in an age-descending order. Stage 2 only commenced once the safety of the vaccine was established in children and enrolled a single group of children aged 1.5 to 11 y (group 5). Participants received two doses of DENVax containing 2 × 104, 5 × 104, 1 × 105 and 3 × 105 PFU of DEN-1, -2, -3 and -4 respectively.Citation72 The vaccine was well tolerated and no serious adverse events were reported in the vaccine groups. However, injection site pain, itching and erythema were more frequently reported in the vaccine recipients compared with placebo group. Up to 97% of participants in all groups seroconverted for DENV 1 to 4 after two doses of TDV. However, the seropositivity for DENV-4 was lower in groups 1 to 4 (87.5%) but higher in the group 5 (94.3%). The geometric mean neutralizing antibody titres were highest for DENV-1 and -2 and lowest for DENV-4. Titres ranged from 582 to 1187 for DENV-1, 582 to 1187 for DENV-2, 196 to 630 for DENV-3, and from 41 to 210 for DENV-4 among the 5 groups.Citation72
LAV Delta 30 (NIAID/Butantan)
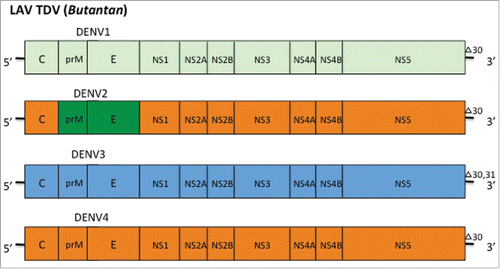
In a separate approach the NIAID undertook the development of serotype specific live attenuated viral vaccines that were similar with respect to immunogenicity and replication in naïve individuals. The attenuation was achieved by producing recombinant DEN viruses that carry nucleotide deletions or that were chimerized between DEN viruses. The DEN-1 and -4 viruses were produced by introducing a 30 nucleotide deletion in the 3’ untranslated region (UTR) of the genome (rDEN1-delta30 and rDEN4-delta30). The DEN-3 virus was produced by introducing a 30 and 31 nucleotide deletion in to the 3’UTR of DEN3 (rDEN3 delta30/31). For DEN2 the delta 30 deletion did not sufficiently attenuate the viral replication. However, by replacing the prM and E proteins of the rDEN4delta30 virus with those of DEN2 it was possible to produce a chimeric virus (rDEN2/4delta30) which was attenuated and remained immunogenic ().Citation73,Citation74 The vaccine was licensed to Butanan for further development and production of master and working virus banks in Vero cultures before producing the Butantan-DV lyophilized formulation. This vaccine contains 103 ± 0.5 PFU per dose of rDEN1delta30, rDEN2/4delta30, rDEN3delta30/31 and rDEN4delta30 attenuated virus strains and is referred to as admixture TV003.Citation73,Citation75 The vaccine has been shown to produce both neutralizing antibody and broad multispecific cytotoxic and memory CD8+ T cell responses that are comparable to natural infection.Citation74-76
Figure 3. LAV Delta 30 consists of live attenuated viral vaccines that carry nucleotide deletions or that have been chimerized. The DEN-1 and -4 viruses carry a 30 nucleotide deletion in the 3’ untranslated region (UTR) of the genome (rDEN1-delta30 and rDEN4-delta30). The DEN-3 virus carries a 30 and 31 nucleotide deletion in to the 3’UTR of DEN3 (rDEN3 delta30/31). The DEN-2 virus was produced by replacing the prM and E proteins of the rDEN4delta30 virus with those of DEN-2 to produce a chimeric attenuated virus (rDEN2/4delta30).
In a Phase 1 trial randomized double-blind placebo-controlled trial examining the safety and immunogenicity of two lots of rDEN1delat30 participants received two doses of vaccine on day 0 and again on day 120 or 180 77(ClinicalTrials.gov NCT00473135). Neutralizing antibody titres were determined by PRNT60 42 d after the second dose. Overall, 82% of participants who received rDEN1delta30, developed at least one solicited clinical or laboratory adverse event, compared with 45% among recipients receiving placebo. Rash, headache, and neutropenia were the most common adverse events. Rash was the only solicited adverse event that was more common in vaccine recipients (27% of vaccinees vs 0% placebo). Transient neutropenia developed in 45%, although 5/51 vaccinees developed severe neutropenia (500–749/mm3). Thirty-four of 51 vaccine recipients (67%) developed a transient low viremia lasting approximately 3.5 d. Overall, 93% vaccine recipients seroconverted and developed an NAb titer of 145 (range <5 to 965) after a single dose of vaccine and this was not increased after a second dose of vaccine.Citation77
Similar phase 1 studies have been completed to determine the safety and immunogenicity of rDEN2/4delta30Citation78 and rDEN4delta30.Citation79 These studies demonstrated the safety and immunogenicity of both vaccine viruses. The vaccines resulted in seroconversion rates of over 95% and high titer NAb. In a subsequent randomized double blind placebo-controlled trial a single dose of 4 different admixtures of a live attenuated tetravalent dengue vaccine were evaluated in 113 flavivirus-naive adults for safety and immunogenicity (ClinicalTrials.gov NCT01072786).Citation80 A single dose of each live attenuated viral admixture induced a trivalent or better neutralizing antibody response in 75–90% of vaccinees. The vaccine TV003 admixture resulted in the highest seroconversion rates (100% against DENV-1 and DENV-4, 85% against DENV-3 and 50% against DENV-2). This vaccine resulted in the most balanced neutralizing antibody response and was well tolerated although an asymptomatic rash developed in 52 (64.2%) of vaccine recipients compared to none in the placebo group. A transient low-level viremia lasting 1 to 2.8 d developed in up to 85% of vaccine recipients.
A recent report of two phase 1 randomized, double-blind, placebo-controlled trials compared the safety and immunognicity of two admixtures of the live attenuated tetravalent dengue vaccineCitation75 (). The first vaccine conatined the TV003 admixture while the second, TV005, had an increased DENV-2 component of 4 log10 PFU together with 3 log10 PFU of DEN-1, -3 and -4 in the admixture. As reported in the previous trial a mild rash was common occurring in approximately 60% of volunteers after the first dose of vaccine. A small proportion of vaccine recipients also developed mild neutropenia. Low-level viremia (<1 log10 PFU) lasting 1 to 3 d was detected in in up to 76% of recipients in both the TV003 and TV005 groups. A high proportion of the vaccine recipients seroconverted to all four serotypes and although seroconversion was lower against DEN-2 (76%) with the TV003 vaccine, the DEN-2 enhanced TV005 admixture resulted in a 95% seroconversion against DEN-2. Seroconversion against the other serotypes ranged from 92 to 100% with both TV003 and TV005. The administration of a second dose of vaccine increased the serconoversion rate against DEN-2 for the TV003 group to 94% but had marginal benefit in all other groups. Serotype–specific GMTs of neutralizing antibodies ranged from 11 to 144 in the TV003 group and 10 to 205 in the TV005 group. The administration of a second dose of vaccine did not increase GMTs ().Citation75
DEN protein subunit V180 vaccine (DEN1–80E) (Hawaii Biotech Inc./Merck)
Another approach to developing a DENV vaccine has been based on a recombinant envelope glycoprotein subunit vaccine.Citation81-84 This vaccine (developed by Hawaii Biotech and now licensed to Merck) consists of a recombinant truncated protein containing 80% of the N-terminal DENV E protein (DEN-80E). By constructing an E protein with a C-terminal truncation at amino acid 395 the membrane anchor sequence of the protein is removed, resulting in a recombinant E protein with improved secretion, purification and immunogenicityCitation81-84 (). The DEN-80E protein for each of the four dengue serotypes has been expressed in the Drosophila S2 expression system to produce a tetravalent vaccine.Citation81-84 The recombinant DEN-80E proteins have been shown to induce NAb in preclinical models and to prevent viremia in a non-human primate challenge model.Citation82-84
Figure 4. DEN protein subunit V180 vaccine (DEN-80E) is a recombinant envelope glycoprotein subunit vaccine that consists of a recombinant E truncated protein containing 80% of the N-terminal DENV E protein (DEN-80E). The truncated dengue envelope proteins (DEN-80E) for all four dengue virus types are expressed in the Drosophila S2 expression system to produce a tetravalent vaccine.

Studies in mice have demonstrated that DEN-80E formulated with ISCOMATRIX™ adjuvant elicited high NAb titers that persisted for at least 6 months and exhibited memory responses. Mice were also protected against intracranial challenge with a mouse adapted DENV-2.Citation82 Non-human primate studies have also evaluated various monovalent and tetravalent DEN-80E formulations with several different adjuvants. DEN2–80E formulated with aluminum hydroxide AS04, AS05, AS08 and ISCOMATRIX™ resulted in high seroconversion rates with high titer NAb responses.Citation82,Citation83 In addition, animals were protected against DENV2 and 4 challenge.Citation82,Citation83
To prepare for human trials a large study in non-human primates was conducted to test the immunogenicity and efficacy of tetravalent DEN-80E.Citation84 The study evaluated low, medium and high doses of the tetravalent DEN-80E subunit vaccines adjuvanted with ISCOMATRIX™ and two dosing regimens (0, 1, 2 months for medium and high dose groups and 0, 1, 2, and 6 months for low dose groups). The vaccine produced balanced high titer NAb responses against all four serotypes although the durability of antibody response was superior in the 0, 1, 2, and 6 month regimen. Animals in the low and medium dose groups were protected against viral challenge while 2 of 12 animal in the high dose group developed short-lived viremia.
The first-in-human, proof-of-principle Phase I trial of DEN1–80E adjuvanted with Alhydrogel™ sponsored by Hawaii Biotech Inc. (HBV-001-C-101; ClinicalTrials.gov NCT00936429) was conducted to assess the safety, tolerability and immunogenicity of the vaccine in flavivirus naïve adults aged between 18 and 45 y.Citation81 Six adults received 10 µg of DEN1–80E vaccine and six received 50µg of DEN1–80E in three monthly doses. The vaccine was well tolerated in all vacinees and none developed any serious adverse events. Of vaccine recipients in the low dose group 80% seroconverted compared with 83% in the high dose group. The NAb GMTs were 29 (range 10–91) for the low dose group and 41 (range 10 to 502) in the high dose group. However, antibody response waned rapidly over the first 26 weeks following the third dose.Citation81 A larger Phase I trial with DEN-80E formulations adjuvanted with ISCOMATRIX™ to determine whether this will result in a stronger and more durable antibody response has been proposed.
DENV DNA vaccines
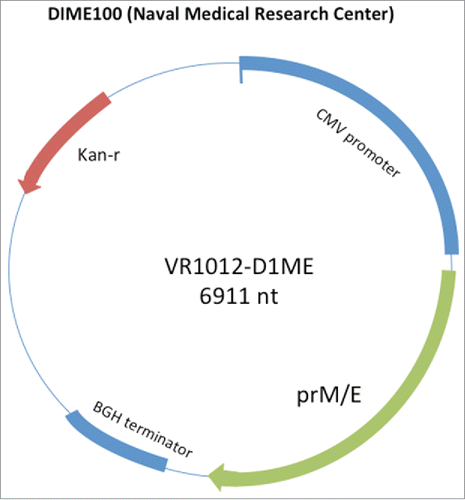
Nucleic acid vaccines have also been developed for dengue and now progressed to Phase 1 clinical trials. The D1ME100 vaccine (US Naval Medical Research Center) is a monovalent DNA plasmid vaccine containing the pre-membrane (prM) and envelope (E) genes of DENV-1 virusCitation85–Citation90 (). Mice immunized with a monovalent DEN-2 DNA vaccine containing the prM gene and a truncated E gene encoding 92% of the envelope protein developed anti-DEN-2 neutralizing antibodies and were protected against intracerebral challenge with DEN-2 virus.Citation89 Subsequent studies comparing the immunogenicity of DNA vaccines encoding truncated or the full length E gene of DEN-1 demonstrated that only constructs containing 80% of the E gene or the prM plus 100% of the E gene generated high levels of NAb. In addition, transfection of 293 cells with the prM 100%E construct also resulted in the production of DEN-1 virus like particles which could be detected in the culture supernatants.Citation88
Figure 5. The D1ME100 vaccine is a monovalent DNA plasmid vaccine containing the pre-membrane (prM) and envelope (E) genes of DENV-1 virus. The plasmid contains regulatory elements including a CMV promoter, and CMV intron A, a BGH terminator and kanamycin resistance gene. Coding sequences of the DENV prM/E, genes and their orientation in the plasmid are indicated by arrows. Adapted from ref. Citation90.
In an effort to develop a vaccine that could protect against all four serotypes, chimeric DNA vaccine constructs encoding antigens containing epitopes from all four dengue serotypes have been produced and tested in macaques.Citation91 The macaques developed NAb responses against all four seroptypes of dengue but when challenged with DEN-1 or DEN-2 virus only partial protection against DEN-1 was observed.Citation91 In a subsequent study, immunization of rhesus monkeys with a tetravalent vaccine containing equal amounts of monovalent DNA vaccines encoding the prM and E genes of dengue-1, -2, -3 or -4 viruses adjuvanted with Vaxfectin® (Vical Inc.) resulted in the production of NAb responses against DEN-1, -3 and -4 viruses. However, animals were protected against DEN-2 challenge compared with control animals.Citation87 The inclusion of Vaxfectin® also significantly improved the vaccine immunogenicity.
Having shown that the DEN-1 monovalent DNA vaccine was immunogenic in non-human primate models and protective against challenge with live DEN-1 virus a Phase 1 clinical trial was performed using the dengue virus serotype-1 (DENV-1) vaccine construct (D1ME100). The study was an open-label, dose escalation, safety and immunogenicity trial.Citation86 Healthy flavivirus-naïve adults were assigned to receive three intramuscular injections (0, 1, and 5 months) of either a high dose (5.0 mg) or a low dose (1.0 mg) DNA vaccine using a needle-free Biojector® 2000. The vaccine was safe and well tolerated. Only 41.6% in the high dose group developed anti-dengue NAb in contrast to none in the low dose group. Also, T-cell responses in the high dose group were stronger (detectable in 83% of vaccine recipients) than the low dose group (detectable in 50% of vaccine recipients). As a further extension on the development of this vaccine a phase 1 dose escalation study evaluating the safety, tolerability and immunogenicity of a tetravalent vaccine formulated in Vaxfectin® has been completed and results are now pending (ClinicalTrials.gov NCT01502358).
Perspectives and challenges for future vaccine development
Tremendous progress has been made toward the development of effective dengue vaccines, however, a number of important challenges remain. The absence of suitable humanized animal model that completely recapitulates the events in natural infection, pathogenesis and the immune response to dengue remains an important obstacle to the development of a dengue vaccine. However, the recent development of Human Immune System (HIS) mice engrafted with human haematopoietic progenitor cells may provide an alternative model to study humanized humoral and cellular immune responses in a single animal model.Citation92,Citation93 Also, HIS mice expressing HLA-DR4 have also been shown to develop high frequencies of human CD4+ T cells, are highly reconstituted with functional human B cells, express serum levels of human IgM, IgG (all four subclasses), IgA, and IgE comparable to humans and elicit high titers of specific human IgG antibodies upon tetanus toxoid vaccination.Citation92 HIS mice have been used to study immune responses to several viruses including HIV,Citation94 EBVCitation95 and DENV.Citation96,Citation97 DENV has been shown to infect HIS mice, recapitulating clinical disease typical of DENV,Citation96,Citation97and these mice have been used to study both cellular and humoral immune responses to DENV.Citation98–Citation100 Further development of this humanized mouse model for dengue virus infection could provide a unique opportunity to test dengue vaccines before proceeding to human clinical trials.
`Our understanding of the nature of protective immune responses in humans is still incomplete. This was highlighted in the recent CYD TDV Phase IIb and III clinical trials in which neutralizing antibody titres did not correlate with protective efficacy. It will be important to develop optimized assays to measure vaccine immunogenicity and predict vaccine efficacy. Measuring dengue-specific neutralizing antibody is a commonly used readout of vaccine response. However, there is no recognized seroprotective neutralising antibody threshold for any dengue serotype. In addition, the maturation state and the dynamic nature of the viral envelope of dengue viruses can impact on the results of neutralization assays. The recent CYD TDV trials highlighted that measuring in vitro neutralizing antibodies alone was insufficient to predict the ability of the vaccine to protect across all dengue serotypes. Other vaccine manufacturers who currently rely on similar in vitro assays to measure neutralizing antibodies will encounter similar problems. The evolution of new dengue virus quasispecies may also impact on the development of long-term protective neutralizing antibody responses. Also, the nature of immune responses that follow repeated vaccination may not be analogous to responses that follow repeated natural infection, which are associated with long-term protection. This may imply that vaccine manufacturers will need to repeatedly update vaccines in order to maintain efficacy against circulating stains of DENV. More reliable measures of protective immune responses are therefore required.
To date current dengue vaccines have focused on the development protective neutralising antibody responses. However, the results of the CYD TDV trials has highlighted the need to better understand the role of non-neutralising antibody and cellular immune (both CD4+ and CD8+ T cell) responses against dengue virus and to dengue vaccines. This may be of greater significance for vaccines based on recombinant live attenuated viruses as these vaccines contain the full compliment of dengue non-structural proteins that are responsible for the strongest CD4+ and CD8+ T cell responses against dengue virus. One such measure could be the analysis of circulating Tfh CD4+ cells that are known to be strongly correlated with the development of protective antibody responses against viral infections. However, measuring this subset of CD4+T cells in blood may not be entirely practical in clinical trials. Determining the nature of cellular immune responses that may be associated with protection against dengue will present a complex challenge, particularly in a dengue or flavivirus primed population. Furthermore, the nature of protective cell mediated immune responses may also vary according to dengue serotype. However, addressing the role of protective cell mediated immune responses as a part of vaccine trials could provide critical information that would help to better design more effective dengue vaccines.
The CYD TDV trials and studies with LAV dengue vaccines in humans also raise the issue of the number of doses required over extended dosing schedules. The impact of extended dosing schedules on the potential development of incomplete immunity in endemic populations and the possibility of developing enhanced dengue infection will need to be determined. However, it must be stressed that there were no cases of DHF reported in the Phase III CYD TDV trials and that the longer-term follow-up safety data has shown that this vaccine has not been associated with severe dengue infection. The CYD TDV studies also demonstrated that vaccine efficacy was higher in individuals with pre-existing flavivirus immunity. It is therefore possible that exposure to dengue antigens through natural infection may contribute to the durability of dengue immune responses and this may result in the need for fewer doses of vaccine. Finally, extended dosing intervals will also pose a significant challenge for the use of dengue vaccines in travelers. Further research is required to determine whether shorter dosing schedules will be possible.
Conclusion
The intense research that is being focused on the development of DENV vaccines underscores the urgency, enormity and economic impact of the clinical problem. Although great progress has been made, indeed we now have a licensed vaccine, there is substantial room for improvement. But with the expansion in commercial interest in DENV vaccines it has become apparent that what is critically required is a better understanding of the early surrogates markers that best predict clinical efficacy. It would appear that a simple determination of NAb titres is not sufficient. The discrepancies between NAb titres and clinical efficacy highlighted in the review of clinical trials above is testament to the fact that our understanding of DENV protective immune responses is incomplete. With the myriad of DENV vaccines being progressed we urgently need better surrogate markers of vaccine efficacy that reliably predict clinical protection.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci U S A 2006; 103:6242-7; PMID:16606847; http://dx.doi.org/10.1073/pnas.0508391103
- World Health Organization. Global strategy for dengue prevention and control 2012
- Tapia-Conyer R, Betancourt-Cravioto M, Mendez-Galvan J. Dengue: an escalating public health problem in Latin America. Paediatr Int Child Health 2012; 32 Suppl 1:14-7; PMID:22668444; http://dx.doi.org/10.1179/2046904712Z.00000000046
- Dantes HG, Farfan-Ale JA, Sarti E. Epidemiological trends of dengue disease in Mexico (2000-2011): a systematic literature search and analysis. PLoS Negl Trop Dis 2014; 8:e3158; PMID:25375162; http://dx.doi.org/10.1371/journal.pntd.0003158
- Bravo L, Roque VG, Brett J, Dizon R, L'Azou M. Epidemiology of dengue disease in the Philippines (2000-2011): a systematic literature review. PLoS Negl Trop Dis 2014; 8:e3027; PMID:25375119; http://dx.doi.org/10.1371/journal.pntd.0003027
- Mohd-Zaki AH, Brett J, Ismail E, L'Azou M. Epidemiology of dengue disease in Malaysia (2000-2012): a systematic literature review. PLoS Negl Trop Dis 2014; 8:e3159; PMID:25375211; http://dx.doi.org/10.1371/journal.pntd.0003159
- Limkittikul K, Brett J, L'Azou M. Epidemiological trends of dengue disease in Thailand (2000-2011): a systematic literature review. PLoS Negl Trop Dis 2014; 8:e3241; PMID:25375766; http://dx.doi.org/10.1371/journal.pntd.0003241
- Teixeira MG, Siqueira JB, Jr, Ferreira GL, Bricks L, Joint G. Epidemiological trends of dengue disease in Brazil (2000-2010): a systematic literature search and analysis. PLoS Negl Trop Dis 2013; 7:e2520; PMID:24386496; http://dx.doi.org/10.1371/journal.pntd.0002520
- Villar L, Dayan GH, Arredondo-Garcia JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. Efficacy of a tetravalent dengue vaccine in children in Latin America. N Engl J Med 2015; 372:113-23; PMID:25365753; http://dx.doi.org/10.1056/NEJMoa1411037
- Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, et al. The global distribution and burden of dengue. Nature 2013; 496:504-7; PMID:23563266; http://dx.doi.org/10.1038/nature12060
- Ratnam I, Black J, Leder K, Biggs BA, Matchett E, Padiglione A, Woolley I, Panagiotidis T, Gherardin T, Pollissard L, et al. Incidence and seroprevalence of dengue virus infections in Australian travellers to Asia. Eur J Clin Microbiol Infect Dis 2012; 31:1203-10; PMID:21983919; http://dx.doi.org/10.1007/s10096-011-1429-1
- Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, Keystone JS, Pandey P, Cetron MS. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med 2006; 354:119-30; PMID:16407507; http://dx.doi.org/10.1056/NEJMoa051331
- Leder K, Mutsch M, Schlagenhauf P, Luxemburger C, Torresi J. Seroepidemiology of dengue in travellers: A paired sera analysis. Travel Med Infect Dis 2013; 11:210-3; PMID:23890678; http://dx.doi.org/10.1016/j.tmaid.2013.06.008
- Gomez-Dantes H, Willoquet JR. Dengue in the Americas: challenges for prevention and control. Cad Saude Publica 2009; 25 Suppl 1:S19-31; PMID:19287863; http://dx.doi.org/10.1590/S0102-311X2009001300003
- Flipse J, Smit JM. The Complexity of a Dengue Vaccine: A Review of the Human Antibody Response. PLoS Negl Trop Dis 2015; 9:e0003749; PMID:26065421; http://dx.doi.org/10.1371/journal.pntd.0003749
- Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 2011; 11:532-43; PMID:21760609; http://dx.doi.org/10.1038/nri3014
- Screaton G, Mongkolsapaya J, Yacoub S, Roberts C. New insights into the immunopathology and control of dengue virus infection. Nat Rev Immunol 2015; 15:745-59; PMID:26603900; http://dx.doi.org/10.1038/nri3916
- Green AM, Beatty PR, Hadjilaou A, Harris E. Innate immunity to dengue virus infection and subversion of antiviral responses. J Mol Biol 2014; 426:1148-60; PMID:24316047; http://dx.doi.org/10.1016/j.jmb.2013.11.023
- Wu MF, Chen ST, Yang AH, Lin WW, Lin YL, Chen NJ, Tsai IS, Li L, Hsieh SL. CLEC5A is critical for dengue virus-induced inflammasome activation in human macrophages. Blood 2013; 121:95-106; PMID:23152543; http://dx.doi.org/10.1182/blood-2012-05-430090
- Midgley CM, Bajwa-Joseph M, Vasanawathana S, Limpitikul W, Wills B, Flanagan A, Waiyaiya E, Tran HB, Cowper AE, Chotiyarnwong P, et al. An in-depth analysis of original antigenic sin in dengue virus infection. J Virol 2011; 85:410-21; PMID:20980526; http://dx.doi.org/10.1128/JVI.01826-10
- Malavige GN, Huang LC, Salimi M, Gomes L, Jayaratne SD, Ogg GS. Cellular and cytokine correlates of severe dengue infection. PLoS One 2012; 7:e50387; PMID:23209731; http://dx.doi.org/10.1371/journal.pone.0050387
- Pandey N, Jain A, Garg RK, Kumar R, Agrawal OP, Lakshmana Rao PV. Serum levels of IL-8, IFNgamma, IL-10, and TGF beta and their gene expression levels in severe and non-severe cases of dengue virus infection. Arch Virol 2015; 160:1463-75; PMID:25860648; http://dx.doi.org/10.1007/s00705-015-2410-6
- Schmid MA, Diamond MS, Harris E. Dendritic cells in dengue virus infection: targets of virus replication and mediators of immunity. Front Immunol 2014; 5:647; PMID:25566258; http://dx.doi.org/10.3389/fimmu.2014.00647
- Rivino L, Kumaran EA, Jovanovic V, Nadua K, Teo EW, Pang SW, Teo GH, Gan VC, Lye DC, Leo YS, et al. Differential targeting of viral components by CD4+ versus CD8+ T lymphocytes in dengue virus infection. J Virol 2013; 87:2693-706; PMID:23255803; http://dx.doi.org/10.1128/JVI.02675-12
- Rothman AL, Currier JR, Friberg HL, Mathew A. Analysis of cell-mediated immune responses in support of dengue vaccine development efforts. Vaccine 2015; 33:7083-90; PMID:26458801; http://dx.doi.org/10.1016/j.vaccine.2015.09.104
- Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology 2011; 411:306-15; PMID:21255816; http://dx.doi.org/10.1016/j.virol.2010.12.020
- Dejnirattisai W, Wongwiwat W, Supasa S, Zhang X, Dai X, Rouvinski A, Jumnainsong A, Edwards C, Quyen NT, Duangchinda T, et al. A new class of highly potent, broadly neutralizing antibodies isolated from viremic patients infected with dengue virus. Nat Immunol 2015; 16:170-7; PMID:25501631; http://dx.doi.org/10.1038/ni.3058
- Fibriansah G, Ibarra KD, Ng TS, Smith SA, Tan JL, Lim XN, Ooi JS, Kostyuchenko VA, Wang J, de Silva AM, et al. DENGUE VIRUS. Cryo-EM structure of an antibody that neutralizes dengue virus type 2 by locking E protein dimers. Science 2015; 349:88-91; PMID:26138979; http://dx.doi.org/10.1126/science.aaa8651
- Smith SA, de Alwis AR, Kose N, Harris E, Ibarra KD, Kahle KM, Pfaff JM, Xiang X, Doranz BJ, de Silva AM, et al. The potent and broadly neutralizing human dengue virus-specific monoclonal antibody 1C19 reveals a unique cross-reactive epitope on the bc loop of domain II of the envelope protein. MBio 2013; 4:e00873-13; PMID:24255124
- de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE Jr, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A 2012; 109:7439-44; PMID:22499787; http://dx.doi.org/10.1073/pnas.1200566109
- Fibriansah G, Tan JL, Smith SA, de Alwis AR, Ng TS, Kostyuchenko VA, Ibarra KD, Wang J, Harris E, de Silva A, et al. A potent anti-dengue human antibody preferentially recognizes the conformation of E protein monomers assembled on the virus surface. EMBO Mol Med 2014; 6:358-71; PMID:24421336
- de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brien JD, Tsai WY, et al. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl Trop Dis 2011; 5:e1188; PMID:21713020; http://dx.doi.org/10.1371/journal.pntd.0001188
- Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 2009; 392:103-13; PMID:19631955; http://dx.doi.org/10.1016/j.virol.2009.06.037
- Fibriansah G, Ng TS, Kostyuchenko VA, Lee J, Lee S, Wang J, Lok SM. Structural changes in dengue virus when exposed to a temperature of 37 °C. J Virol 2013; 87:7585-92; PMID:23637405; http://dx.doi.org/10.1128/JVI.00757-13
- Lok SM, Kostyuchenko V, Nybakken GE, Holdaway HA, Battisti AJ, Sukupolvi-Petty S, Sedlak D, Fremont DH, Chipman PR, Roehrig JT, et al. Binding of a neutralizing antibody to dengue virus alters the arrangement of surface glycoproteins. Nat Struct Mol Biol 2008; 15:312-7; PMID:18264114; http://dx.doi.org/10.1038/nsmb.1382
- Austin SK, Dowd KA, Shrestha B, Nelson CA, Edeling MA, Johnson S, Pierson TC, Diamond MS, Fremont DH. Structural basis of differential neutralization of DENV-1 genotypes by an antibody that recognizes a cryptic epitope. PLoS Pathog 2012; 8:e1002930; PMID:23055922; http://dx.doi.org/10.1371/journal.ppat.1002930
- Sukupolvi-Petty S, Austin SK, Engle M, Brien JD, Dowd KA, Williams KL, Johnson S, Rico-Hesse R, Harris E, Pierson TC, et al. Structure and function analysis of therapeutic monoclonal antibodies against dengue virus type 2. J Virol 2010; 84:9227-39; PMID:20592088; http://dx.doi.org/10.1128/JVI.01087-10
- Zust R, Toh YX, Valdes I, Cerny D, Heinrich J, Hermida L, Marcos E, Guillén G, Kalinke U, Shi PY, et al. Type I interferon signals in macrophages and dendritic cells control dengue virus infection: implications for a new mouse model to test dengue vaccines. J Virol 2014; 88:7276-85; PMID:24741106; http://dx.doi.org/10.1128/JVI.03827-13
- Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 2013; 110:E2046-53; PMID:23580623; http://dx.doi.org/10.1073/pnas.1305227110
- Zellweger RM, Miller R, Eddy WE, White LJ, Johnston RE, Shresta S. Role of humoral versus cellular responses induced by a protective dengue vaccine candidate. PLoS Pathog 2013; 9:e1003723; PMID:24204271; http://dx.doi.org/10.1371/journal.ppat.1003723
- Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM, Crotty S, Peters B, Sette A. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci U S A 2015; 112:E4256-63; PMID:26195744; http://dx.doi.org/10.1073/pnas.1505956112
- Schmitt N, Bentebibel SE, Ueno H. Phenotype and functions of memory Tfh cells in human blood. Trends Immunol 2014; 35:436-42; PMID:24998903; http://dx.doi.org/10.1016/j.it.2014.06.002
- Spensieri F, Borgogni E, Zedda L, Bardelli M, Buricchi F, Volpini G, Fragapane E, Tavarini S, Finco O, Rappuoli R, et al. Human circulating influenza-CD4+ ICOS1+IL-21+ T cells expand after vaccination, exert helper function, and predict antibody responses. Proc Natl Acad Sci U S A 2013; 110:14330-5; PMID:23940329; http://dx.doi.org/10.1073/pnas.1311998110
- Bentebibel SE, Lopez S, Obermoser G, Schmitt N, Mueller C, Harrod C, Flano E, Mejias A, Albrecht RA, Blankenship D, et al. Induction of ICOS+CXCR3+CXCR5+ TH cells correlates with antibody responses to influenza vaccination. Sci Transl Med 2013; 5:176ra32; PMID:23486778; http://dx.doi.org/10.1126/scitranslmed.3005191
- Li Y, Ma S, Tang L, Wang W, Huang X, Lai Q, Zhang M, Sun J, Li CK, Abbott WG, et al. Circulating chemokine (C-X-C Motif) receptor 5(+) CD4(+) T cells benefit hepatitis B e antigen seroconversion through IL-21 in patients with chronic hepatitis B virus infection. Hepatology 2013; 58:1277-86; PMID:23703545; http://dx.doi.org/10.1002/hep.26489
- Spaan M, Kreefft K, de Graav GN, Brouwer WP, de Knegt RJ, Ten Kate FJ, Baan CC, Vanwolleghem T, Janssen HL, Boonstra A. CD4(+)CXCR5(+) T cells in chronic HCV infection produce less IL-21, yet are efficient at supporting B cell responses. J Hepatol 2015; 62:303-10; PMID:25281860; http://dx.doi.org/10.1016/j.jhep.2014.09.024
- Guy B, Saville M, Lang J. Development of Sanofi Pasteur tetravalent dengue vaccine. Hum Vaccin 2010; 6; PMID:20861669; http://dx.doi.org/10.4161/hv.6.9.12739
- Guirakhoo F, Kitchener S, Morrison D, Forrat R, McCarthy K, Nichols R, Yoksan S, Duan X, Ermak TH, Kanesa-Thasan N, et al. Live attenuated chimeric yellow fever dengue type 2 (ChimeriVax-DEN2) vaccine: Phase I clinical trial for safety and immunogenicity: effect of yellow fever pre-immunity in induction of cross neutralizing antibody responses to all 4 dengue serotypes. Hum Vaccin 2006; 2:60-7; PMID:17012873; http://dx.doi.org/10.4161/hv.2.2.2555
- Guy B. Immunogenicity of sanofi pasteur tetravalent dengue vaccine. J Clin Virol 2009; 46:S16-S9; PMID:19800561; http://dx.doi.org/10.1016/S1386-6532(09)70290-2
- Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine 2011; 29:7229-41; PMID:21745521; http://dx.doi.org/10.1016/j.vaccine.2011.06.094
- Guy B, Briand O, Lang J, Saville M, Jackson N. Development of the Sanofi Pastuer tetravalent dengue vaccine: one more step forward. Vaccine 2015; 33:7100-11; PMID:26475445; http://dx.doi.org/10.1016/j.vaccine.2015.09.108
- Guy B, Guirakhoo F, Barban V, Higgs S, Monath TP, Lang J. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 2010; 28:632-49; PMID:19808029; http://dx.doi.org/10.1016/j.vaccine.2009.09.098
- Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol 2016; 14:45-54; PMID:26639777; http://dx.doi.org/10.1038/nrmicro.2015.2
- Guy B, Nougarede N, Begue S, Sanchez V, Souag N, Carre M, Chambonneau L, Morrisson DN, Shaw D, Qiao M, et al. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008; 26:5712-21; PMID:18762226; http://dx.doi.org/10.1016/j.vaccine.2008.08.019
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet (London, England) 2012; 380:1559-67; PMID:22975340; http://dx.doi.org/10.1016/S0140-6736(12)61428-7
- Capeding MR, Tran NH, Hadinegoro SR, Ismail HI, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: a phase 3, randomised, observer-masked, placebo-controlled trial. Lancet (London, England) 2014; 384:1358-65; PMID:25018116; http://dx.doi.org/10.1016/S0140-6736(14)61060-6
- Torresi J, Heron LG, Qiao M, Marjason J, Chambonneau L, Bouckenooghe A, Boaz M, van der Vliet D, Wallace D, Hutagalung Y, et al. Lot-to-lot consistency of a tetravalent dengue vaccine in healthy adults in Australia: a randomised study. Vaccine 2015; 33:5127-34; PMID:26279339; http://dx.doi.org/10.1016/j.vaccine.2015.08.008
- Wren LH, Chung AW, Isitman G, Kelleher AD, Parsons MS, Amin J, Cooper DA. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 2013; 138:116-23; PMID:23173935; http://dx.doi.org/10.1111/imm.12016
- Jegaskanda S, Job ER, Kramski M, Laurie K, Isitman G, de Rose R, Winnall WR, Stratov I, Brooks AG, Reading PC, et al. Cross-reactive influenza-specific antibody-dependent cellular cytotoxicity antibodies in the absence of neutralizing antibodies. J Immunol 2013; 190:1837-48; PMID:23319732; http://dx.doi.org/10.4049/jimmunol.1201574
- Hadinegoro SR, Arredondo-Garcia JL, Capeding MR, Deseda C, Chotpitayasunondh T, Dietze R, Muhammad Ismail HI, Reynales H, Limkittikul K, Rivera-Medina DM, et al. Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N Engl J Med 2015; 373:1195-206; PMID:26214039; http://dx.doi.org/10.1056/NEJMoa1506223
- Gailhardou S, Skipetrova A, Dayan GH, Jezorwski J, Saville M, Van der Vliet D, Wartel TA. Safety Overview of a Recombinant Live-Attenuated Tetravalent Dengue Vaccine: Pooled Analysis of Data from 18 Clinical Trials. PLoS Negl Trop Dis 2016; 10:e0004821; PMID:27414655; http://dx.doi.org/10.1371/journal.pntd.0004821
- Olivera-Botello G, Coudeville L, Fanouillere K, Guy B, Chambonneau L, Noriega F, Jackson N. Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue infections in healthy children and adolescents aged 2–16 years in Asia and Latin America. J Infect Dis 2016; 214(7):994-1000; PMID:27418050
- Osorio JE, Partidos CD, Wallace D, Stinchcomb DT. Development of a recombinant, chimeric tetravalent dengue vaccine candidate. Vaccine 2015; 33:7112-20; PMID:26585500; http://dx.doi.org/10.1016/j.vaccine.2015.11.022
- Huang CY, Kinney RM, Livengood JA, Bolling B, Arguello JJ, Luy BE, Silengo SJ, Boroughs KL, Stovall JL, Kalanidhi AP, et al. Genetic and phenotypic characterization of manufacturing seeds for a tetravalent dengue vaccine (DENVax). PLoS Negl Trop Dis 2013; 7:e2243; PMID:23738026; http://dx.doi.org/10.1371/journal.pntd.0002243
- Osorio JE, Huang CY, Kinney RM, Stinchcomb DT. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine 2011; 29:7251-60; PMID:21777638; http://dx.doi.org/10.1016/j.vaccine.2011.07.020
- Brewoo JN, Kinney RM, Powell TD, Arguello JJ, Silengo SJ, Partidos CD, Huang CY, Stinchcomb DT, Osorio JE. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine 2012; 30:1513-20; PMID:22178727; http://dx.doi.org/10.1016/j.vaccine.2011.11.072
- Henriques HR, Rampazo EV, Goncalves AJ, Vicentin EC, Amorim JH, Panatieri RH, Amorim KN, Yamamoto MM, Ferreira LC, Alves AM, et al. Targeting the non-structural protein 1 from dengue virus to a dendritic cell population confers protective immunity to lethal virus challenge. PLoS Negl Trop Dis 2013; 7:e2330; PMID:23875054; http://dx.doi.org/10.1371/journal.pntd.0002330
- Osorio JE, Brewoo JN, Silengo SJ, Arguello J, Moldovan IR, Tary-Lehmann M, Powell TD, Livengood JA, Kinney RM, Huang CY, et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in Cynomolgus macaques. Am J Trop Med Hyg 2011; 84:978-87; PMID:21633037; http://dx.doi.org/10.4269/ajtmh.2011.10-0592
- Fuchs J, Chu H, O'Day P, Pyles R, Bourne N, Das SC, Milligan GN, Barrett AD, Partidos CD, Osorio JE. Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice. Vaccine 2014; 32:6537-43; PMID:25239488; http://dx.doi.org/10.1016/j.vaccine.2014.08.087
- Osorio JE, Velez ID, Thomson C, Lopez L, Jimenez A, Haller AA, Silengo S, Scott J, Boroughs KL, Stovall JL, et al. Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study. Lancet Infect Dis 2014; 14:830-8; PMID:25087476; http://dx.doi.org/10.1016/S1473-3099(14)70811-4
- George SL, Wong MA, Dube TJ, Boroughs KL, Stovall JL, Luy BE, Haller AA, Osorio JE, Eggemeyer LM, Irby-Moore S, et al. Safety and Immunogenicity of a Live Attenuated Tetravalent Dengue Vaccine Candidate in Flavivirus-Naive Adults: A Randomized, Double-Blinded Phase 1 Clinical Trial. J Infect Dis 2015; 212:1032-41; PMID:25791116; http://dx.doi.org/10.1093/infdis/jiv179
- Sirivichayakul C, Barranco-Santana EA, Esquilin-Rivera I, Oh HM, Raanan M, Sariol CA, Shek LP, Simasathien S, Smith MK, Velez ID, et al. Safety and Immunogenicity of a Tetravalent Dengue Vaccine Candidate in Healthy Children and Adults in Dengue-Endemic Regions: A Randomized, Placebo-Controlled Phase 2 Study. J Infect Dis 2016; 213:1562-72; PMID:26704612; http://dx.doi.org/10.1093/infdis/jiv762
- Precioso AR, Palacios R, Thome B, Mondini G, Braga P, Kalil J. Clinical evaluation strategies for a live attenuated tetravalent dengue vaccine. Vaccine 2015; 33:7121-5; PMID:26458796; http://dx.doi.org/10.1016/j.vaccine.2015.09.105
- Lindow JC, Durbin AP, Whitehead SS, K.K. P, Carmolli MP, Kirkpatrick BD. Vaccination of volunteers with low-dose, live-attenuated, dengue viruses leads toserotype-specific immunologic and virologic profiles. Vaccine 2013; 31:3347-52; PMID:23735680; http://dx.doi.org/10.1016/j.vaccine.2013.05.075
- Kirkpatrick BD, Durbin AP, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Diehl SA, Elwood D, Jarvis AP, et al. Robust and Balanced Immune Responses to All 4 Dengue Virus Serotypes Following Administration of a Single Dose of a Live Attenuated Tetravalent Dengue Vaccine to Healthy, Flavivirus-Naive Adults. J Infect Dis 2015; ;212(5):702-10; PMID:25801652
- Weiskopf D, Angelo MA, Bangs DJ, Sidney J, Paul S, Peters B, de Silva AD, Lindow JC, Diehl SA, Whitehead S, et al. The Human CD8 T Cell Responses Induced by a Live Attenuated Tetravalent Dengue Vaccine Are Directed against Highly Conserved Epitopes. J Virol 2015; 89:120-8; PMID:25320311; http://dx.doi.org/10.1128/JVI.02129-14
- Durbin AP, Whitehead SS, Shaffer D, Elwood D, Wanionek K, Thumar B, Blaney JE, Murphy BR, Schmidt AC. A single dose of the DENV-1 candidate vaccine rDEN1Delta30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl Trop Dis 2011; 5:e1267; PMID:21829748; http://dx.doi.org/10.1371/journal.pntd.0001267
- Durbin AP, McArthur JH, Marron JA, Blaney JE, Thumar B, Wanionek K, Murphy BR, Whitehead SS. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum Vaccin 2006; 2:255-60; PMID:17106267; http://dx.doi.org/10.4161/hv.2.6.3494
- Durbin AP, Whitehead SS, McArthur J, Perreault JR, Blaney JE, Jr, Thumar B, Murphy BR, Karron RA. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis 2005; 191:710-8; PMID:15688284; http://dx.doi.org/10.1086/427780
- Durbin AP, Kirkpatrick BD, Pierce KK, Elwood D, Larsson CJ, Lindow JC, Tibery C, Sabundayo BP, Shaffer D, Talaat KR, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double-blind clinical trial. J Infect Dis 2013; 207:957-65; PMID:23329850; http://dx.doi.org/10.1093/infdis/jis936
- Manoff SB, George SL, Bett AJ, Yelmene ML, Dhanasekaran G, Eggemeyer L, Sausser ML, Dubey SA, Casimiro DR, Clements DE, et al. Preclinical and clinical development of a dengue recombinant subunit vaccine. Vaccine 2015; 33:7126-34; PMID:26458804; http://dx.doi.org/10.1016/j.vaccine.2015.09.101
- Clements DE, Coller BA, Lieberman MM, Ogata S, Wang G, Harada KE, Putnak JR, Ivy JM, McDonell M, Bignami GS, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine 2010; 28:2705-15; PMID:20097152; http://dx.doi.org/10.1016/j.vaccine.2010.01.022
- Coller BA, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine 2011; 29:7267-75; PMID:21777637; http://dx.doi.org/10.1016/j.vaccine.2011.07.021
- Govindarajan D, Meschino S, Guan L, Clements DE, ter Meulen JH, Casimiro DR, Coller BA, Bett AJ. Preclinical development of a dengue tetravalent recombinant subunit vaccine: Immunogenicity and protective efficacy in nonhuman primates. Vaccine 2015; 33:4105-16; PMID:26144900; http://dx.doi.org/10.1016/j.vaccine.2015.06.067
- Porter KR, Raviprakash K. Nucleic acid (DNA) immunization as a platform for dengue vaccine development. Vaccine 2015; 33:7135-40; PMID:26458805; http://dx.doi.org/10.1016/j.vaccine.2015.09.102
- Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, Wu SJ, Sun P, Kochel T, Raviprakash K, et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine 2011; 29:960-8; PMID:21111785; http://dx.doi.org/10.1016/j.vaccine.2010.11.050
- Raviprakash K, Apt D, Brinkman A, Skinner C, Yang S, Dawes G, Ewing D, Wu SJ, Bass S, Punnonen J, et al. A chimeric tetravalent dengue DNA vaccine elicits neutralizing antibody to all four virus serotypes in rhesus macaques. Virology 2006; 353:166-73; PMID:16814355; http://dx.doi.org/10.1016/j.virol.2006.05.005
- Raviprakash K, Kochel TJ, Ewing D, Simmons M, Phillips I, Hayes CG, Porter KR. Immunogenicity of dengue virus type 1 DNA vaccines expressing truncated and full length envelope protein. Vaccine 2000; 18:2426-34; PMID:10738100; http://dx.doi.org/10.1016/S0264-410X(99)00570-8
- Kochel T, Wu SJ, Raviprakash K, Hobart P, Hoffman S, Porter K, Hayes C. Inoculation of plasmids expressing the dengue-2 envelope gene elicit neutralizing antibodies in mice. Vaccine 1997; 15:547-52; PMID:9160523; http://dx.doi.org/10.1016/S0264-410X(97)00215-6
- Thomas SJ. Developing a dengue vaccine: progress and future challenges. Ann N Y Acad Sci 2014; 1323:140-59; PMID:24689974; http://dx.doi.org/10.1111/nyas.12413
- Porter KR, Ewing D, Chen L, Wu SJ, Hayes CG, Ferrari M, Teneza-Mora N, Raviprakash K. Immunogenicity and protective efficacy of a vaxfectin-adjuvanted tetravalent dengue DNA vaccine. Vaccine 2012; 30:336-41; PMID:22085548; http://dx.doi.org/10.1016/j.vaccine.2011.10.085
- Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, Casares S. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS One 2011; 6:e19826; PMID:21611197; http://dx.doi.org/10.1371/journal.pone.0019826
- Ippolito GC, Hoi KH, Reddy ST, Carroll SM, Ge X, Rogosch T, Zemlin M, Shultz LD, Ellington AD, Vandenberg CL, et al. Antibody repertoires in humanized NOD-scid-IL2Rgamma(null) mice and human B cells reveals human-like diversification and tolerance checkpoints in the mouse. PLoS One 2012; 7:e35497; PMID:22558161; http://dx.doi.org/10.1371/journal.pone.0035497
- Singh M, Singh P, Gaudray G, Musumeci L, Thielen C, Vaira D, Vandergeeten C, Delacroix L, Van Gulck E, Vanham G, et al. An improved protocol for efficient engraftment in NOD/LTSZ-SCIDIL-2Rgammanull mice allows HIV replication and development of anti-HIV immune responses. PLoS One 2012; 7:e38491; PMID:22675567; http://dx.doi.org/10.1371/journal.pone.0038491
- Thomas S, Klobuch S, Sommer M, van Ewijk R, Theobald M, Meyer RG, Herr W. Human CD8+ memory and EBV-specific T cells show low alloreactivity in vitro and in CD34+ stem cell-engrafted NOD/SCID/IL-2Rgammac null mice. Exp Hematol 2014; 42:28-38 e1-2; http://dx.doi.org/10.1016/j.exphem.2013.09.013
- Mota J, Rico-Hesse R. Humanized mice show clinical signs of dengue fever according to infecting virus genotype. J Virol 2009; 83:8638-45; PMID:19535452; http://dx.doi.org/10.1128/JVI.00581-09
- Mota J, Rico-Hesse R. Dengue virus tropism in humanized mice recapitulates human dengue fever. PLoS One 2011; 6:e20762; PMID:21695193; http://dx.doi.org/10.1371/journal.pone.0020762
- Jaiswal S, Smith K, Ramirez A, Woda M, Pazoles P, Shultz LD, Greiner DL, Brehm MA, Mathew A. Dengue virus infection induces broadly cross-reactive human IgM antibodies that recognize intact virions in humanized BLT-NSG mice. Exp Biol Med (Maywood) 2015; 240:67-78; PMID:25125497; http://dx.doi.org/10.1177/1535370214546273
- Chung YS, Son JK, Choi B, Joo SY, Lee YS, Park JB, Moon H, Kim TJ, Kim SH, Hong S, et al. Co-transplantation of human fetal thymus, bone and CD34(+) cells into young adult immunodeficient NOD/SCID IL2Rgamma(null) mice optimizes humanized mice that mount adaptive antibody responses. Clin Immunol 2015; 157:156-65; PMID:25725428; http://dx.doi.org/10.1016/j.clim.2015.02.005
- Chung YS, Son JK, Choi B, Park JB, Chang J, Kim SJ. Transplantation of human spleen into immunodeficient NOD/SCID IL2Rgamma(null) mice generates humanized mice that improve functional B cell development. Clin Immunol 2015; 161:308-15; PMID:26360254; http://dx.doi.org/10.1016/j.clim.2015.09.001
