ABSTRACT
Background: Maternal antibodies, acquired passively via placenta and/or breast milk, may contribute to the reduced efficacy of oral rotavirus vaccines observed in children in developing countries. This study aimed to investigate the effect of rotavirus specific maternal antibodies on the serum IgA response or stool excretion of vaccine virus after any dose of an oral rotavirus vaccine, RV3-BB, in parallel to a Phase IIa clinical trial conducted at Dunedin Hospital, New Zealand. At the time of the study rotavirus vaccines had not been introduced in New Zealand and the burden of rotavirus disease was evident.
Methods: Rotavirus specific IgG and serum neutralizing antibody (SNA) levels in cord blood and IgA levels in colostrum and breast milk samples collected ∼4 weeks, ∼20 weeks and ∼28 weeks after birth were measured. Infants were randomized to receive the first dose of vaccine at 0–5 d (neonatal schedule) or 8 weeks (infant schedule). Breast feeding was with-held for 30 minutes before and after vaccine administration. The relationship between rotavirus specific IgG and SNA levels in cord blood and IgA in colostrum and breast milk at the time of first active dose of RV3-BB vaccine and level of IgA response and stool excretion after 3 doses of vaccine was assessed using linear and logistic regression.
Results: Forty infants received 3 doses of RV3-BB rotavirus vaccine and were included in the analysis of the neonatal and infant groups. Rotavirus specific IgA in colostrum (neonatal schedule group) and breast milk at 4 weeks (infant schedule group) was identified in 14/21 (67%) and 14/17 (82%) of infants respectively. There was little evidence of an association between IgA in colostrum or breast milk IgA at 4 weeks, or between cord IgG or SNA level, and IgA response or stool excretion after 3 doses of RV3-BB, or after one dose (neonatal schedule) (all p>0.05).
Conclusions: The level of IgA in colostrum or breast milk and level of placental IgG and SNA did not impact on the serum IgA response or stool excretion following 3 doses of RV3-BB Rotavirus Vaccine administered using either a neonatal or infant schedule in New Zealand infants.
Introduction
Diarrhea due to rotavirus causes significant morbidity and accounted for nearly half a million deaths in children under 5 y in 2008, with the majority of the deaths occurring in low-income countries.Citation1 In 2009, the World Health Organization's Strategic Advisory Group of Experts (SAGE) made a global recommendation for a monovalent (RV1, Rotarix, GSK Biologicals, Belgium) and a pentavalent (RV5, RotaTeq, Merck, USA) rotavirus vaccine to be included in National Immunisation Programs (NIP).Citation2 Vaccine efficacy of these rotavirus vaccines in high income countries with low child mortality has consistently been shown to be between 85–100% against severe rotavirus disease,Citation3-6 with significantly lower vaccine efficacy (39–77%) in low income countries with high child mortality.Citation7-9 Similarly, vaccine effectiveness against severe rotavirus disease in post-licensure studies have shown greater protection in high income countries (89–100% in Australia and USA),Citation10-12 compared with low or middle income countries (40–76% in Brazil, El Salvador and Nicaragua).Citation13-16
Several hypotheses have been suggested to explain the difference in vaccine efficacy between lower and higher-income countries, including maternal antibodies acquired via breast milk or transplacentally.Citation17,18 Traditionally maternal antibodies are defined as antibodies actively transported across the placenta but for this study, maternal antibodies were defined as both placentally-transferred and antibodies transferred through breast milk during breast feeding, including both IgG and IgA antibodies.Citation19,20 Malnutrition, concomitant intestinal infections or other diseases, such as HIV or malaria, differing immune responses to specific rotavirus strains or co-administration with oral polio vaccine have also been postulated to play a role.Citation21,22
Maternal serum (IgG) and breast milk (IgA) antibodies to rotavirus are highly prevalent in low income countries compared with high income countries, with the hypothesis that this is due to repeat exposure to wild-type rotavirus infections.Citation17,23 Higher titres of anti-rotavirus IgA and neutralising activity in breast milk have been shown in Indian mothers compared with American mothers,Citation24 and in colostrum compared with transitional breast milk.Citation23 Higher maternally transferred IgG levels have also been shown in infants who did not demonstrate IgA seroconversion to RV1 compared with those who had a positive serum immune response. However, more recent studies that have compared the effect of with-holding breast feeding before and after administration of currently licensed rotavirus vaccines with no with-holding, have not shown improved vaccine immune responses.Citation25-27 Given these conflicting results surrounding the role of maternally acquired antibodies, it is not clear whether breast feeding restriction could affect vaccine efficacy in low income countries. In particular, the impact of maternally acquired antibodies has not been examined in relation to a rotavirus vaccine given at birth.
The primary objective of this study was to determine the relationship between rotavirus specific IgA in colostrum or breast milk at the time of first active dose of a rotavirus vaccine and level of IgA response after 3 doses of vaccine when received as a neonatal or an infant schedule, and after one dose in the neonatal schedule. Secondary objectives explored the effect of cord IgG and serum neutralisation antibodies (SNA) on level of IgA response and also the outcomes of IgA response, cumulative stool excretion and cumulative vaccine take. Finally we describe the level of IgA in breast milk from birth to 28 weeks. Conduct of a phase II trial of a new rotavirus vaccine, RV3-BB,Citation28 provided an opportunity to explore these objectives in New Zealand infants. Although a high income country, rotavirus infections in New Zealand are associated with an estimated 1500 hospitalisations in children under 5 y of age, 3000 emergency department presentations and 10,000 general practitioner visits per year, with an annual cost of $NZ7.07 million.Citation29 At the time of the study, rotavirus vaccines had not been introduced in New Zealand so we anticipated that mothers would still be exposed to circulating rotavirus in the community and thus some would have high levels of maternal antibodies in breast milk. We hypothesized that maternal antibodies may inhibit the immune response to RV3-BB rotavirus vaccine, with a more pronounced effect seen when the first dose was administered at birth compared with 8 weeks due to the high levels of IgA in colostrum compared with transitional (milk produced after colostrum and lasts for approximately 2 weeks) or mature milk (milk produced after 2 or 3 weeks).Citation23
Results
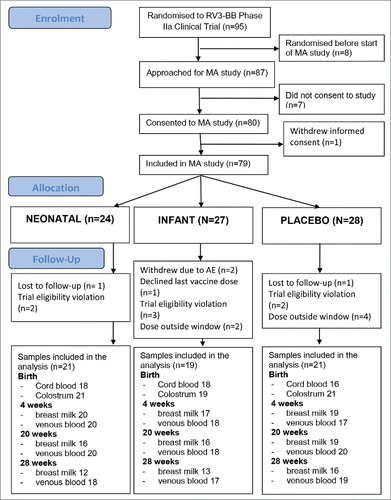
There were 79 participants enrolled into the Maternal Antibody study, and 61 infants were included in the analysis, with 40 study infants receiving 3 doses of RV3-BB rotavirus vaccine administered in either a neonatal (neonatal group: n = 21) or infant schedule (infant group: n = 19) (). The 21 participants in the placebo group were used as a negative control. The demographic characteristics of the study participants included in the analysis (n = 61) are presented in . 21/21 (100%) of infants in the neonatal group had colostrum at birth and 17/19 (89%) from the infant group had breast milk at 4 weeks included in the analysis. In the placebo group, 21/21 (100%) provided colostrum and 19/21 (90%) had breast milk at 4 weeks included in the analysis ().
Table 1. Summary of demographic data for Maternal Antibody study participants included in the analysis.
Exclusive breastfeeding
Of all infants included in the analysis, 19/21 (90%) in the neonatal group and 15/19 (79%) in the infant group were exclusively breast fed at birth and at 4 weeks respectively (). After 3 doses of vaccine, 13/21 (62%) were exclusively breast fed in the neonatal group at 20 weeks, and 9/19 (47%) were exclusively breast fed in the infant group at 28 weeks.
IgA in colostrum or breast milk and serum IgA and stool excretion following 3 doses of RV3-BB vaccine
Neonatal group
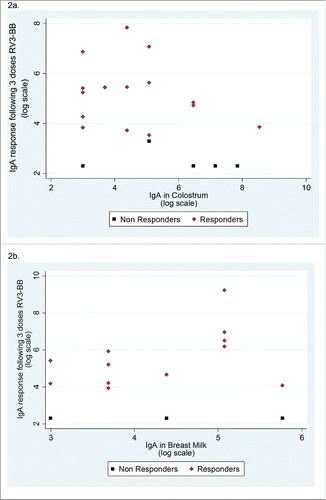
A serum IgA response after 3 doses of RV3-BB vaccine was identified in 16 (76%) of 21 infants in the neonatal group. The median IgA in colostrum was higher in serum IgA non-responders compared with responders (640 vs 80) (), however there was no evidence of an association between IgA in colostrum and log serum IgA response following 3 doses of vaccine (regression coefficient = −0.004 (95% confidence interval [CI] −0.0011, 0.0002)). Of note, there was large variability in colostrum IgA levels among participants, particularly in those who did not exhibit IgA sero-conversion (). Positive cumulative stool excretion was identified in 15 (71%) of 21 infants. The median IgA level in colostrum was higher in neonates with no stool vaccine virus excretion compared with those who demonstrated stool virus excretion (). Twenty (95%) of the 21 infants had a positive vaccine take.
Table 2. Summary of IgA in colostrum and Breast Milk at time of first active vaccine dose for participants with and without serum IgA or stool excretion and vaccine take after 3 doses of RV3-BB.
Figure 2. Scatterplots of IgA in colostrum and breast milk at time of first active vaccine dose against serum IgA response after 3 doses of RV3-BB (Log scale) 2a. Participants who received the neonatal schedule – after 3 doses 2b. Participants who received the infant schedule – after 3 doses Note: : one missing post dose 3 serum IgA level. : 2 missing post dose 4 serum IgA levels and 2 missing breast milk at 4 week levels.
Infant schedule
A serum IgA response post 3 doses of RV3-BB vaccine was identified in 14 (74%) of 19 infants in the infant schedule group. The median IgA level in breast milk at 4 weeks was the same in serum IgA responders and non-responders (80) (). There was no evidence of an association between IgA in breast milk and log serum IgA response following 3 doses of vaccine (regression coefficient = −0.0007 (95% CI −0.0106, 0.012) (). Positive cumulative stool excretion was identified in 15 (79%) of the 19 infants and the median IgA in breastmilk at 4 weeks level was higher in infants with evidence of stool excretion of vaccine virus compared with those who demonstrated no stool virus excretion. Seventeen (89%) of the 19 infants had a positive vaccine take ().
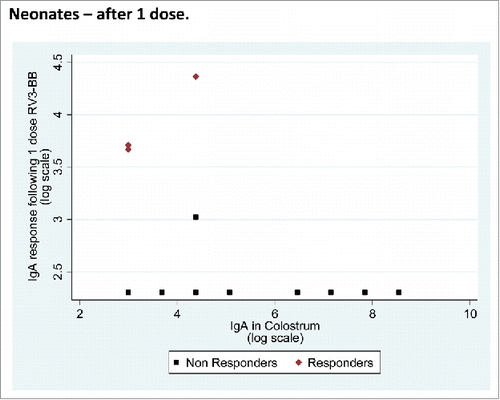
IgA in colostrum and serum IgA and stool excretion after the first dose of RV3-BB vaccine, neonatal schedule
A serum IgA response was identified in 3 (14%) of 21 and 5 (24%) of 21 participants had a positive vaccine take following a single dose of RV3-BB in the neonatal group. The median and IQR for IgA in colostrum was lower in serum IgA responders 3/21 (median 20; IQR 20–80) compared with the non-responders 17/21 (median 160; IQR 20–640). There was no evidence of an association between IgA in colostrum and log serum IgA response (regression coefficient = −0.0001 (95%CI −0.0003, 0.0001)) (). Stool excretion was identified in 3/21 (14%) following 1 dose of RV3-BB vaccine. The median IgA in colostrum of the participants exhibiting stool vaccine excretion was 1280 (IQR 20–2560) compared with those who did not have vaccine excretion who had a median IgA in colostrum of 80 (IQR 20–160).
IgG and SNA in cord blood and serum IgA and stool excretion following 3 doses of RV3-BB vaccine
There was no evidence of an association between cord IgG and either serum IgA response or cumulative stool excretion post 3 doses of RV3-BB vaccine in either the neonatal or infant groups, or post 1 dose of RV3-BB vaccine in the neonatal group. However, median cord IgG levels appeared to be higher in serum IgA non-responders in both groups although there was wide variability (serum IgA non-responders: 28437 units (range 26397–34566 units) (neonatal) and 31100 units (range 24303–41746 units) (infant) vs serum IgA responders: 18599 units (range 7169–29223 units) (neonatal) and 15879 units (range 8485–29157 units) (infant)).
There was no evidence of an association between cord SNA and either serum IgA response or cumulative stool excretion after 3 doses of RV3-BB vaccine in either the neonatal or infant groups, or after a single dose of RV3-BB vaccine in the neonatal group. However, median cord SNA levels appeared to be higher in the serum IgA non-responders in the infant group compared with responders although there was wide variability (infants: 1280 units (range 847–1631 units) for non-responders vs 251 units (range 141–408 units) for responders; neonates: 448 units (range 263–1046 units) for non-responders and 474 units (range 147–1331 units) for responders).
Placebo group – Negative control
Five of 21 (24%) participants in the placebo group developed a positive serum anti-rotavirus IgA level following receipt of the placebo vaccination, of which 1 (5%) participant had positive serum IgA level detected from 20 weeks of age, and 4 (19%) from 28 weeks of age. One participant in this group exhibited stool excretion of RV3-BB vaccine at 20 weeks and 28 weeks and developed a positive serum anti-rotavirus IgA level at 28 weeks.
Colostrum/breast milk IgA levels over the study duration
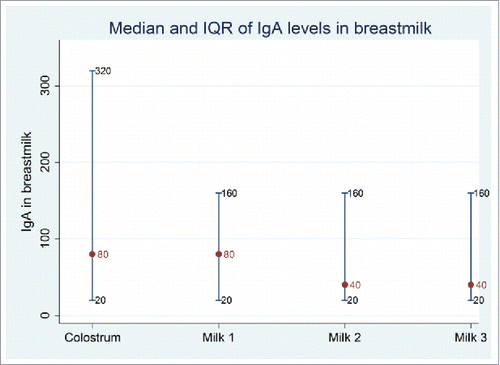
Median IgA levels were similar in colostrum and breast milk at 4 weeks (80), with a wider IQR for colostrum specimens (). At 20 and 28 weeks, the median IgA level in breast milk level was 40. In the neonatal schedule group, 14/21 (67%) were positive for rotavirus specific IgA in colostrum and 14/17 (82%) in the infant schedule group were positive for rotavirus specific IgA at 4 weeks (2 participants in this group did not provide breast milk samples at 4 weeks) ().
Figure 4. Mean and IQR IgA Titer in Colostrum and Breast Milk over first 28 weeks postpartum, for all participants in the maternal antibodies study irrespective of randomized group (N = 79). Note: Colostrum (n = 79) was collected at birth; milk1 (n = 71) at 4 weeks; Milk 2 (n = 62) at ∼20 weeks and milk 3 (n = 50) at ∼28 weeks.
Discussion
This study examined the relationship between IgA in colostrum and the immunogenicity of a single birth dose of an oral rotavirus vaccine, RV3-BB, following 3 doses of vaccine using 2 different administration schedules – a neonatal and infant schedule. Vaccine take was very high in participants in both groups (95% and 93% respectively)Citation28 in this setting and group sizes were relatively small, which limited our ability to identify an association between responders and non-responders. However, the study did provide a unique opportunity to describe breast milk and placentally derived maternal antibodies in parallel to a rotavirus vaccine trial.
Breast milk is known to have a protective effect against rotavirus disease due to both innate immune and antibody factors which have been shown to inhibit rotavirus replication in vitro.Citation30,31 However, there is clearly a complex relationship between maternal antibody titers at the time of immunisation and viral load that affects the neutralisation of live viral vaccines. It has been postulated that when there is an excess of maternal antibody, such as in rotavirus endemic settings, and/or low viral load, there may be inhibition of viral replication, and thus possibly of both B and T cell infant responses.Citation32 Conversely, low titer maternal antibody in breast milk may have both a suppressing and enhancing effect on B cell responsesCitation33 and other factors may also play a role, such as the rotavirus vaccine strain. The RV3-BB vaccine is derived from a neonatal rotavirus strain which binds to specific receptors in the newborn gut and is integrin independent and, may inherently offer protection from maternal antibodies.Citation34
In this study, high levels of IgA in colostrum or breast milk did not appear to inhibit the serum IgA response or stool excretion following 3 doses of RV3-BB rotavirus vaccine in either a neonatal schedule with the first dose at age 0–5 d or an infant schedule with the first dose at 8 weeks of age. These findings are consistent with studies from North IndiaCitation25 and South AfricaCitation26 which found little difference in anti-rotavirus IgA seroconversion irrespective of whether breastfeeding was withheld for either 30 minutes or one hour before and after RV1 vaccine administration. Interestingly, in a recent Pakistani study, immediate breast feeding following vaccination improved the seroconversion rate following both 2 and 3 doses of RV1 compared with with-holding breast feeding for one hour.Citation27 Although we did not aim to study the timing of breast feeding on vaccine take, breast milk would most likely still have been present in the infant's stomach at the time of vaccination in this study, despite a feeding restriction time of 30 minutes pre- and post-administration of the vaccine, as half gastric emptying time in infants is estimated to be 86 minutes.Citation35
Given that this study was conducted in parallel to the Phase IIa trial, breast milk was taken at 4 weeks which aligned with a study visit and was used as an approximation of breast milk before the first vaccine dose in the infant arm given at 8 weeks. As breast milk at 4 and 8 weeks is considered mature milk and beyond the stage of transitional milk, there was expected to be minimal decline in the rotavirus specific IgA level in breast milk between 4 and 8 weeks. If indeed there was a slight decline in the IgA levels in breast milk from 4 to 8 weeks, it would have meant that should our hypothesis be true, the infant group should have higher vaccine take than the neonatal group, which was not the case. A slight decline in IgA levels in breast milk from 4 to 8 weeks would not affect the comparison of responders and non-responders within the infant group, as both the responders and non-responders should have the same rate of decline in breast milk IgA levels. The background rate of natural rotavirus infection in the first few months of life is low in this setting (< 5% by 20 weeks as seen in the placebo group), therefore we would not expect there to be a rise in the IgA levels in breast milk from 4 to 8 weeks.
Although this and other studies have not demonstrated an association between IgA levels in colostrum or breast milk and vaccine take, an inverse relationship has been reported between maternal serum anti-rotavirus IgG antibody and serum anti-rotavirus IgA level after 2 doses of RV1.Citation25 High titres of pre-existing rotavirus IgG, presumed to be a measure of transplacental IgG in the absence of elevated serum IgA titer, were associated with a lower serum IgA response to the 116E rotavirus vaccine (a monovalent human-bovine oral rotavirus vaccine) in Indian infants. This was overcome in a dose-dependent manner.Citation36 However, in our study anti-rotavirus IgG and SNA in cord blood did not appear to affect vaccine take following 3 doses or one dose of RV3-BB vaccine.
It has been postulated that there may be a critical level of IgA antibody titer that could be associated with sustained protection after rotavirus vaccination. In a recent systematic review which found that rotavirus vaccine efficacy during the first 2 years of life was lower among countries where the serum IgA geometric mean concentration or titer (GMC) was < 90 compared with countries with a GMC >90.Citation37 It could be that IgA in colostrum or breast milk reduces the duration of the serum immune response despite evidence of an immediate post-immunisation response.
It is hard to ascertain the true impact of colostrum IgA levels on the first dose of RV3-BB vaccine from the current study given the small sample size. A meta-analysis stated that immediate breast feeding impaired the immune response to a single dose of rhesus rotavirus vaccine.Citation38 However, subsequent doses of vaccine were shown to be associated with IgA sero-conversion.Citation39 It could be that the first dose of a rotavirus vaccine primes the immune system, with a serum IgA response only detected after 2 or 3 doses of rotavirus vaccine. However, a potential advantage of a vaccine based on a human neonatal rotavirus strain delivered at birth is the proposed intrinsic structural characteristics that enable it to avoid neutralisation by maternal and breast milk antibodies.Citation34
Data from the placebo group provided a negative control for the rate of natural rotavirus infection in this study population. Interestingly one of the participants in the placebo group was excreting RV3-BB rotavirus vaccine in the stool at both 20 weeks and 28 weeks, which suggested presence of this strain in the community.
Only 1 participant included in the analysis group (1/61, 1.6%) had a positive cord blood anti-rotavirus IgA level, which was just above detection limit. This participant was from the neonatal group. Excluding participants in the neonatal arm who received a birth dose of RV3-BB vaccine, none of the other study participants (in the infant and placebo arms) had positive serum anti-rotavirus IgA at 4 weeks, indicating that early neonatal natural rotavirus infection rate is minimal in this population In contrast, the background pre-vaccination (at 6 weeks) positive serum rotavirus IgA rate ranged from 9/353 (2.5%) in PakistanCitation27 to 62/359 (17.2%) in India,Citation36 though in the Indian study an IgA level double the lowest detection limit was used as the cut off to indicate early neonatal rotavirus infection.
There was a wide range of cord blood rotavirus IgG from the participants in this study. Over 98% (60/61) of our participants had detectable rotavirus IgG in the cord blood. Our cord rotavirus IgG level appeared higher than that reported in Indonesia (range 372–28,000, median 2446, IQR 1276–5760),Citation23 but was comparable to the pre-vaccination rotavirus IgG levels at 8 weeks of age reported in India (median 9140–14962, range 894–85,360).Citation36 Given that transplacental rotavirus IgG decreases over time, it can be assumed that the cord rotavirus IgG level in India is higher than that seen in this setting.
Limitations
Our study is limited by the small sample size and high vaccine take found in this setting which provided little power to identify associations. Given this we regard the findings from this study as exploratory and hypothesis generating for future studies. Despite this, the findings presented here are important as there is currently no other available data examining the impact of maternally acquired antibodies on a dose of rotavirus vaccine given at birth. The exploratory findings from this study need to be explored in a larger study and in particular in a resource poor setting where the level of maternal antibody is likely to be higher and have a greater impact on vaccine efficacy, which is currently being conducted as part of a phase IIb trial of RV3-BB vaccine in Indonesia. The lack of an unrestricted feeding group in the current study did not allow comparison of the effect of immediate exposure to breast milk IgA in the gastrointestinal tract.
Conclusion
This study found no evidence that passively acquired breast milk antibodies, including IgA in colostrum and breast milk and IgG and SNA in cord blood, were associated with a reduction in IgA response or stool excretion to RV3-BB vaccine administered in a neonatal or infant schedule in New Zealand infants. These data provide evidence to support the administration of the human neonatal rotavirus vaccine, RV3-BB, in either a neonatal or an infant schedule, although further studies involving a larger infant cohort in a high rotavirus disease burden setting are required.
Methods
The Maternal Antibody Study was conducted in the Department of Women's and Children's Health, University of Otago and the Dunedin Hospital, New Zealand. Ethics approval was obtained from the Lower South Regional Ethics Committee, New Zealand (LRS/12/01/004) and approved by the Royal Children's Hospital HREC.
Participant populations
All mothers of the 95 eligible infants recruited into the Phase IIa trial were invited to participate in the Maternal Antibody Study after birth. Study inclusions included their intention to breast-feed and willingness to provide breast milk samples at each blood collection time point (detailed below). Exclusion criteria were as for the Phase IIa RV3-BB vaccine trial.Citation28
In the Phase IIa trial participants were randomized to receive 3 oral doses of RV3-BB vaccine prepared at Meridian Life Sciences (Memphis, TN, USA), at a titer of ∼8·3 × 106 focus-forming units per mL in a concealed syringe, administered in either a neonatal schedule (doses administered at birth (0–5 days), ∼8 weeks and ∼15 weeks), an infant schedule (doses administered at 8, 15 and 24 weeks) or placebo.Citation28 RV3-BB is a monovalent human neonatal rotavirus vaccine derived from G3P[6] human neonatal rotavirus strain found circulating in healthy newborn babies in the obstetric hospitals in Melbourne, Australia in 1975. Vaccine doses were separated from routine NIP immunisations by at least 10 d and breast-feeding was withheld for 30 minutes before and after vaccine administration. The second, third, and fourth doses of vaccine or placebo were preceded by a 2 mL dose of antacid solution (Mylanta Original; Johnson & Johnson, New Brunswick, NJ, USA).
Specimen collection
Cord blood samples (5ml) were obtained at delivery and venous blood was collected at ∼4 weeks, ∼20 weeks and ∼28 weeks after birth to assess the immune response after RV3-BB vaccine. Serum was isolated from whole blood and stored at −70C until analyzed.
Colostrum (2 ml) was collected 0–5 d after delivery, and breast milk (3 ml) samples were obtained at each of the 3 blood collection visits conducted as part of the Phase IIa RV3-BB vaccine trial.Citation28 Breast milk at 4 weeks was used as an approximation of breast milk before the first active vaccine dose in the infant arm given at 8 weeks.
Stool samples were obtained on days 3–7 following each dose of vaccine to assess RV3-BB virus excretion. All the breastmilk, serum and stool samples were stored at −70° freezer at Dunedin Hospital before being sent to the Enteric Virus Laboratory at Murdoch Childrens Research Institute (MCRI) in Melbourne, Australia for analysis.Citation28
Laboratory analysis
Rotavirus-specific IgA and IgG antibodies were measured in serum samples by enzyme linked immunosorbent assays (ELISA) as described previously.Citation28 The levels of anti-rotavirus specific IgA and IgG were determined using a standard curve generated from sera known to contain specific anti-rotavirus IgA or IgG. The positive sera known to contain anti-rotavirus IgA was arbitrarily assigned a titer of 250,000 Units (U)/ml, and positive sera containing anti-rotavirus IgG was arbitrarily assigned a value of 250,000 U/ml.Citation28,40 The lower detection limit of the assays was 20 U/ml, negative sera samples were assigned a concentration 50% of the lower limit of detection of the ELISA (i.e. 10 U/ml).
Neutralising antibody titres against RV3-BB were measured in cord blood samples using fluorescent focus reduction neutralisation assay, as described previously.Citation40,41 The neutralising titer was determined as the reciprocal of the dilution where a 50% reduction in fluorescence was observed.Citation42 Rotavirus specific IgA in colostrum and breast milk were measured by end point ELISA as described previously.Citation23 Data was reported as the highest reciprocal titer with an OD 2-fold higher than background. Rotavirus excretion was assessed in stool samples with a rotavirus VP6-specific RT-PCR assay. Method for detection of RV3 vaccine virus by sequence analysis was as described previously.Citation28
Statistical analysis
The analysis is based on participants in the 2 active vaccination arms, with the placebo group as a negative control to indicate the rate of natural infection with rotavirus in this study population. Demographic characteristics of the participants in the analysis are presented using means and standard deviations (SD) for continuous variables and numbers and proportions for categorical variables. Level of IgA response is presented as a mean and SD, and IgA response, stool excretion and cumulative vaccine take are presented as numbers and proportions, all of which are presented separately for the 2 schedules. Medians and interquartile ranges (IQRs) of IgA in colostrum and breast milk at 4 weeks are reported by schedule.
The analysis of the relationship between maternal antibodies and all outcomes included all eligible participants who received all 3 doses of RV3-BB vaccine according to the Phase IIa trial protocolCitation28 and had at least one of IgA or stool excretion data following at least one dose of vaccine, and a sample of colostrum or breast milk at the time of administration of the first dose of vaccine and/or cord blood for analysis of IgG or SNA. Only participants meeting these criteria were included in the analysis. The level of IgA response was determined from serum collected from blood samples. A positive IgA response was defined as > = a threefold increase from baseline in serum anti-rotavirus IgA. Cumulative stool excretion of vaccine virus was defined as RV3-BB excretion in stools any day from day 3 to 7 following administration of any dose of vaccine. Vaccine Take was assessed by serum immune response (> = threefold increase from baseline in serum anti-rotavirus IgA or serum neutralising antibodies (SNA) in the 28 d following vaccine administration) and/or stool excretion of vaccine virus following any dose of vaccine. Cumulative vaccine take was defined as a positive vaccine take following any dose of vaccine.
Initially our primary objective was to examine the relationship between the level of IgA in colostrum/breastmilk and cumulative vaccine take following 3 doses of RV3-BB rotavirus vaccine, using both schedules (neonatal and infant). However given the cumulative vaccine take was so high we focused on the components of cumulative vaccine take – serum IgA response and stool excretion as continuous as well as dichotomous variables in a post hoc analysis. Placebo participants were excluded from this analysis given the focus on the immune response to the vaccine. Statistical analysis was performed using Stata 13.
Medians and IQRs of IgA in colostrum and Breast Milk at time of first active vaccine dose for participants are also presented separately for those with and without serum IgA or stool excretion and vaccine take after 3 doses of RV3-BB, by vaccine schedule.
Separate linear regression models were used to explore the relationship between the i) level of IgA in colostrum/breast milk at 4 weeks ii) SNA and iii) Cord IgG and the serum log-transformed IgA levels following 3 doses of vaccine, in each vaccine schedule. In the neonatal schedule only, linear regression was also used to explore the relationship between the level of IgA in colostrum and serum log-transformed IgA levels following the first dose of RV3-BB. Logistic regression models were used to explore the relationship between the level of IgA in colostrum and breast milk at 4 weeks and cumulative stool excretion.
Finally, the level of anti-rotavirus IgA titres over time, up to 28 weeks after birth are displayed graphically as medians and IQR at each time point. Median levels instead of mean levels were chosen as the data was highly skewed.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would sincerely like to thank the participants and their families for participating in this study. We would also like to thank the Freemasons (New Zealand) Postgraduate Fellowship in Pediatrics and Child Health for salary support to Mee-Yew Chen during the time of this study. We sincerely thank Dr Pam Jackson at Department of Women's and Children's Health, Dunedin School of Medicine, University of Otago for her supervision and assistance of the study in New Zealand. We acknowledge Professor Ruth Bishop and A/Prof Jim Buttery in the RV3 Rotavirus Research Group, Murdoch Children's Research Institute and Professor Barry Taylor and Associate Professor David Reith at Dunedin School of Medicine for their assistance and support with this study. We also acknowledge the help from the RV3 team at Department of Women's and Children's Health, Dunedin School of Medicine with this study. CDK was supported by an NHMRC CDA fellowship (607347). This research was also supported by the Victorian Government's Operational Infrastructure Support Program.
Funding
This study received grants from:
Dunedin School of Medicine Dean's Bequest Round
Maurice and Phyllis Paykel Trust
The Dunedin School of Medicine Dean's Bequest Round and Maurice and Phyllis Paykel Trust have no involvement in the study design, the collection, analysis and interpretation of data, the writing of the report, and the decision to submit the article for publication.
References
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD, WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12(2):136-41; http://dx.doi.org/10.1016/S1473-3099(11)70253-5
- World Health Organisation. WHO recommends global use of rotavirus vaccines. WHO Weekly Epidemiological Record 2009; 23:232–236.
- Vesikari T, Matson DO, Dennehy P, Van Damme P, Santosham M, Rodriguez Z, Dallas MJ, Heyse JF, Goveia MG, Black SB, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Eng J of Med 2006; 354(1):23-33; PMID:16394299; http://dx.doi.org/10.1056/NEJMoa052664
- Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis B, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Eng J Med 2006; 354(1):11-22; PMID:16394298; http://dx.doi.org/10.1056/NEJMoa052434
- Phua KB, Lim FS, Lau YL, Nelson EAS, Huang LM, Quak SH, Lee BW, Teoh YL, Tang H, Boudville I, et al. Safety and efficacy of human rotavirus vaccine during the first 2 years of life in Asian infants: randomised, double-blind, controlled study. Vaccine 2009; 27(43):5936-41; PMID:19679216; http://dx.doi.org/10.1016/j.vaccine.2009.07.098
- Vesikari T, Karvonen A, Prymula R, Schuster V, Tejedor JC, Cohen R, Meurice F, Han HH, Damaso S, Bouckenooghe A. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet 2007; 370(9601):1757-63; PMID:18037080; http://dx.doi.org/10.1016/S0140-6736(07)61744-9
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, Ngwira B, Victor JC, Gillard PH, Cheuvart BB, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. N Eng J Med 2010; 362(4):289-98; PMID:20107214; http://dx.doi.org/10.1056/NEJMoa0904797
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376(9741):606-14; PMID:20692030; http://dx.doi.org/10.1016/S0140-6736(10)60889-6
- Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 376(9741):615-23; PMID:20692031; http://dx.doi.org/10.1016/S0140-6736(10)60755-6
- Field EJ, Vally H, Grimwood K, Lambert SB. Pentavalent rotavirus vaccine and prevention of gastroenteritis hospitalizations in Australia. Pediatrics 2010; 126(3):e506-12; PMID:20732946; http://dx.doi.org/10.1542/peds.2010-0443
- Eberly MD, Gorman GH, Eide MB, Olsen CH, Rajnik M. The effect of rotavirus immunization on rotavirus gastroenteritis hospitalization rates in military dependents. Vaccine 2011; 29(4):650-9; PMID:21129394; http://dx.doi.org/10.1016/j.vaccine.2010.11.041
- Wang FT, Mast TC, Glass RJ, Loughlin J, Seeger JD. Effectiveness of the pentavalent rotavirus vaccine in preventing gastroenteritis in the United States. Pediatrics 2010; 125(2):e208-13; PMID:20100757; http://dx.doi.org/10.1542/peds.2009-1246
- Justino MC, Linhares A, Lanzieri T, Miranda Y, Mascarenhas JD, Abreu E, Guerra SF, Oliveira AS, da Silva VB, Sanchez N, et al. Effectiveness of the monovalent G1P[8]Human rotavirus vaccine against hospitalization for severe G2P[4]rotavirus gastroenteritis in Belem, Brazil. Pediat Infect Dis J 2011; 30(5):396-401; PMID:21150692; http://dx.doi.org/10.1097/INF.0b013e3182055cc2
- de Palma O, Cruz L, Ramos H, de Baires A, Villatoro N, Pastor D, de Oliveira LH, Kerin T, Bowen M, Gentsch J, et al. Effectiveness of rotavirus vaccination against childhood diarrhoea in El Salvador: Case-control study. BMJ 2010;340:c2825.
- Mast TC, Khawaja S, Espinoza F, Paniagua M, Del Carmen LP, Cardellino A, Sánchez E. Case-control study of the effectiveness of vaccination with pentavalent rotavirus vaccine in Nicaragua. Pediat Infect Dis J 2011; 30(11):e209-15; PMID:21768920; http://dx.doi.org/10.1097/INF.0b013e31822a8527
- Patel M, Pedreira C, De Oliveira LH, Tate J, Orozco M, Mercado J, Gonzalez A, Malespin O, Amador JJ, Umaña J, et al. Association between pentavalent rotavirus vaccine and severe rotavirus diarrhea among children in Nicaragua. JAMA 2009; 301(21):2243-51; PMID:19491186; http://dx.doi.org/10.1001/jama.2009.756
- Serazin AC, Shackelton LA, Wilson C, Bhan MK. Improving the performance of enteric vaccines in the developing world. Nat Immunol 2010; 11(9):769-73; PMID:20720580; http://dx.doi.org/10.1038/ni0910-769
- Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? J Infect Dis 2009; 200(Suppl 1):S39-48.
- Niewiesk S. Maternal antibodies: clinical significance, mechanism of interference with immune responses, and possible vaccination strategies. Front Immunol 2014; 5:446; PMID:25278941; http://dx.doi.org/10.3389/fimmu.2014.00446
- Edwards KM. Maternal antibodies and infant immune responses to vaccines. Vaccine 2015; 33(47):6469-72; PMID:26256526; http://dx.doi.org/10.1016/j.vaccine.2015.07.085
- Patel M, Steele AD, Parashar UD. Influence of oral polio vaccines on performance of the monovalent and pentavalent rotavirus vaccines. Vaccine 2012; 30(Supplement 1):A30-A5; http://dx.doi.org/10.1016/j.vaccine.2011.11.093
- Kirkwood CD. Genetic and antigenic diversity of human rotaviruses: potential impact on vaccination programs. J Infect Dis 2010; 202(Supplement 1):S43-S8; PMID:20684716; http://dx.doi.org/10.1086/653548
- Chan J, Nirwati H, Triasih R, Bogdanovic-Sakran N, Soenarto Y, Hakimi M, Duke T, Buttery JP, Bines JE, Bishop RF, et al. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine 2011; 29(6):1242-7; PMID:21147127
- Moon S-S, Wang Y, Shane AL, Nguyen T, Ray P, Dennehy P, Baek LJ, Parashar U, Glass RI, Jiang B. Inhibitory effect of breast milk on infectivity of live oral rotavirus vaccines. Pediat Infect Dis J 2010; 29(10):919-23; PMID:20442687; http://dx.doi.org/10.1097/INF.0b013e3181e232ea
- Rongsen-Chandola T, Strand TA, Goyal N, Flem E, Rathore SS, Arya A, Winje BA, Lazarus R, Shanmugasundaram E, Babji S, et al. Effect of withholding breastfeeding on the immune response to a live oral rotavirus vaccine in North Indian infants. Vaccine 2014; 32(Suppl 1):A134-9; http://dx.doi.org/10.1016/j.vaccine.2014.04.078
- Groome MJ, Moon S-S, Velasquez D, Jones S, Koen A, van Niekerk N, Jiang B, Parashar UD, Madhi SA. Effect of breastfeeding on immunogenicity of oral live-attenuated human rotavirus vaccine: a randomized trial in HIV-uninfected infants in Soweto, South Africa. Bull World Health Org 2014; 92(4):238-45; PMID:24700991; http://dx.doi.org/10.2471/BLT.13.128066
- Ali A, Kazi AM, Cortese MM, Fleming JA, Moon S, Parashar UD, Jiang B, McNeal MM, Steele D, Bhutta Z, et al. Impact of withholding breastfeeding at the time of vaccination on the immunogenicity of oral rotavirus vaccine—a randomized trial. PloS One 2015; 10(6):e0127622; PMID:26035743; http://dx.doi.org/10.1371/journal.pone.0127622
- Bines JE, Danchin M, Jackson P, Handley A, Watts E, Lee KJ, West A, Cowley D, Chen MY, Barnes GL, et al. Safety and immunogenicity of RV3-BB human neonatal rotavirus vaccine administered at birth or in infancy: A randomised, double-blind, placebo-controlled trial. Lancet Infect Dis 2015; 15:1389-97; PMID:26318715
- Milne RJ, Grimwood K. Budget impact and cost-effectiveness of including a pentavalent rotavirus vaccine in the New Zealand childhood immunization schedule. Value in Health 2009; 12(6):888-98; PMID:19490550; http://dx.doi.org/10.1111/j.1524-4733.2009.00534.x
- Newburg DS, Peterson JA, Ruis-Palacios GM, Matson DO, Morrow AL, Shults J, Guerrero ML, Chaturvedi P, Newburg SO, Scallan CD, et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998; 351(9110):1160; PMID:9643686; http://dx.doi.org/10.1016/S0140-6736(97)10322-1
- Yolken RH, Peterson JA, Vonderfecht SL, Fouts ET, Midthun K, Newburg DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest 1992; 90(5):1984-91; PMID:1331178; http://dx.doi.org/10.1172/JCI116078
- Siegrist CA. Mechanisms by which maternal antibodies influence infant vaccine responses: review of hypotheses and definition of main determinants. Vaccine 2003; 21(24):3406-12; PMID:12850349; http://dx.doi.org/10.1016/S0264-410X(03)00342-6
- Nguyen TV, Yuan L, Azevedo MS, Jeong K-I, Gonzalez AM, Iosef C, Lovgren-Bengtsson K, Morein B, Lewis P, Saif LJ. Low titer maternal antibodies can both enhance and suppress B cell responses to a combined live attenuated human rotavirus and VLP-ISCOM vaccine. Vaccine 2006; 24(13):2302-16; PMID:16361002; http://dx.doi.org/10.1016/j.vaccine.2005.11.043
- Graham KL, Halasz P, Tan Y, Hewish MJ, Takada Y, Mackow ER, Robinson MK, Coulson BS. Integrin-using rotaviruses bind α2β1 integrin α2 I domain via VP4 DGE sequence and recognize αXβ2 and αVβ3 by using VP7 during cell entry. J Virol 2003; 77(18):9969-78; PMID:12941907; http://dx.doi.org/10.1128/JVI.77.18.9969-9978.2003
- Van Den Driessche M, Peeters K, Marien P, Ghoos Y, Devlieger H, Veereman-Wauters G. Gastric emptying in formula-fed and breast-fed infants measured with the 13C-octanoic acid breath test. J Pediat Gastroenterol Nutrit 1999; 29(1):46-51; PMID:10400103; http://dx.doi.org/10.1097/00005176-199907000-00013
- Appaiahgari MB, Glass R, Singh S, Taneja S, Rongsen-Chandola T, Bhandari N, Mishra S, Vrati S. Transplacental rotavirus IgG interferes with immune response to live oral rotavirus vaccine ORV-116E in Indian infants. Vaccine 2014; 32(6):651-6; PMID:24374502; http://dx.doi.org/10.1016/j.vaccine.2013.12.017
- Patel M, Glass RI, Jiang B, Santosham M, Lopman B, Parashar U. A systematic review of anti-rotavirus serum IgA antibody titer as a potential correlate of rotavirus vaccine efficacy. J Infect Dis 2013; 208(2):284-94; PMID:23596320; http://dx.doi.org/10.1093/infdis/jit166
- Pichichero ME. Effect of breast-feeding on oral rhesus rotavirus vaccine seroconversion: a metaanalysis. J Infect Dis 1990; 162(3):753-5; PMID:2167344; http://dx.doi.org/10.1093/infdis/162.3.753
- Rennels MB. Influence of breast-feeding and oral poliovirus vaccine on the immunogenicity and efficacy of rotavirus vaccines. J Infect Dis 1996; 174(Suppl 1):S107-11; http://dx.doi.org/10.1093/infdis/174.Supplement_1.S107
- Bishop RF, Cipriani E, Lund JS, Barnes GL, Hosking CS. Estimation of rotavirus immunoglobulin G antibodies in human serum samples by enzyme-linked immunosorbent assay: expression of results as units derived from a standard curve. J Clin Microbiol 1984; 19(4):447-52; PMID:6325495
- Bishop RF, Bugg HC, Masendycz PJ, Lund JS, Gorrell RJ, Barnes GL. Serum, fecal, and breast milk rotavirus antibodies as indices of infection in mother-infant pairs. J Infect Dis 1996; 174(Suppl 1):S22-9; http://dx.doi.org/10.1093/infdis/174.Supplement_1.S22
- Coulson BS, Fowler KJ, Bishop RF, Cotton RG. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol 1985; 54(1):14-20; PMID:2579249