ABSTRACT
Considering the pandemic potential of avian influenza A/H5N1, development of an effective and well-tolerated vaccine is an essential part of pandemic preparedness plans. This phase III, randomized, double-blind study was conducted to assess the immunogenicity and safety profile of an alum-adjuvanted, whole virion, pre-pandemic influenza A/H5N1 vaccine (MG1109). Healthy individuals were randomly assigned, in a 3:1 ratio, to receive two doses of either MG1109 or placebo containing alum gel. Immunogenicity was determined by hemagglutination inhibition (HI) and microneutralization (MN) assays. Solicited and unsolicited adverse events were assessed after vaccination. Among 420 enrolled subjects, 418 were available for safety analysis, and 298 MG1109 recipients were available for per-protocol immunogenicity analyses. According to the HI assays, after two vaccine doses, all three of the Committee for Medicinal Products for Human Use (CHMP) criteria were met against the vaccine strain for all age groups: seroprotection rate = 74.8% (95% CI: 69.9 – 79.8), seroconversion rate = 67.8% (95% CI: 62.5–73.1), and geometric mean titer ratio (GMTR) = 5.9 (95% CI: 5.4 – 6.4). According to the MN assays, the GMTR was 2.4 (95% CI: 2.1 – 2.7) and 7.0 (95% CI: 6.3 – 7.9) three weeks after the first and second vaccine doses, respectively. Solicited local and systemic adverse events were mostly mild to moderate and were not significantly different between MG1109 and placebo recipients. In conclusion, two-dose administration of alum-adjuvanted H5N1 pre-pandemic influenza vaccine (MG1109) was highly immunogenic and tolerable in adults.
Introduction
Highly pathogenic avian influenza A/H5N1 was first detected in geese in China in 1996. One year later, human cases of avian influenza A/H5N1 were identified in Hong Kong. Since then, avian influenza A/H5N1 viruses spread widely to more than 50 countries in Asia, Africa, Europe, and America.Citation1 The virus is endemic in six countries, including Bangladesh, China, Egypt, Indonesia, and Vietnam. As of October 2016, 856 laboratory-confirmed cases were reported with a high case-fatality rate (452 death, 52.8%).Citation1 Among avian influenza viruses, influenza A/H5N1 and A/H7N9 viruses have particularly high pandemic potential.
As experienced during previous 2009 influenza A/H1N1 pandemics, vaccination is the most important measure to mitigate an outbreak. Thus, development and stockpiling of pre-pandemic influenza vaccines should be an essential component of any pandemic preparedness plan.
This phase III, randomized, double-blind study was conducted to assess the immunogenicity and safety of an alum-adjuvanted, pre-pandemic influenza A/H5N1 vaccine in healthy adults.
Results
Study subjects
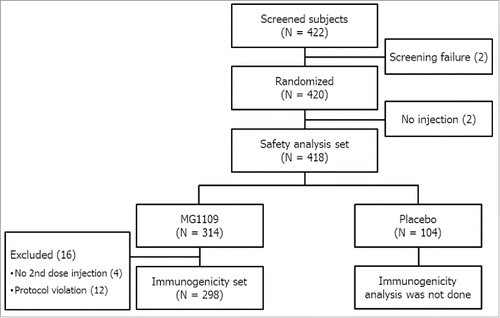
Among 422 screened subjects, a total of 420 healthy subjects ≥ 18 y old were enrolled and organized into age groups: 18 – 29 y (N = 135), 30 – 49 y (N = 223), and 50 – 60 y (N = 60). Enrolled subjects were randomized, in a 3:1 ratio, into either the MG1109 (315 subjects) or placebo control (105 subjects) group (). Among them, 418 subjects were included in the study; two subjects (one from each group) were excluded because they received neither MG1109 nor placebo. Demographic and baseline characteristics were well matched between the two groups, as shown in . Among the 418 subjects evaluated in the safety analysis, 314 received MG1109 and 104 received the placebo (). Among 314 MG1109 recipients, 298 were available for per-protocol immunogenicity analyses.
Table 1. Demographic characteristics of the study subjects.
Immunogenicity
Antibody responses against the vaccine antigen of the NIBRG-14 virus strain are shown in . None of the subjects had seroprotective HI titers at baseline before vaccination and all their GMT levels were low (6.6, 95% CI: 6.3 – 6.9). After the first vaccine dose (day 22), most CHMP criteria were not met. After the second vaccine dose (day 43), all three CHMP criteria were met against the vaccine strain in all age groups, according to the HI assay: seroprotection rate = 74.8% (95% CI: 69.9 – 79.8), seroconversion rate = 67.8% (95% CI: 62.5 – 73.1) and GMTR = 5.9 (95% CI: 5.4 – 6.4).
Table 2. Immune responses after immunization with MG1109, as measured using a hemagglutination-inhibition (HI) assay.
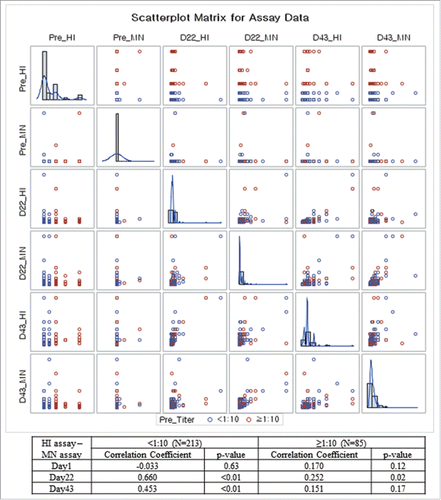
In this study, 85 (28.5%) subjects had detectable HI titers (≥ 1:10) before vaccination, although their levels fell below the seroprotective level: 67 (22.5%) subjects had a 1:10 titer while 18 (6.01%) subjects had a 1:20 titer. In , demographic characteristics and immune responses are compared between subjects with detectable HI titers (≥ 1:10) and those with non-detectable HI titers. Subjects with detectable HI titers (≥ 1:10) had higher GMTs after the first vaccination dose, but these subjects’ titers were indistinguishable after the second dose.
Table 3. Comparison of demographic characteristics and immune responses based on pre-vaccination hemagglutinin-inhibition (HI) titers: subjects with detectable HI titers (≥ 1:10) vs. those with non-detectable HI titers.
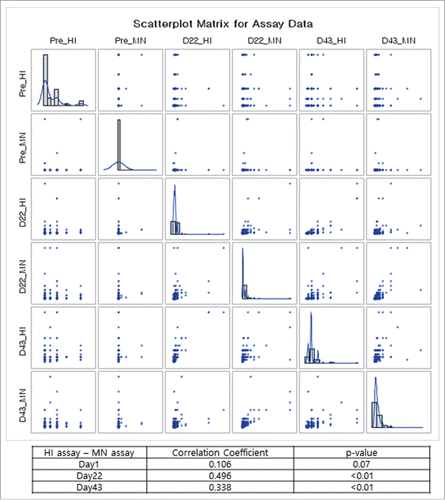
When analyzed by MN assay, GMTs increased from 5.1 at baseline (before vaccination) to 12.2 at day 22 (after first vaccine dose) and to 35.6 at day 43 (after second dose) (). Compared to baseline GMT at day 1, GMTR was 2.4 (first dose, 95% CI: 2.1 – 2.7) and 7.0 (second dose, 95% CI: 6.3 – 7.9). As shown in , the MN and HI assay results showed similar trends in GMT increase after the first and second vaccination doses. HI and MN assays were positively correlated at day 22 (r = 0.496, p < 0.01) and day 43 (r = 0.338, p < 0.01) (A). In the subgroup analyses, HI and MN assays were highly correlated at day 22 (r = 0.660, p < 0.01) and day 43 (r = 0.453, p < 0.01) among subjects with pre-vaccination HI titers <1:10. However, the correlation was weak among those with HI titers ≥ 1:10 at day 22 (r = 0.252, p = 0.02) and day 43 (r = 0.151, p = 0.17).
Table 4. Immune response after immunization with MG1109 at 3 and 6 weeks post-vaccination: hemagglutinin-inhibition assay and micro-neutralization assay.
Safety
Serial two-dose injections of the A/H5N1 study vaccine (MG1109) were well tolerated without any vaccine-related SAEs. Solicited local and systemic adverse events reported within seven days of vaccination are shown in . Overall, 78.7% (247/314) and 51.0% (160/314) of MG1109 recipients reported solicited local or systemic adverse events, respectively, after the first vaccination. Most SEAs were grade 1 (85.9%) or grade 2 (13.0%) and there were no significant differences in SEA-report rates between the study group and the placebo group in local adverse events (78.7% versus 72.1%, p = 0.17) and systemic adverse events (51.0% vs. 42.3%, p = 0.13). The most common solicited local adverse event was injection-site pain (78.7% after first dose and 59.9% after second dose). As for solicited systemic adverse events, myalgia (35.4%) was the most common, followed by fatigue (33.4%), and headache (25.5%). Myalgia (p < 0.01), headache (p = 0.01), chill (p = 0.04), and hyperhidrosis (p = 0.04) were more common among MG1109 recipients than placebo recipients after the first vaccination dose, but not after the second dose. Local and systemic SEAs were both less frequent after the second dose than the first dose in both the MG1109 and placebo groups.
Table 5. Solicited local and systemic adverse events within seven days after vaccination: MG1109 vs. placebo control.
Discussion
This study showed that two-dose priming of alum-adjuvanted, inactivated, whole-virus influenza A/H5N1 vaccine (MG1109, Green Cross Corporation) is immunogenic and well tolerated. The HI assay has been reported as being insensitive for measuring H5 antibodies, but our MG1109 study vaccine fulfilled all CHMP criteria, according to the HI assay. HI titers correlated well with those from the MN assay. Moreover, although a whole-virion influenza vaccine has been considered less safe than split formulations, MG1109 had a good safety profile. The rate of solicited AEs among MG1109 recipients was comparable to those from previous clinical trials of influenza vaccines, including both H5N1 monovalent and conventional trivalent influenza vaccines.Citation2-6 According to the meta-analysis of trials that assessed influenza H5N1 vaccine safety and immunogenicity, the rates of adverse events were reported variably: fever (0–8.7%), headache (8.3–46%), malaise (4–55%), myalgia (4–47%), local pain (14–89%) and erythema (0–42%).Citation7 In addition to the vaccine composition, the difference in the definition and severity threshold of adverse events might responsible for the wide range observed. Adverse events were not dose-dependent, and no SAE was reported in any trial.Citation7
In this study, subjects with either a history of A/H5N1 exposure or previous immunization with an A/H5N1 vaccine were excluded. Nevertheless, 85 (28.5%) subjects had detectable HI titers against the H5 antigen. One explanation for this could be a prior H1N1 influenza infection, which would affect assay results. In a mouse model, influenza A/H1N1 virus infection induced hetero-subtypic cross-reactivity to the avian influenza A/H5N1 virus.Citation8 In subjects with detectable pre-vaccination HI titers (≥ 1:10), the results from the HI and MN assays were not well correlated, indicating non-specific cross-reactive immune responses. Primed subjects were expected to produce higher antibody levels after the first vaccination, but booster effects were not remarkable in subjects with detectable baseline HI titers compared with those with undetectable HI titers. Secondly, some study subjects might have been exposed to influenza A/H5N1 or A/H5N8 viruses in the past, although symptomatic human cases have not yet been reported in the Republic of Korea (ROK). Since 2003, there have been five highly pathogenic avian influenza (HPAI) virus outbreaks in the ROK: four HPAI H5N1 outbreaks and one HPAI H5N8 outbreak.Citation9 Migratory birds were thought to be the initial source of HPAI in Korea. Since early 2014, HPAI H5N8 has been frequently detected in migratory birds and poultry.Citation10,Citation11
A single-dose of A/H5N1 influenza vaccine (15 µg) was insufficient to induce a protective immune response. According to the meta-analysis, high-dose (≥ 30 µg) un-adjuvanted H5N1 influenza vaccine was better immunogenic compare with 15 µg-dose, but the difference was insignificant in case of alum- and non-alum adjuvanted vaccines.Citation7 As presented in this study, either a two-dose priming or a priming-booster vaccination strategy is required. In previous studies, priming with a clade-mismatched pandemic influenza A/H5N1 vaccine improved the rapidity and magnitude of the immunological response following a heterologous single-dose pandemic H5N1 vaccine.Citation3,Citation12,Citation13 In fact, it is impossible to accurately predict which clade/subclade of H5N1 will cause the next pandemic. Considering the possibility of cross-reactive immunogenicity, strategic priming with the pre-pandemic H5N1 influenza vaccine-not just vaccine stockpiling-is necessary as part of a pandemic preparedness plan. Furthermore, it would be useful for testing diverse dose-priming and boosting regimens.
The MG1109 vaccine strain, A/Vietnam/1194/2004 /H5N1, was first isolated in 2004 from an infected human and was classified as clade 1.Citation14 Since then, avian influenza H5N1 viruses have continued to spread, evolve, and diversify genetically. There are currently seven clades of H5N1 (1, 2.1.3, 2.2, 2.2.1, 2.3.2, 2.3.4, and 7) in worldwide circulation.Citation15 Fortunately, a number of studies showed cross-reactive immunogenicity between different H5N1 clades.Citation12,Citation13 Cross-reactive immunity of MG1109 needs to be thoroughly investigated in light of the current H5N8 epidemic in the ROK and other H5 avian influenza viruses, including H5N1 and H5N2 subtypes.
This study has several limitations. First, cross-reactive and long-term immunogenicity were not evaluated. Further studies would be required after licensure of MG1109 from the Korean FDA. Second, licensed H5N1 vaccines were not used as controls because they were not available in the ROK. Third, data on prior seasonal influenza vaccinations were not collected. Given the cross-reactivity of some of the stem epitopes of the group-1 influenza virus strains (including H1 and H5), it is possible that the H5N1 vaccine stimulates a small number of pre-existing H1 stem plasmablasts rather than inducing a new response to the H5 hemagglutinin head in seasonal vaccine recipients, thereby giving a negative influence to the immunogenicity of the H5N1 influenza vaccine.Citation16,Citation17 Finally, some subjects had detectable antibodies against H5 antigen before vaccination, but available data were limited.
In summary, MG1109, a pre-pandemic influenza A/H5N1 vaccine showed excellent immunogenicity and a good safety profile in adults. Pre-pandemic influenza vaccine should be an important component of any pandemic preparedness plan. During early pandemic periods, stepwise targeted use of pre-pandemic vaccines may mitigate the rapid spread of influenza.
Materials and methods
Study design
A phase III, randomized, double-blind study was conducted to assess the immunogenicity and safety profile of an alum-adjuvanted, pre-pandemic influenza vaccine (MG1109) containing 15 µg of NIBRG-14 viral antigen in healthy adults aged 18 – 60 y at four centers in the ROK (clinical trial number: NCT01987011).
Subjects were excluded from this study for any of the following reasons: history of exposure to A/H5N1 virus or previous immunization with an A/H5N1 vaccine; allergy to eggs; history of Guillain-Barre syndrome; immunodeficiency or receipt of immunosuppressive therapy; active neoplastic disease; pregnant or breast-feeding; coagulation disorder; administration of blood products or immunoglobulins within the past three months; fever (≥ 38°C) within the past 72 hours or any other serious diseases ≤ 14 d prior to study enrollment; or any condition that interfered with evaluation of the study objectives as judged by the investigators.
Study subjects were recruited and stratified into three age groups: 18 – 29, 30 – 49, and 50 – 60 y. Each subject was randomly assigned, in a 3:1 ratio, to receive two doses of either the experimental vaccine (MG1109) or a placebo containing alum gel; doses were given three weeks apart. Randomization was performed using an interactive web response system (IWRS) at enrollment time. IWRS is an automated randomization system to ensure quick, accurate, balanced and valid subject assignments by using the latest statistical methods, programming and validation procedures. An unblended pharmacist provided the study drug (vaccine/placebo) according to the unique randomization number. The study was double blinded to study subjects, investigators and sponsor until the end of clinical trial; the database was locked until the completion of laboratory test and review.
Blood samples were collected for immunogenicity analyses at baseline (day 1), three weeks after administration of the first vaccine dose (day 22), and three weeks after administration of the second dose (day 43). Seven to ten days after each vaccination dose, a phone interview was conducted to assess subject safety.
The primary immunogenicity objective was to evaluate whether MG1109-induced hem-agglutination inhibition (HI) responses met the Committee for Medicinal Products for Human Use (CHMP) criteria among healthy adults. The secondary immunogenicity objective aimed to assess immune responses (geometric mean titer and geometric mean titer ratio) based on micro-neutralization (MN) assays. Safety objectives included evaluation of solicited local and systemic adverse events (AEs), spontaneously reported unsolicited AEs, and serious adverse events (SAEs).
The study was conducted in accordance with the Declaration of Helsinki and the standards of Good Clinical Practice as defined by the International Conference on Harmonization. The protocol and consent forms were approved by the Institutional Review Boards of each participating study site. Informed written consent was obtained from all subjects following a detailed explanation of schedules and study protocols.
Vaccines
Our study vaccine (MG1109) was an alum-adjuvanted, inactivated, whole-virus vaccine (Green Cross Corporation, Yongin, ROK). Each 0.5-mL vaccine dose contained 15 µg of NIBRG-14 viral antigen, which is a reassortant prepared by reverse genetics from the A/Vietnam/1194/2004 (H5N1) virus (in which the polybasic hemagglutinin cleavage site was excised) and the A/PR/8/34(H1N1) virus. The placebo control (0.5 mL) was composed of alum gel and all the same vaccine ingredients except for the H5N1 antigen. Vaccine and placebo were administered intramuscularly into the deltoid region of the arm.
Immunogenicity assessment
Immunogenicity was determined using HI and MN assays, which were run in the Green Cross Laboratories. Sera were tested in duplicate at each time point by blinded personnel. The GMT of duplicates was calculated and used in our analyses.
The HI test was performed using the standard assay method, modified to utilize an equine erythrocyte suspension instead of an avian erythrocyte suspension.Citation18 HI titers were assessed against the hemagglutinin antigen of the NIBRG-14 virus strain. The three post-vaccination immunogenicity end points were the proportion of subjects with antibody titers of 1:40 or more according to HI assay (seroprotection rate), the proportion of subjects who seroconverted (a change in HI titer from <1:10 to ≥ 1:40) or experienced a 4-fold or more increase in antibody titer (seroconversion rate), and the geometric mean titer ratio (GMTR) (i.e., the ratio of the geometric mean titer after and before vaccination).Citation19 Serologic response, as measured by HI antibody titers, was assessed using the criteria set by the CHMP of the European Medicines Agency (EMA). Protective immunogenicity based on the CHMP criteria was confirmed when all of the following criteria were met: a seroprotection rate >70% for subjects aged 19 – 60 y old and >60% for subjects over 60 y old, seroconversion rates >40% for subjects aged 19 – 60 y old and >30% for subjects over 60 y old, or GMT ratios >2.5 for subjects aged 19 – 60 y old and >2.0 for subjects over 60 y old.Citation19
MN assays were performed according to the World Health Organization (WHO) protocol, with serial dilutions of serum starting at 1:10. The reciprocals of the 2-fold dilutions that achieved ≥ 50% viral growth neutralization were considered positive.Citation20 GMTs were estimated at each time point, and GMTRs (day 22/day 1 and day 43/day 1) were calculated.
Safety assessment
For the safety assessment, subjects were given a digital thermometer and a diary card containing of a list of solicited adverse events and a numerical scale to grade each event experienced. For seven days post-vaccination, subjects were asked to record the severity of solicited local and systemic adverse events, their body temperature via oral measurement, and other current medications on the diary card. Solicited local adverse events included pain, tenderness, redness and swelling, and solicited systemic adverse events included fever, headache, malaise/fatigue, myalgia, arthralgia, vomiting, and diarrhea. We requested reports of any unsolicited AEs for up to 24 weeks after the second vaccination. Subjects used a standard scale to grade adverse events.Citation21 During the study period, subjects were required to report any SAEs within 24 hours.
Statistical analyses
Immunogenicity analyses were run for each protocol set and included all enrolled subjects who received the vaccine correctly, provided evaluable serum samples at the appropriate time points, and had no other major protocol deviations. Safety was analyzed for all subjects who received the study vaccine.
The sample size for the immunogenicity was estimated to demonstrate that the lower limit of the 95% CI for seroprotection rate would be ≥ 70% in the vaccinated subjects at day 43, using a 2-sided 1-proportion test (α = 2.5%). We calculated a target sample size for the MG1109 group of 289 with at least 95% power. Considering a 10% drop-out rate, a sample size of 320 subjects was required to examine the primary immunogenicity objective. To assess the safety of MG1109, 100 additional subjects were included for the placebo control group in a 3:1 ratio. For the safety assessment, the sample size with the randomization ratio of 3: 1 was considered enough to maintain the statistical power.
All statistics were generated using SAS software (version 9.2; SAS Institute, Cary, NC, USA). Immunogenicity data were expressed in terms of GMTs (mean ± standard deviation) and CHMP criteria with two-sided 95% confidence intervals (CIs). Two-sided 95% CIs for GMTs and GMT ratios were calculated using the normal approximation of log-transformed titers, and percentages were calculated with approximate or exact 95% CIs. Safety data are presented as the proportion of study subjects reporting local and systemic adverse reactions. Independent two-sample t-tests (by log-normal distribution) were used to compare GMTs and GMT ratios between the two time points. The chi-square test or Fisher's exact test were conducted to analyze categorical variables. Independent two-sample t-tests were conducted to analyze continuous variables. Correlation analyses were performed by Pearson's method. Results were considered statistically significant if the P-value was less than 0.05.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful to the MG1109 study team and the subjects for providing samples and other data for this work. The authors also thank the laboratory staff at each of the participating institutions for assistance with sample management and testing.
Funding
This work was supported by a grant (code# 2014ER430200) from the Research of Korea Centers for Disease Control and Prevention.
References
- World Health Organization. Cumulative number of confirmed human cases of avian influenza A(H5N1) reported to WHO. Geneva, Switzerland: World Health Organization, 2016. [ accessed 2016 November 05]. http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/
- Izurieta P, Kim WJ, Wie SH, Lee J, Lee JS, Drame M, Vaughn DW, Schuind A. Immunogenicity and safety of an AS03-adjuvanted H5N1 pandemic influenza vaccine in Korean adults: a phase IV, randomized, open-label, controlled study. Vaccine 2015; 33:2800–7; PMID:25910919; http://dx.doi.org/10.1016/j.vaccine.2015.04.027
- Langley JM, Frenette L, Jeanfreau R, Halperin SA, Kyle M, Chu L, McNeil S, Dramé M, Moris P, Fries L, et al. Immunogenicity of heterologous H5N1 influenza booster vaccination 6 or 18 months after primary vaccination in adults: a randomized controlled clinical trial. Vaccine 2015; 33:559–67; PMID:25448092; http://dx.doi.org/10.1016/j.vaccine.2014.11.018
- Song JY, Cheong HJ, Lee J, Wie SH, Park KH, Kee SY, Jeong HW, Kim YS, Noh JY, Choi WS, et al. Phase IV: randomized controlled trial to evaluate lot consistency of trivalent split influenza vaccines in healthy adults. Hum Vaccin Immunother 2014; 10:2958–64; PMID:25483462; http://dx.doi.org/10.4161/21645515.2014.970950
- Phan TL, Ho VT, Vu MH, Nguyen TN, Duong HT, Holt R, Wahid R, Donnelly J, Flores J. Clinical testing of an inactivated influenza A/H5N1 vaccine candidate in a double-blinded, placebo-controlled, randomized trial in healthy adults in Vietnam. Vaccine 2016; 34:5449–56; PMID:27591953; http://dx.doi.org/10.1016/j.vaccine.2016.08.055
- Song JY, Cheong HJ, Lee J, Woo HJ, Wie SH, Lee JS, Kim SW, Noh JY, Choi WS, Kim H, et al. Immunogenicity and safety of a cell culture-derived inactivated trivalent influenza vaccine (NBP607): A randomized, double-blind, multi-center, phase 3 clinical trial. Vaccine 2015; 33:5437–44; PMID:26314625; http://dx.doi.org/10.1016/j.vaccine.2015.08.030
- Manzoli L, Salanti G, De Vito C, Boccia A, Ioannidis JP, Villari P. Immunogenicity and adverse events of avian influenza A H5N1 vaccine in healthy adults: multiple-treatments meta-analysis. Lancet Infect Dis 2009; 9:482–92; PMID:19628173; http://dx.doi.org/10.1016/S1473-3099(09)70153-7
- Richards KA, Chaves FA, Sant AJ. Infection of HLA-DR1 transgenic mice with a human isolate of influenza a virus (H1N1) primes a diverse CD4 T-cell repertoire that includes CD4 T cells with heterosubtypic cross-reactivity to avian (H5N1) influenza virus. J Virol 2009; 83:6566–77; PMID:19386707; http://dx.doi.org/10.1128/JVI.00302-09
- Shin JH, Woo C, Wang SJ, Jeong J, An IJ, Hwang JK, Jo SD, Yu SD, Choi K, Chung HM, et al. Prevalence of avian influenza virus in wild birds before and after the HPAI H5N8 outbreak in 2014 in South Korea. J Microbiol 2015; 53:475–80; PMID:26115997; http://dx.doi.org/10.1007/s12275-015-5224-z
- Kwon JH, Lee DH, Swayne DE, Noh JY, Yuk SS, Erdene-Ochir TO, Hong WT, Jeong JH, Jeong S, Gwon GB, et al. Highly Pathogenic Avian Influenza A(H5N8) Viruses Reintroduced into South Korea by Migratory Waterfowl, 2014-2015. Emerg Infect Dis 2016; 22:507–10; PMID:26890406; http://dx.doi.org/10.3201/eid2203.151006
- Lee DH, Kwon JH, Noh JY, Park JK, Yuk SS, Erdene-Ochir TO, Lee JB, Park SY, Choi IS, Lee SW, et al. Pathogenicity of the Korean H5N8 highly pathogenic avian influenza virus in commercial domestic poultry species. Avian Pathol 2016; 45:208–11; PMID:26814367; http://dx.doi.org/10.1080/03079457.2016.1142502
- Winokur PL, Patel SM, Brady R, Chen WH, El-Kamary SS, Edwards K, Creech CB, Frey S, Keitel WA, Belshe R, et al. Safety and Immunogenicity of a Single Low Dose or High Dose of Clade 2 Influenza A(H5N1) Inactivated Vaccine in Adults Previously Primed With Clade 1 Influenza A(H5N1) Vaccine. J Infect Dis 2015; 212:525–30; PMID:25712967; http://dx.doi.org/10.1093/infdis/jiv087
- Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci U S A 2009; 106:7962–7; PMID:19416838; http://dx.doi.org/10.1073/pnas.0903181106
- Sansyzbay AR, Erofeeva MK, Khairullin BM, Sandybayev NT, Kydyrbayev ZK, Mamadaliyev SM, Kassenov MM, Sergeeva MV, Romanova JR, Krivitskaya VZ, et al. An inactivated, adjuvanted whole virion clade 2.2 H5N1 (A/Chicken/Astana/6/05) influenza vaccine is safe and immunogenic in a single dose in humans. Clin Vaccine Immunol 2013; 20:1314–9; PMID:23803900; http://dx.doi.org/10.1128/CVI.00096-13
- World Health Organization. Updated unified nomenclature system for the highly pathogenic H5N1 avian influenza viruses. Geneva, Switzerland: World Health Organization, October 2011. [ accessed 2016 November 05]. http://www.who.int/influenza/gisrs_laboratory/h5n1_nomenclature
- Li GM, Chiu C, Wrammert J, McCausland M, Andrews SF, Zheng NY, Lee JH, Huang M, Qu X, Edupuganti S, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A 2012; 109:9047–52; PMID:22615367; http://dx.doi.org/10.1073/pnas.1118979109
- Smith DJ, Forrest S, Ackley DH, Perelson AS. Variable efficacy of repeated annual influenza vaccination. Proc Natl Acad Sci U S A 1999; 96:14001–6; PMID:10570188; http://dx.doi.org/10.1073/pnas.96.24.14001
- Cox NJ, Ziegler T. Influenza viruses. In: Murray PR, Barron EJ, Jorgensen JH, Pfaller MA, Yolken RH, eds. Manual of clinical microbiology. Washington, DC: ASM Press, 2003:1360–65
- European Committee for Proprietary Medicinal Products. Note for guidance on harmonisation of requirements for influenza vaccines (CPMP/BWP/214/96). London, UK: The Europen Agency for the Evaluation of Medicinal Products, 1997. [ Internet]. [accessed 2010 August 17]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf
- World Health Organization. Serological diagnosis of influenza by the microneutralization assay. Geneva, Switzerland: World Health Organization, 6 December 2010. [ accessed 2016 March 27]. http://www.who.int/influenza/gisrs_laboratory/national_influenza_centres/en/
- U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Silver Spring, MD: U.S. Food and Drug Administration, September 2007. [ Internet]. [accessed 2010 August 17]. http://www.fda.gov/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm074775.html