ABSTRACT
The serogroup epidemiology of invasive meningococcal disease (IMD), which varies considerably by geographic region and immunization schedule, changes continuously. Meningococcal carriage data are crucial for assessing IMD epidemiology and designing f potential vaccination strategies. Meningococcal seroepidemiology in Turkey differs from that in other countries: serogroups W and B are the predominant strains for IMD during childhood, whereas no serogroup C cases were identified over the last 10 y and no adolescent peak for IMD was found. There is a lack of data on meningococcal carriage that represents the whole population. The aims of this multicenter study (12 cities in Turkey) were to evaluate the prevalence of Neisseria meningitidis carriage, the serogroup distribution and the related risk factors (educational status, living in a dormitory or student house, being a household contact with Hajj pilgrims, smoking, completion of military service, attending bars/clubs) in 1518 adolescents and young adults aged 10–24 y. The presence of N. meningitidis DNA was tested, and a serogroup analysis was performed using polymerase chain reaction. The overall meningococcal carriage rate was 6.3% (n = 96) in the study population. A serogroup distribution of the 96 N. meningitidis strains isolated from the nasopharyngeal specimens revealed serogroup A in 5 specimens (5.2%), serogroup B in 9 specimens (9.4%), serogroup W in 64 specimens (66.6%), and serogroup Y in 4 specimens (4.2%); 14 were classified as non-grouped (14.4%). No serogroup C cases were detected. The nasopharyngeal meningococcal carriage rate was 5% in the 10–14 age group, 6.4% in the 15–17 age-group, and 4.7% in the 18–20 age group; the highest carriage rate was found in the 21–24 age group (9.1%), which was significantly higher than those of the other age groups (p < 0.05). The highest carriage rate was found in 17-year-old adolescents (11%). The carriage rate was higher among the participants who had had close contact with Hajj/Umrah pilgrims (p < 0.01) or a history of upper respiratory tract infections over the past 3 months (p < 0.05). The nasopharyngeal carriage rate was 6.3% among adolescents and young adults in Turkey and was similar to the recent rates observed in the same age groups in other countries. The most prevalent serogroup was W, and no serogroup C cases were found. In conclusion, the present study found that meningococcal carriage reaches its peak level by age 17, the highest carriage rate was found in 21 - to 24 - year-olds and the majority of the carriage cases were due to serogroup W. Adolescents and young adult carriers seem to be a potential reservoir for the disease, and further immunization strategies, including adolescent immunization, may play a role in the control of IMD.
Neisseria meningitidis is one of the major causes of meningitis, sepsis and bacteremia, although it rarely results in clinical presentations such as focal infections, arthritis, and pneumonia. Invasive meningococcal infections are among the infections associated with the highest risk of morbidity and mortality worldwide.Citation1 In addition to a higher mortality rate, despite all therapeutic interventions, 10–20% of survivors may suffer from serious sequelae (e.g., amputation, skin necrosis, deafness, mental retardation) to meningococcal infections.Citation2 N. meningitidis is divided into 13 different serotypes based on the type of polysaccharide capsule, among which 6 serotypes (A, B, C, W, X and Y) account for the most common life-threatening invasive meningococcal infections.Citation2-5 N. meningitidis is a microorganism that is naturally found in the flora of the human throat, which is its only reservoir. Determining the carriage rates and serotypes for meningococcal infections are closely related to the epidemiology of invasive infections. Age is one of the most important factors that influence the meningococcal disease and carriage. Meningococcal infections commonly occur in children under the age of 5 y. In some countries (e.g., the United States, the United Kingdom), a secondary peak associated with the disease is seen in adolescents and young adults. Although they do not have a clinical presentation, adolescents and young adults play a role in the transmission of the disease.Citation2,6 In addition to age, changes in the carriage rate are associated with gender, crowding, geographical conditions, smoking, current viral or bacterial upper respiratory tract infections and lower socioeconomic status.Citation2,5-7 Visiting Saudi Arabia for the Hajj/Umrah pilgrimage has also been shown be one of the risk factors affecting carriage and disease.Citation1,8
A surveillance study showed that invasive meningococcal infections in Turkey differ from those in other countries.Citation8 It was shown that the disease was most common in children under 5 y of age, and no secondary peak was observed in adolescence between 2005 and 2014.Citation9-11 It has been demonstrated that serogroup W, which is considered the most common cause of disease worldwide, was also the most common cause of invasive disease in Turkey, followed by serogroup B; however, no cases of serogroup C were observed.Citation8-11 No carriage data are available for Turkey because all of these studies were conducted either in a single center or in wide range of age groups; further, the meningococcal carriage rate varied from 1.23% to 28%.Citation12 Characterizing N. meningitidis carriage in adolescents and young adults and serogroups in Turkey will improve our understanding of the epidemiology of invasive meningococcal infections and vaccination schedules. The objective of the present study was to characterize the nasopharyngeal carriage of N. meningitidis, determine the serogroups among the N. meningitidis isolates and evaluate demographic and other risk factors associated with carriage in adolescents and young adults aged 10–24 y in Turkey.
Results
The study included 1518 patients aged 10 and 24 y who were enrolled at 13 sites in 12 provinces between January 1, 2015, and May 31, 2015. Of these 1518 adolescents and young adults, 747 were male, and 771 were female, with no significant difference in sex (p > 0.05). The participants were divided into 4 age groups. The participants aged between 10 and 14 y were included in Group 1 (n = 522), the participants aged between 15 and 17 y were included in Group 2 (n = 311), the participants aged between 18 and 20 y were included in Group 3 (n = 279), and the participants aged between 21 and 24 y were included in Group 4 (n = 406). The analysis was based on 1501 participants who completed the risk factors section in the questionnaire for evaluating the risk factors associated with meningococcal carriage in the study participants. The number of participants who also attended a private teaching school was 237 (15.7%); 300 (19.9%) lived in a dormitory or a student house; 40 (2.7%) had a family history of meningitis; 40 (2.7%) had completed military service; 137 (9.1%) had relatives who were Hajj or Umrah pilgrims; 254 (16.9%) smoked or living with smokers; 704 (46.9%) attended mass areas such as cafes/cinemas and/or bars once a week or more; and 801 (53.3%) had a history of upper respiratory tract infection over the past 3 y.
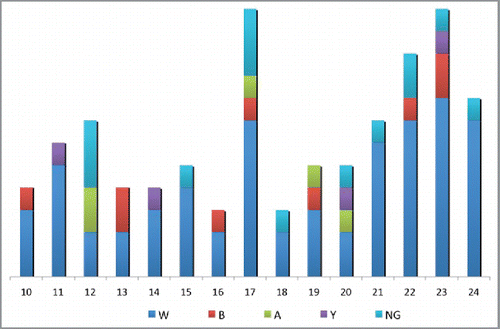
Of these 1518 adolescents and young adults, 96 (6.3%) had N. meningitidis isolated from the nasopharyngeal samples by PCR. An analysis of the entire study group showed a carriage rate of 0.3% for serogroup A, 0.6% for serogroup B, 0.9% for serogroup NG, 4.2% for serogroup W, and 0.3% for serogroup Y.
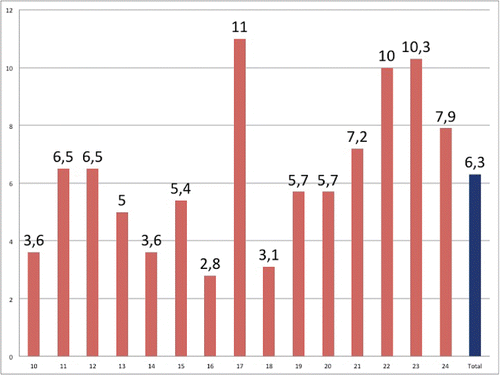
The meningococcal carriage rates by age and age groups are shown in . The highest carriage rate (11%) was found in those aged 17 y. An evaluation of the carriage rate by age groups showed a carriage rate of 5% in the 10–14 age group, 6.4% in the 15–17 age group, 4.7% in the 18–20 age group, and 9.1% in the 21–24 age group. The highest carriage rate was found in the 21–24 age group and was significantly higher than those of the other age groups (p < 0.05) ().
Table 1. The prevalence of Neisseria meningitidis carriage rate with serogroup distribution among adolescents/young adults and age groups.
A serogroup distribution of the 96 N. meningitidis samples isolated from the nasopharyngeal specimens revealed serogroup A in 5 specimens (5.2%), serogroup B in 9 specimens (9.4%), serogroup W in 64 specimens (66.6%), and serogroup Y in 4 specimens (4.2%). The N. meningitidis samples isolated from 14 (14.4%) specimens were classified as non-grouped. Serogroup W was the most dominant, with a rate of 66.6%, and no serogroup C specimens were identified ().
An analysis of the effect of risk factors on carriage showed no correlation between the meningococcal carriage and attending a private teaching school, living in a dormitory or student house, presence of a relative with a history of meningitis, military service, current smoking or exposure to smoking, and frequently attending mass areas such as cafes, cinemas, and bars (p > 0.05). However, the risk factor “being a household contact of Hajj or Umrah pilgrims” was significantly higher in the participants with meningococcal carriage than in those without (17.7% vs. 8.5%, respectively; p = 0.04). Another risk factor, a history of URTI in the past 3 months, was 58% in the group of participants with meningococcal carriage but was 45.8% in those without meningococcal carriage (p < 0.05). ().
Table 2. Risk factors for Neisseria meningitidis carriers and non-carriers.
An individual analysis of age groups by risk factors showed that the number of household contacts with Hajj and Umrah pilgrims was statistically higher in the 10–14 age group with meningococcal carriage compared with those without meningococcal carriage (30.7% vs. 9.95%, respectively p = 0.04). No statistically significant difference was found between the carriage group and non-carriage group of the 14–17 and 18–20 age groups for “being a household contact of Hajj or Umrah pilgrims” (p > 0.05). In the 21–24 age group, with the highest rate of carriage, the rate of participants “being a household contact of Hajj or Umrah pilgrims” was significantly higher in the carriage group than in the non-carriage group (16.2% vs 5.73%, respectively; p < 0.05).
Discussion
The present study showed that the carriage rate (6.3%) of N. meningitidis among adolescents and young adults aged 10–25 y in Turkey was similar to the rate reported globally. A serogroup analysis showed that the most dominant serogroup isolated from the specimens with meningococcal carriage was serogroup W (66.6%), followed by non-groupable (14.4%), serogroup B (9.4%), serogroup A (5.2%), and serogroup Y (4.2%). No serogroup C was detected among our participants. Our results for meningococcal carriage in the 10–24 age group (dominance of serogroup W, absence of serogroup C) are compatible with the epidemiology of the childhood invasive meningococcal infections in Turkey.Citation9-11 An analysis of the age distribution showed that the highest rate of carriage was in the 21–24 age group and in those aged 17 y. The present study showed that the risk factors included being a household contact of a Hajj or Umrah pilgrim and having a history of upper respiratory tract infection over the past 3 months. We found that the other risk factors evaluated, including attending a private teaching school, residence in a dormitory or a student house, family history of meningitis, smoking or exposure to smoking, and military service, were not significantly significant.
Meningococcal carriage rarely causes systemic infection, but it is a prerequisite for the development of an infection.Citation13 There is a complex relationship between carriage and invasive disease. Although the disease is common in hyper-invasive strains, it can also occur in non-pathogenic strains.Citation5 It has been demonstrated that although meningococci are carried in nasopharynx in 5–10% of individuals, the carriage rate increases to 40–50% in closed and crowded places such as dormitories and barracks and can even reach 100% during outbreaks; further, the risk of acquiring the infection increases 100- to 1000-fold in cases of having close contact with a meningococcemia patient compared with the overall population.Citation14 The present study found a meningococcal carriage rate of 6.3% in the 10–24 age group. In 2004, Pavlopoulou et al.Citation15 found an overall carriage rate of 4% in a study of 554 cases aged 2–29 y in Greece. In a study performed in Spain in 2000, Garcia et al.Citation16[showed that the carriage rate varied from 3.1% to 9.7%, whereas another Spanish study reported that the carriage rate was 8% before 20 y of age and decreased to 3.3% after 20 y of age.Citation17 Claus et al.Citation18 found that the carriage rate was 1.7% in children aged 3 to 6 y and increased to 18.1% between 15 and 21 y of age. In a 2004 Czech study in students, Krizova et al.Citation19 reported a carriage rate of 16% in the 15–19 age group. In a 1998 study in Norway, Bevanger et al.Citation20 found that the carriage rate was 36.4% in the 18–19 y age group, whereas Patrick et al.Citation21 reported a carriage rate of 7.9% in 2003 in the UK. In a study conducted in Brazil in 2012,Citation22 the overall carriage rate was 9.8% in 1208 adolescents and young adults aged 11–19 years; serogroup C was the most common serogroup, whereas serogroup W was at found a rate of 0.25%. In 2005, the carriage rate was found to be 2% in 750 children and adolescents aged 2–19 y in Uganda, and the highest carriage rate was found in the 16–19 y age group, at 4.7%.Citation23 Similarly, other studies found a carriage rate of 9.9% in Czech Republic, 10.6% in Greece, 9.6% in Norway in 1991,Citation24 6.2% in Nigeria in 1999,Citation25 and 2.7% in Morocco, 4.5% in Umman, and 1.7% in Sudan.Citation26 The figures that we found are similar to those reported very recently in Chile and Colombia. In 2013, the carriage rate was 6.5% in the 10–19 age group in Chile,Citation27 and an overall carriage rate of 6.85% was detected in Colombia in a study of 1459 cases aged 15–21 y.Citation28 A review of studies in Turkey shows that they are usually performed in a single region and include a limited number of participants, finding a carriage rate ranging from 1.2% to 28%.Citation12 Bakir et al.Citation29 isolated N. meningitidis in 17 out of 1382 asymptomatic children aged 0–10 y and found a carriage rate of 1.23%; the most prevalent serogroup was Y, and there was no presence of serogroup C, similar to our study. The present study included adolescents and young adults from 12 provinces of Turkey and found a carriage rate of 6.3%, which is the most representative of the country so far. In a UK study including 1040 children and young adults aged 10–25 y over 6 months, Jeppesen et al. found that the carriage was most prevalent in serogroups B and Y.Citation30 Similarly, carriage studies in Europe found that serogroups B and C were common, but serogroup A was among the major serogroups in the sub-Saharan meningitis belt. Serogroup W has been reported to be dominant in the Middle Eastern countries.Citation31-32
The carriage rate varies with age and the incidence of invasive disease. Although the carriage rate in Europe and North America is very low in early years of life, it increases rapidly during adolescence and young adulthood. In a 2013 meta-analysis, Cohn et al. showed that the meningococcal carriage rate increased from 4.5% in infants to 23.7% in 19-year-olds.Citation33 Similarly, in our study, an analysis of carriage rates by age showed that the carriage rate was 5% in the 10–14 age group, 6.4% in the 15–17 age group, and 4.7% in the 18–20 age group and was highest in the 21–24 age group, at 9.1%. An individual analysis of each age group showed that the carriage rate was the highest in 17-year-olds in the 10–24 age group, which suggests the presence of an adolescent peak for carriage that was not observed for invasive diseases in Turkey. In a study of 500 Chilean young adults aged 18–24 y conducted in 2012, Rodriguez et al.Citation34 found a meningococcal carriage rate of 4%. During this period, there was an outbreak of invasive meningococcal infection associated with serogroup W in Chile. The carriage rate in our 21–24 age group was 9.1%, which was higher than the rate in Chile. In the same age group, the carriage rate was 11.8% in KoreaCitation35 and 35% in North America.Citation36 The lower rate in Chile compared with other regions was attributed to the fact that none of the participants in the study resided in a dormitory, a student house or a crowded room.
The epidemiological data on invasive meningococcal infections have been regularly monitored in Turkey since 2005.Citation9-11 In an ongoing study in 14 provinces over 8 years, Ceyhan et al. indicated that there was an increase in serogroup W, although N. meningitidis is responsible for the childhood meningitis in Turkey.Citation10 It can be explained by the Hajj pilgrimage, which is among the effective risk factors associated with carriage in Turkey. In a study of specimens collected before and after the Hajj, Ceyhan et al.Citation37 showed that the prevalence of carriage was 13% before and reached 27% after pilgrimage; 91% of the specimens were positive for serogroup W. In a review of 27 reports on invasive meningococcal infections over the past 40 y in Turkey, Bakir et al.Citation12 found that the serogroup W was not common during the 1970s but became the most common invasive pathogen over time. Although serogroup W does not seem to be the most common cause in Turkish studies on carriage, we found that it was the most frequent cause, with a rate of 66.6%. A review of serogroup W in Turkey showed that it was first isolated from a soldier in 200138; however, most meningitis cases were reported to be associated with the serogroup W between 2005 and 2012.Citation10 After demonstrating the association of serogroup W with Hajj and Umrah pilgrims, the meningococcal ACWY polysaccharide vaccine is now being used in travelers partaking in the Hajj and Umrah pilgrimage, and the conjugated vaccine has been introduced in individuals younger than 55 y of age since 2014 in Turkey.Citation8 During the 2005–2013 period, there was no infection associated with serogroup C, which was considered the most common meningococcal serogroup in many European countries.Citation10 Similarly, we found no serogroup C carriage in the present study.
An analysis of the risk factors that were described previously as being associated with meningococcal infections, including attending a private school, residence in a dormitory or student house, military service, and attending crowded places such cafes, cinemas, and bars, showed that these factors did not have a significant effect on carriage. An individual analysis of age groups indicated no association between meningococcal carriage and these risk factors in any age groups. Unlike our study, a 2014 Japanese study in a dormitory of 900 people, including those with serogroup carriage, found that the mean age of students was 21 y and that the overall carriage rate was 32.2%. This study showed that the carriage rate was increased in students who had lived in the dormitory for longer periods.Citation39 A UK study in 14057 adolescents aged 15–19 y reported that the overall carriage rate was 16.7% and that active and/or passive smoking, attending crowded places such as pubs and bars, and intimate kissing increased the risk of meningococcal carriage 4-fold.Citation40 In our study, smoking and/or exposure to smoking was not described as a risk factor. Murray et al.Citation41 published an analysis of 18 reports that examined smoking and exposure to smoking in children in 2012 and showed that smoking significantly increased the risk of meningococcal disease. This increased risk is also reflected in carriage, and even passive smoking represents an increased risk for carriage. Similar to our study, Rodriguez et al.Citation34 found that active or passive smoking was not a risk factor for carriage in Chile. Additionally, in a study of 554 cases aged 2–19 y (with a carriage rate of 4%), Pavlopoulou et al.Citation15 showed that passive smoking was not a statistically significant risk factor.
For the risk factor of being a household contact of a Hajj pilgrim, we found that close contact with a Hajj pilgrim was a significant risk factor in all age groups compared with those without any contact. With respect to age groups, the risk was higher particularly in the 10–14 and 21–24 age groups. Ceyhan et al. evaluated the carriage by the Hajj pilgrims who acquired serogroup W carriage at the Hajj and found that 10 of the 11 family members (91%) were positive for serogroup W carriage.Citation37 In a study on characterizing the meningococcal serogroup with acquired carriage, all of the W isolates were identical to W135:2a:P1.5.2, a Hajj-associated clone of the serogroup W isolates.Citation42 A US study reported that vaccinated pilgrims leaving the country with no serogroup W carriage returned home with serogroup W at a rate of 0.8%.Citation43
The present study found that URTI experience over the past 3 months was a significant risk factor for meningococcal carriage. In a study of 1208 cases in Brazil, de Moraes et al.Citation22 showed that crowded places, passive smoking, attending night clubs and having a history of URTI increased the risk of meningococcal carriage. In this respect, a history of URTI represents a risk, as shown in our study. Unlike our study, Pavlopoulou et al.Citation15 reported that a recent history of URTI was not a significant risk factor.
The limitations of the present study included the fact that we conducted a meningococcal screening on the nasopharyngeal specimens by using PCR method but not a routine bacterial culture. Therefore, we could not perform a gene lineage analysis of the strains in our isolates. It would be beneficial to perform this analysis to examine whether the serogroup W strains were associated with the Hajj.
The second invasive meningococcal infection peak that has been observed in adolescents and young adults in the USA and some European countries is not seen in Turkey. However, the prevalence of meningococcal carriage in adolescents and young adults is similar to those found in other countries. For the distribution of invasive meningococcal infections, Ceyhan et al. found that serogroup W was the most dominant in a study conducted in 2005, and they found that there were no cases with serogroup C carriage. Although there is no invasive disease peak during adolescence in Turkey, the distribution of carriage serogroups was similar to invasive disease in the present study, with serogroup W as the most common and an absence of serogroup C. Our results indicate that the meningococcal carriage profile in adolescents and young adults may provide insight into the serogroup distribution of the invasive disease. With respect to the vaccination of risk groups, which is among the vaccination strategies for preventing meningococcal infections, it may be beneficial to vaccinate adolescents and young adults to eliminate the risk of transmission. In the UK, conjugated vaccines containing serogroup W have been introduced during adolescence following the recent increase in cases with serogroup W. Further large studies are required to evaluate the effect of conjugated vaccine administration to at-risk groups with carriage (the Hajj and Umrah pilgrims and adolescents) on the overall prevalence of disease and distribution of serogroups.
Serogroup W is the most common serogroup isolated in Turkey, and no serogroup C carriage was detected. Because it is similar to the distribution of invasive disease serogroups in Turkey, we believe that the meningococcal carriage in adolescents and young adults may be effective in the seroepidemiology of invasive disease.
Material and method
The aim of the present study was to determine the prevalence of nasopharyngeal carriage of N. meningitidis in adolescents and young adults aged 10–24 y in Turkey. After determining the study sites, an approval was obtained from the Ethics Board of the Eskişehir Osmangazi University (Approval No. 80558721/327 dated 15.11.2014). The study was fully financed by the Scientific Research Projects of the Eskisehir Osmangazi University (2014/455).
In Turkey, meningococcal vaccination is not part of the routine vaccination schedule during childhood. Quadrivalent meningococcal vaccines are administered to children > 9 months of age in private practice (according to the vaccine licensure), but the coverage rate is less than 5%. During the time of the study, a quadrivalent meningococcal polysaccharide vaccine had been administered to people traveling for Hajj or Umrah. The age for mandatory military service is 20 years, and all men enlisted in the army are required to receive a single dose meningococcal polysaccharide vaccine.
We recorded the province, age (birth date), sex, the school attended, number of students in the classroom, if any, residence (dormitory or house), number of roommates/housemates, military service (for cases older than 20 y of age), history of Hajj/Umrah or travel to Saudi Arabia over the past year for householders, smoking exposure, attendance at crowded places such as cafes, cinemas, bars, stadiums, and shopping centers, and history of upper respiratory tract infection (URTI) over the past 3 months for each participant enrolled in the study.
The nasopharyngeal swab samples from the cases were collected into transport medium by principal investigators after receiving consent from the participants. Charcoal-impregnated, cotton-tipped swabs were used for all throat cultures. Samples were transported using Stuart's transport medium. All laboratory analyses in the study were performed by the Laboratory of the Pediatric Infectious Diseases of Hacettepe University.
DNA extraction
DNA was extracted using QuickGene DNA Tissue Kit S DT-S using the semi-automated QuickGene-Mini80 instrument (Autogen/FujiFilm, Holliston, MA, USA) following the manufacturer's instructions. First, 10 μl EDT (proteinase K) and 200 μl LDT (lysis buffer) solution were added to 200 μl samples and mixed thoroughly by vortexing for 15 seconds at the maximum flash spin down. The mixtures were incubated at 56°C for 10 min with flash spin down, and 200 μl >99% ethanol was added. They were then mixed thoroughly by vortexing for 15 sec at maximum flash spin down. Lysate was used to transfer all contents of the micro tube into QuickGene cartridges. Samples were transferred to QuickGene cartridges and placed in the QuickGene Mini80 apparatus, and DNA binding, washing, and elution were accomplished through pressurization. DNA was eluted with 200 μl Elution buffer.
PCR amplification
To identify the bacterial agent, simultaneous single-tube multiplex PCR assay was performed. The specific targets were ctrA gene for N. meningitidis. In each assay, the final reaction mixture of 50 μl contained 15 μl of each DNA sample, 1xPCR buffer, 3 mM MgCl2, 200 µM of each deoxynucleotide triphosphate, 0.6 μl of each corresponding oligonucleotide primer and 1 U of Taq polymerase. The PCR assay was performed using a DNA thermal cycler (Gena Amp PCR SYSTEM 9700 Foster City, CA, USA) under the following conditions: a first cycle of denaturation at 95°C for 5 minutes followed by 35 cycles of 95°C for 25 seconds, 57°C for 40 seconds, and 72°C for 60 seconds. The samples found to be positive for Neisseria meningitidis serogroup prediction (A, B, C, W and Y) were based on the oligonucleotides in the siaD gene for serogroups B, C, W and Y and in orf-2 of a gene cassette required for serogroup A. For serogroup determination, the amplification reactions (50 μl) contained 15 μl of each DNA sample, 60 mM TrisHCl (pH 8.8), 17 mM (NH4)2SO4, 5 mM of MgCl2, 0.5 mM each deoxynucleotide triphosphate, 0.3 μl corresponding oligonucleotides and 1 U of Taq Polymerase. The PCR conditions were as follows: denaturation at 94°C for 3 minutes, followed by 35 cycles of 92°C for 40 seconds, 55°C for 30 seconds, and 72°C for 20 seconds in thermal cycler. A final cycle of elongation at 72°C for 10 minutes was performed following these cycles. All amplicons were analyzed on a standard 2% agarose gel. A negative control consisting of distilled water and a positive control consisting of the reference strain was included in each test.
Sample size calculation and statistical analysis
According to the recent census data, the population of adolescents and young adults aged 10–24 y is 18.750.000. Considering the previous studies. which reported a prevalence rate of N. meningitidis carriage varying from 1% to 20%, we calculated a sample size of 1350 people with a statistical power of 90% and an α cut off of 5%. We increased the sample size by 10 percent to 1500 people, considering the external conditions that might have arose during the study. We selected 12 provinces, which represent the country both demographically and geographically, and included 13 centers located in these provinces in our study. We determined the sample size based on the ratio of the last census data for provinces of each of the 11 study sites to overall population. We planned to enroll cases for each age group between the ages of 10 and 24, equal in number and sex. All statistical analyses were performed using the SPSS 16.5 for Windows (Chicago, IL, USA) software. A frequency analysis was used for all descriptive tests. A 95% confidence interval (CI) for the means and proportions was also calculated. Association of nasopharyngeal meningococcal carriage rate with some risk factors was analyzed with the chi-square test. A p-value < 0.05 was considered statistically significant.
Compliance with ethical standards
Ethical approval: An approval was obtained from the Ethics Board of the Eskisehir Osmangazi University (Approval No. 80558721/327 dated 15.11.2014). All procedures performed in this trial were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent Informed consent was obtained from each participant and/or a parent or legal guardian for every individual participant included to this study.
Abbreviations
| IMD | = | invasive meningococcal disease |
| PCR | = | polymerase chain reaction |
| URTI | = | upper respiratory tract infection |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Author contributions
Dr. Tekin, Dr. EC Dinleyici, Dr Ceyhan participated in protocol development, cites selection, enrollment, statistical analysis, primary data analysis, interpretation and wrote the first version of the manuscript and also finalized the manuscript. Dr Karbuz, Dr. Salman, Dr. Sutcu, Dr. Kurugol, Dr. Balliel, Dr. Celik, Dr. Hacimustafaoglu, Dr. Kuyucu, Dr. Kondolot, Dr. Sensoy, Dr Kara, Dr M Dinleyici, Dr Kilic, Dr. Bayhan, Dr Memedova, Dr Karli, Dr Bozlu, and Dr Celebi participated in patient screening, enrolment, and writing the manuscript. Gurbuz and Aycan participated in the protocol development for labarotory analysis, samples evaluation, primary data analysis and writing the manuscript.
Acknowledgments
We thank the Samet Ece, for their assistance to transfer all samples to central laboratory.
Funding
The study was fully financed by the Scientific Research Projects of the Eskisehir Osmangazi University (2014/455).
References
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global epidemiology of invasive meningococcal disease. Popul Health Metr 2013; 11(1):17; PMID:24016339; http://dx.doi.org/10.1186/1478-7954-11-17
- Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia and Neisseria meningitidis. Lancet 2007; 369(9580):2196-210; PMID:17604802; http://dx.doi.org/10.1016/S0140-6736(07)61016-2
- World Health Organization. Control of Epidemic Meningococcal Disease: WHO Practical Guidelines 2nd edition. Geneva, Switzerland: WHO Health Organization; 1998. Available at: http://www.who.int/csr/resources/publications/meningitis/whoemcbac983.pdf
- Pathan N, Faust SN, Levin M. Pathophysiology of meningococcal meningitis and septicaemia. Arch Dis Child 2003; 88(7):601-7; PMID:12818907; http://dx.doi.org/10.1136/adc.88.7.601
- Yazdankhah SP, Caugant DA. Neisseria meningitidis: an overview of the carriage state. J Med Microbiol 2004; 53(1):821-832; PMID:15314188; http://dx.doi.org/10.1099/jmm.0.45529-0
- Pollard AJ. Global epidemiology of meningococcal disease and vaccine efficacy. Pediatr Infect Dis J 2004; 23(12):274-9
- Caugant DA, Tzanakaki G, Kriz P. Lessons from meningococcal carriage studies. FEMS Microbiol Rev 2007; 31(1):52-63; PMID:17233635; http://dx.doi.org/10.1111/j.1574-6976.2006.00052.x
- Dinleyici EC, Ceyhan M. The dynamic and changing epidemiology of meningococcal disease at the country-based level: the experience in Turkey. Expert Rev Vaccines 2012; 11(5):515-8; PMID:22827237; http://dx.doi.org/10.1586/erv.12.29
- Ceyhan M, Yildirim I, Balmer P, Borrow R, Dikici B, Turgut M, Kurt N, Aydogan A, Ecevit C, Anlar Y, et al. A prospective study of etiology of childhood acute bacterial meningitis, Turkey. Emerg Infect Dis 2008 Jul; 14(7):1089-96; PMID:18598630; http://dx.doi.org/10.3201/eid1407.070938
- Ceyhan M, Gürler N, Ozsurekci Y, Keser M, Aycan AE, Gurbuz V, Salman N, Camcioglu Y, Dinleyici EC, Ozkan S, et al. Meningitis caused by Neisseria Meningitidis, Hemophilus Influenzae Type B and Streptococcus Pneumoniae during 2005–2012 in Turkey. A multicenter prospective surveillance study. Hum Vaccin Immunother 2014; 10(9):2706-12; PMID:25483487; http://dx.doi.org/10.4161/hv.29678
- Ceyhan M, Ozsurekci Y, Gürler N, Karadag Oncel E, Camcioglu Y, Salman N, Celik M, Emiroglu MK, Akin F, Tezer H, et al. Bacterial agents causing meningitis during 2013–2014 in Turkey: A multi-center hospital-based prospective surveillance study. Hum Vaccin Immunother 2016 Jul 25; 12(11):1-6
- Bakır M, Altınel S. Review of invasive meningococcal disease during the last 40 years in Turkey. Expert Rev Vaccines 2015; 14(8):1089-1097; PMID:26132432; http://dx.doi.org/10.1586/14760584.2015.1060859
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344(18):1378-88; PMID:11333996; http://dx.doi.org/10.1056/NEJM200105033441807
- Davies AL, O'Flanagan D, Salmon RL, Coleman TJ. Risk factors for Neisseria meningitidis carriage in a school during a community outbreak of meningococcal infection. Epidemiol Infect 1996; 117(2):259-66; PMID:8870623; http://dx.doi.org/10.1017/S0950268800001436
- Pavlopoulou ID, Daikos GL, Alexandrou H, Petridou E, Pangalis A, Theodoridou M, Syriopoulou VP. Carriage of Neisseria meningitidis by Greek children: risk factors and strain characteristics. Clin Microbiol Infect 2004; 10(2):137-42; PMID:14759238; http://dx.doi.org/10.1111/j.1469-0691.2004.00750.x
- García Rojas A, Bordes Benítez A, Lafarga Capuz B, Vázquez Moreno J, López Villarrubia E, García Castellano P, Solís Romero J. Survey of carriers of Neisseria meningitidis in the health area of Gran Canary. Rev Esp Salud Publica 2004; 74(4):419-24
- Ramos Aceitero JM. Surveys on the rates of healthy carriers of Neisseria meningitidis and charaterization of circulating strains. Rev Esp Salud Publica 2000; 74(4):413-7; PMID:11031851; http://dx.doi.org/10.1590/S1135-57272000000400013
- Claus H, Maiden MC, Wilson DJ, McCarthy ND, Jolley KA, Urwin R, Hessler F, Frosch M, Vogel U. Genetic analysis of meningococci carried by children and young adults. J Infect Dis 2005; 191(8):1263-71; PMID:15776372; http://dx.doi.org/10.1086/428590
- Krízová P, Kalmusová J, Musílek M, Felsberg J, Haugvicová R, Vlcková J. Study of long-term and multiple carriage of Neisseria meningitidis in a healthy population using molecular biology methods. Epidemiol Mikrobiol Imunol 2004; 53(1):25-36; PMID:15052832
- Bevanger L, Bergh K, Gisnas G, Caugant DA, Froholm LO. Identification of nasopharyngeal carriage of an outbreak strain of Neisseria meningitidis by pulsed-field gel electrophoresis versus phenotypic methods. J Med Microbiol 1998; 47(11):993-8; PMID:9822298; http://dx.doi.org/10.1099/00222615-47-11-993
- Patrick DM, Champagne S, Goh SH, Arsenault G, Thomas E, Shaw C, Rahim T, Taha F, Bigham M, Dubenko V, et al. Neisseria meningitidis carriage during an outbreak of serogroup C disease. Clin Infect Dis. Clin Infect Dis 2003; 37(9):1183-8; http://dx.doi.org/10.1086/378743
- Cassio de Moraes J, Kemp B, de Lemos AP, Outeiro Gorla MC, Lemes Marques EG, Ferreira Mdo C, Sacchi C, Carvalhanas TR, Ribeiro AF, Ferreira CM, et al. Prevalence, risk factors and molecular characteristics of meningococcal carriage among Brazilian adolescents. Pediatr Infect Dis J 2015; 34(11):1197-202; PMID:26222063; http://dx.doi.org/10.1097/INF.0000000000000853
- Caugant DA, Fogg C, Bajunirwe F, Piola P, Twesigye R, Mutebi F, Frøholm LO, Rosenqvist E, Batwala V, Aaberge IS, et al. Pharyngeal carriage of Neisseria meningitidis in 2–19-year-old individuals in Uganda. Trans R Soc Trop Med Hyg 2006; 100(12):1159-63; PMID:16765397; http://dx.doi.org/10.1016/j.trstmh.2006.01.004
- Yazdankhah SP, Kriz P, Tzanakaki G, Kremastinou J, Kalmusova J, Musilek M, Alvestad T, Jolley KA, Wilson DJ, McCarthy ND, et al. Distribution of serogroups and genotypes among disease-associated and carried isolates of Neisseria meningitidis from the Czech Republic, Greece, and Norway. J Clin Microbiol 2004; 42(11):5146-53; PMID:15528708; http://dx.doi.org/10.1128/JCM.42.11.5146-5153.2004
- Emele FE, Ahanotu CN, Anyiwo CE. Nasopharyngeal carriage of meningococcus and meningococcal meningitis in Sokoto, Nigeria. Acta Paediatr 1999; 88(3):265-9; PMID:10229035; http://dx.doi.org/10.1111/j.1651-2227.1999.tb01094.x
- Nicolas P, Ait M'barek N, Al-Awaidy S, Al Busaidy S, Sulaiman N, Issa M, Mahjour J, Mölling P, Caugant DA, Olcén P, et al. Pharyngeal carriage of serogroup W135 Neisseria meningitidis in Hajjees and their family contacts in Morocco, Oman and Sudan. APMIS 2005; 113(3):182-6; PMID:15799761; http://dx.doi.org/10.1111/j.1600-0463.2005.apm1130305.x
- Díaz J, Cárcamo M, Seoane M, Pidal P, Cavada G, Puentes R, Terrazas S, Araya P, Ibarz-Pavon AB, Manríquez M, et al. Prevalence of meningococcal carriage in children and adolescents aged 10–19 years in Chile in 2013. J Infect Public Health 2016; 9(4):506-15; http://dx.doi.org/10.1016/j.jiph.2015.12.011
- Moreno J, Hidalgo M, Duarte C, Sanabria O, Gabastou JM, Ibarz-Pavon AB. Characterization of carriage isolates of Neisseria meningitides in the Adolescents and young adults population of Bogota (Colombia). PLoS One 2015; 10(8):e0135497; PMID:26322796; http://dx.doi.org/10.1371/journal.pone.0135497
- Bakir M, Yagci A, Ulger N, Akbenlioglu C, Ilki A, Soyletir G. Asymtomatic carriage of Neisseria meningitidis and Neisseria lactamica in relation to Streptococcus pneumoniae and Haemophilus influenzae colonization in healthy children: apropos of 1400 children sampled. Eur J Epidemiol 2001; 17(11):1015-1018; PMID:12380714; http://dx.doi.org/10.1023/A:1020021109462
- Jeppesen CA, Snape MD, Robinson H, Gossger N, John TM, Voysey M, Ladhani S, Okike IO, Oeser C, Kent A, et al. Meningococcal carriage in adolescents in the United Kingdom to inform timing of an adolescent vaccination strategy. J Infect 2015; 71(1):43-52; PMID:25709085; http://dx.doi.org/10.1016/j.jinf.2015.02.006
- Gabutti G, Stefanati A, Kuhdari P. Epidemiology of Neisseria meningitidis infections: case distribution by age and relevance of carriage. J Prev Med Hyg 2015; 56(3):116-20
- MenAfriCar Consortium. Meningococcal carriage in the African meningitis belt. Trop Med Int Health 2013; 18(8):968-78; PMID:23682910; http://dx.doi.org/10.1111/tmi.12125
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2013; 62:1-28; PMID:23515099
- Rodriguez P, Alvarez I, Torres MT, Diaz J, Bertoglia MP, Carcamo M, Seoane M, Araya P, Russo M, Santolaya ME. Meningococcal carriage prevalence in university students, 1824 years of age in Santiago, Chile. Vaccine 2014; 32(43):5677-80; PMID:25148776; http://dx.doi.org/10.1016/j.vaccine.2014.08.015
- Durey A, Bae SM, Lee HJ, Nah SY, Kim M, Baek JH, Kang YH, Chung MH, Lee JS. Carriage rates and serogroups of Neisseria meningitidis among freshmen in a University dormitory in Korea. Yonsei Med J 2012; 53(4):742-7; PMID:22665340; http://dx.doi.org/10.3349/ymj.2012.53.4.742
- Stephens DS. Uncloaking the meningococcus: dynamics of carriage and disease. Lancet 1999; 353(9157):941-2; PMID:10459897; http://dx.doi.org/10.1016/S0140-6736(98)00279-7
- Ceyhan M, Celik M, Demir ET, Gurbuz V, Aycan AE, Unal S. Acquisition of meningococcal serogroup W-135 carriage in Turkish Hajj pilgrims who had received the quadrivalent meningococcal polysaccharide vaccine. Clin Vaccine Immunol 2013; 20(1):66-8; PMID:23136117; http://dx.doi.org/10.1128/CVI.00314-12
- Doganci L, Baysallar M, Saracli MA, Hascelik G, Pahsa A. Neisseria meningitidis W135, Turkey. Emerg Infect Dis 2004; 10(5):936-7; PMID:15200836; http://dx.doi.org/10.3201/eid1005.030572
- Kamiya H, Takahashi H, Sunagawa T, Oishi K, Ohnishi M. Unexpected high carriage rate of Neisseria Meningitidis among dormitory residents in Tokyo, Japan. Open Forum Infectious Diseases 2015; 2(Suppl 1): S304; http://dx.doi.org/10.1093/ofid/ofv133.875
- MacLennan J, Kafatos G, Neal K, Andrews N, Cameron JC, Roberts R, Evans MR, Cann K, Baxter DN, Maiden MC, et al; United Kingdom Meningococcal Carriage Group. Social behavior and meningococcal carriage in British teenagers. Emerg Infect Dis 2006; 12(6):950-7; PMID:16707051; http://dx.doi.org/10.3201/eid1206.051297
- Murray RL, Britton J, Leonardi-Bee J. Second hand smoke exposure and the risk of invasive meningococcal disease in children: systematic review and meta-analysis. BMC Public Health 2012; 12:1062; PMID:23228219; http://dx.doi.org/10.1186/1471-2458-12-1062
- Taha MK, Achtman M, Alonso JM, Greenwood B, Ramsay M, Fox A, Gray S, Kaczmarski E. Serogroup W135 meningococcal disease in Hajj pilgrims. Lancet 2000; 356(9248):2159; PMID:11191548; http://dx.doi.org/10.1016/S0140-6736(00)03502-9
- Centers for Disease Control and Prevention (CDC). Update: assessment of risk for meningococcal disease associated with the Hajj. MMWR Morb Mortal Wkly Rep 2001; 50(12):221-222; PMID:11300626