ABSTRACT
Although in the last years poliovirus (PV) transmission has been reported at the lowest levels ever recorded, the spread of virus from endemic countries endures; the high levels of immigration flows across the Mediterranean Sea jeopardize Italy for PV reintroduction. The World Health Organization (WHO) strategic plan for global poliomyelitis (polio) eradication indicates the nationwide surveillance of Acute Flaccid Paralysis (AFP) as the gold standard for detecting cases of polio. In addition, the Environmental Surveillance (ES), seeking the presence of PV and Non-Polio Enterovirus (NPEV) in sewage, is recognized as a powerful tool to confirm PV circulation in absence of AFP cases, especially in polio-free countries. Here we report the results of AFP surveillance (AFPS) and ES in Lombardy (Northern Italy) from 2012 to 2015.
Forty-eight AFP cases were identified during the study period. No AFP case was caused by PV infection. NPEVs were identified in 6.3% (3/48) of AFP cases. The annual AFP incidence rate was 0.87/100′000 children <15 y in 2012, 1.42/100′000 in 2013, 1.02/100′000 in 2014, and 0.47/100′000 in 2015; according to WHO indicators, the sensitivity of AFPS was adequate in 2013 and 2014. Completeness of case investigation raised progressively during the study period to achieve the WHO standards in 2014 (92.3%) and 2015 (100%). Completeness of follow-up increased from 72.7% in 2012 to 100% in 2014.
In the framework of the ES conducted in Milan, 268 wastewater samples were collected from 2012 to 2015 and no PVs were isolated. In contrast, NPEVs were detected in 65.3% (175/268) of samples. All NPEVs characterized belonged to enterovirus species B: echovirus type 11, 6 and 3 were the most frequently detected viruses, representing 29.1% (41/141), 20.6% (29/141) and 9.2% (13/141) of genotyped NPEVs, respectively.
Keeping strong and encouraging both AFPS and ES is crucial to ensure that PV will not return unnoticed in Italy - as well as in other polio-free countries - and, as a final point, to achieve the global polio eradication goal.
Introduction
Although in the last years poliovirus (PV) transmission has been reported at the lowest levels ever recorded, the virus is still endemic in 3 countries – Nigeria, Afghanistan and Pakistan.
Until PV transmission is not interrupted, all countries - especially those more vulnerable, with weak public health and immunization services and travel or trade links to endemic countries – remain at risk of virus importation. In May 2014, the World Health Organization (WHO) declared the international spread of wild PV a Public Health Emergency of International Concern (PHEIC) due to PV reintroduction through populations movements in 3 regions: central Asia (from Pakistan to Afghanistan), Middle East (from Syria to Iraq), and central Africa (from Cameroon to Equatorial Guinea).Citation1, 2 Since then, a reinforcement of PV surveillance was encouraged here and there in order to detect any virus importation, even though there were no recommendations in polio-free countries.
In Italy, the last case of indigenous polio due to a wild PV infection was reported in 1982, whereas the declaration of “polio-free country” was obtained in 2002. Since then, polio vaccination schedule consists in 4 doses of inactivated polio vaccine (IPV).Citation3 To date, importation of PV in Italy has never been recorded, albeit the high levels of immigration flows across the Mediterranean Sea jeopardize Italy for PV reintroduction.
The WHO strategic plan for the global polio eradication includes the implementation of infant immunization coverages by routinely vaccination campaign, national immunization days and targeted “mop-up” campaigns, along with the active community-based PV investigation.Citation4 In particular, the nationwide surveillance of Acute Flaccid Paralysis (AFP) is recognized by the WHO as the gold standard for detecting cases of polio.Citation5 The systematic analysis of stool samples collected from patients identified in the framework of AFP surveillance (AFPS) enables to link PV strains to paralysis cases, allowing a detailed examination of these individuals and their community.
Routine systematic Environmental Surveillance (ES) seeks the presence of PVs by the examination of sewage, connects PV strains from uncommon individuals to the population served by the same Waste-Water Treatment Plant (WWTP) and can allow the identification of PV circulation also in absence of AFP cases.Citation5-8 Moreover, ES can detect the presence of vaccine-related virus like Oral Polio Vaccine (OPV) (Sabin-like PV) and Vaccine-Derived PV (VDPV).Citation5 For these reasons, ES is currently recognized as a powerful tool for PV surveillance, especially in polio-free countries.Citation7, 9
Since Non-Polio Enteroviruses (NPEVs) are widespread worldwide and - like PVs - can be transmitted by fecal-oral route, NPEVs detection is expected in ES and epidemiological field study on these viruses are encouraged.Citation5, 10-14
In Italy, nationwide AFPS was established in 1997,Citation15 whereas ES was set up in 2005 only in Milan and other 5 Italian cities (Bolzano, Parma, Sassari, Napoli and Palermo).Citation11
This study described the results of both AFPS and ES in the period spanning from 2012 to 2015 in Lombardy, a region of Northern Italy accounting for nearly 10 million inhabitants (out of 60 million at national level). Critical aspects of the surveillance systems (such as data quality, sensitivity and timeliness) were also discussed. Additionally, the circulation of NPEVs was evaluated in the framework of ES.
Results
Demographical, epidemiological, clinical and virological characteristics of AFP cases
From January 1st, 2012, to December 31st, 2015, 48 AFP cases were reported in Lombardy. The median age of AFP cases was 4.5 y (range: 3.1–5.2 y; inter-quartile range [IQR]: 7.5 y). Thirty-six out of 48 (75%) AFP cases were recorded in children ≤ 5 y old. Half of cases (24/48) were males. The majority of cases came from Milan (14/48; 29.2%) and Bergamo (10/48; 20.8%) districts.
Most (30/48; 62.5%) AFP cases were notified by pediatric units, and the diagnosis at follow-up was Guillain-Barrè syndrome (GBS) in 47.9% (23/48) cases, followed by myelitis (7/48; 14.6%). Genetic disorders/malignancies and hypotonia/asthenia were reported at the same extent (6/48; 12.5%); a “not definitive diagnosis” was indicated in 12.5% (6/48) cases.
The AFP cases distribution by month showed 2 peaks: one in July (8/48; 16.7%) and one from October to December (16/48; 33.4%).
As concern virological investigations, no PVs were identified in the AFP cases during the 4-year study period. NPEVs were detected in 3 (3/48; 6.3%) samples collected from AFP cases; echovirus type 11 was detected in a 5-y-old male with GBS in 2012, echovirus type 6 in an 8-y-old female affected by myelitis in 2013, and echovirus 11 in a 3-y-old female with clinical paralysis of undefined origin in 2014. No NPEVs were identified in 2015.
About 94% (45/48) of AFP cases had at least one serum sample tested and the serological detection of anti-PV antibodies. The proportion of children with protective antibody levels (≥1:8) against PV1 was 93.3% (42/45) and against PV2 and PV3 was 95.6% (43/45). No seroconversions in paired serum samples of AFP cases (33/45; 73.3%) were reported. The immunization status of AFP cases was available for 85.4% (41/48) of AFP cases: 3 children were aged less than 3 months and thus not vaccinated against PV; no information regarding PV vaccine administration was available for 14.6% (7/48) AFP cases. The remaining children were reported to be vaccinated with IPV. All children with unknown immunization status had protective antibody titers.
Performance indicators of AFPS
According to the size of Lombardy population aged less than 15 y, 13 AFP cases per year were expected. The annual AFP incidence rate was 0.87/100′000 children <15 y in 2012, 1.42/100′000 in 2013, 1.02/100′000 in 2014, and 0.47/100′000 in 2015 (). According to WHO indicators, the sensitivity of AFPS was adequate (≥1.00/100′000) in 2013 and 2014. Completeness of case investigation (≥80%) was achieved in 2014 (12/13; 92.3%) and 2015 (6/6; 100%), whereas in 2012 and 2013 paired stool samples were collected in 63.6% (7/11) and 77.8% (14/18) of AFP cases, respectively (). Completeness of follow-up increased from 72.7% (8/11) in 2012 to 100% (13/13) in 2014. In 2015, follow-up examinations were available for about one-third of AFP cases ().
Table 1. Performance of AFPS carried out in Lombardy (Northern Italy) from January 1st, 2012, to December 31st, 2015. Indicators (incidence index, completeness of case investigation and completeness of follow-up) are reported by year of study. Results that fulfil the WHO target are highlighted in bold.
ES
During the 4-year study, 268 wastewater samples were collected from 3 WWTP. Viral isolation in L20b cell culture showed no virus growth, thus excluding PV presence in tested samples. NPEVs were detected in 65.3% (175/268) of samples. The proportion of NPEV-positive samples by year of study was 70% (42/60) in 2012, 56.9% (41/72) in 2013, 66.7% (48/72) in 2014, and 68.8% (44/64) in 2015.
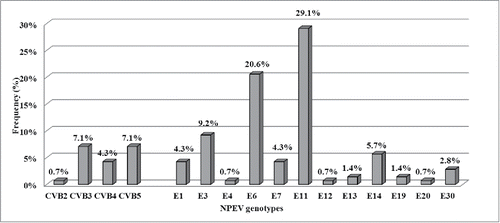
Overall, 80.6% (141/175) of NPEV-positive wastewater samples were molecularly characterized. All characterized NPEVs belonged to species B, and according to sequencing results 16 different NPEV genotypes were identified: 4 were group B coxsackievirus and 12 echovirus (). Echovirus type 11 was the genotype detected most frequently (41/141; 29.1%) followed by echovirus type 6 (29/141; 20.6%), and echovirus type 3 (13/141; 9.2%) (), accounting for 58.9% (83/141) of all characterized NPEVs.
Figure 1. Frequency of NPEV genotypes identified in Lombardy (Northern Italy) from January 1st, 2012, to December 31st, 2015 in the framework of ES. Genotype results were available for 141 out of 175 (80.6%) NPEVs. (CVB: coxsackievirus B; E: echovirus).
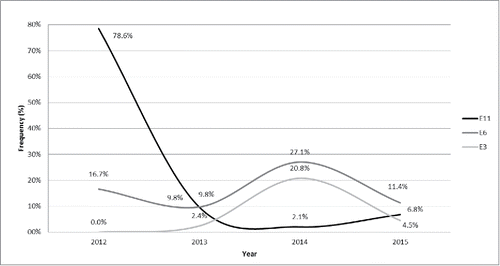
As shown in , a distinctive temporal distribution of echovirus type 6, 11 and 3 was observed. The analysis of circulating NPEV genotypes per year showed that echovirus type 11 was detected in 78.6% (33/42) of wastewater samples collected in 2012, 9.8% (4/41) in 2013, 2.1% (1/48) in 2014, and 6.8% (3/44) in 2015. Echovirus type 3 and type 6 started to increase from 2012 reaching a detection rate of 20.8% (10/48) and 27.1% (13/48), respectively, in 2014 ().
Discussion
Since the launch of the Global Polio Eradication Initiative in 1998, the incidence of wild PV cases has decreased by >99%; nonetheless, international spread of wild PV from endemic countries remains possible, as recognized in 2014 by the WHO with the PHEIC declaration.Citation1, 2
Italy is a country of huge immigration impact and this may facilitate the re-introduction of wild PV. In this setting, it is mandatory to strengthen the AFPS at regional and national level in order to detect rapidly any virus importation or emergence, and enable a prompt public health response. Although AFPS remains the gold standard for PV control, systematic ES has been recognized as a powerful tool to detect PV in the absence of AFP cases, especially in polio-free countries.Citation5, 7-9
According to Centralized Information System for Infectious Diseases (CISID), Italy - as several European countries - achieves barely the WHO target of AFPS performance indicators,Citation16 probably because of the low priority given to AFPS by national public health authorities and because Italy was declared “polio-free” in 2002 and since then PV has not been considered a health hazard anymore.
In our 4-year study, AFPS met the WHO criteria for sensitivity from 2013 onwards and the level of completeness of case investigation improved significantly from 2014 after the PHEIC declaration.Citation2 Physicians and healthcare workers involved in surveillance activities have proved to give special attention to AFPS thanks to several initiatives proposed by SNRL and implemented since 2013, such as the sharing of surveillance reports, epidemiological alerts and updates on polio eradication proceedings. The utmost attention was paid in 2014 after the PHEIC declaration when all AFPS performance indicators of sensitivity were achieved.
Overall, results of AFPS in the period spanning from 2012 to 2015 were adequate in terms of data quality, sensitivity, and timeliness, thus underlining an improvement of AFPS compared with the results obtained from 1997 to 201115 in the same surveyed area and those reached in other Italian regions.Citation17-20
Epidemiological characteristics of AFP cases were similar to those reported in the current scientific literature; no differences of the AFP frequency between males and females were observed. 75% of AFP cases were recorded in children aged below 5 y, as also reported by others.Citation21, 22
The temporal distribution of AFP was characteristic with notifications peaks in July (16.7%) and in the period spanning from October to December (33.5%), differently to the results reported in Lombardy in the pastCitation15 and in another region of Central Italy.Citation21
A number of causes can bring about AFP, and GBS is the most frequently recognized clinical manifestation of AFP onset, particularly after the interruption of wild PV transmission.Citation23 Here, GBS was confirmed as the most common conclusive diagnosis linked to AFP; this is consistent with other findings reported in ItalyCitation15,21,24 and in other countries such as South Africa and Hong Kong.Citation25, 26
No wild PV were detected during this 4-year study supporting the epidemiological data deriving from AFPS at national level. Three children (6.3% of AFP cases) resulted positive to NPEV; a 5-y-old male presented GBS was echovirus type 11-positive, an 8-y-old female with myelitis was echovirus type 6-positive, and a 3-y-old female with paralysis of undefined origin was echovirus 11-positive. The rate of NPEVs detected in our AFP series is lower than that noticed in India (21.4%)Citation27 but it is in line with findings of WHO European region (unpublished data). It remains challenging to associate the detection of NPEV in clinical samples to the onset of AFP, even if several NPEVs, such as Enterovirus (EV) 71 and D68, have been recognized to cause paralysis.Citation28, 29
As long as in the current polio endgame PV outbreaks reflect serious gaps in immunity to PV due to the weakness of routine immunization coverage in otherwise polio-free countries, all nations should maintain uniformly high routine immunization coverage at the district level to minimize the consequences of any new virus introduction. In Italy, a satisfactory vaccination coverage with 3 doses of vaccine in children less than 2-y-old is currently reached.Citation30 Our data confirm adequate levels of immune protection in the study population since more than 93% of children showed a protective antibody titer against PV. In Italy, PV vaccination is realized by the administration of IPV only; since IPV does not elicit a consistent mucosal immunity,Citation31 silent transmission of PV can happen through IPV-immunized individuals, favoring possible infection of unvaccinated subjects or children receiving delayed IPV vaccination.
In this setting, ES is able to detect the silent introduction and circulation of wild PVCitation8 and VDPV.Citation32 In Italy, ES was set up in 2005, since then no wild PV were detected whereas Sabin-like PV were identified here and there,Citation10,11,33,34 even if in Italy OPV administration was stopped in 2002. These findings underline the ability of ES to detect any PV strains and the need to implement this surveillance to monitor the effectiveness of OPV containment after the interruption of OPV administration in these latest eradication stages.
In the framework of ES, no PV reintroduction was detected from 2012 to 2015 in Lombardy. In contrast, NPEVs were detected in 65.3% of tested samples, in accord to our previous dataCitation11 and to results obtained by ES conducted in Tehran (60.2%) in 200714 and in Poland (77%)Citation12 in 2011. In our study, all characterized NPEVs belonged to enterovirus species B and echovirus type 11, 6 and 3 were the predominant genotypes. Overall, echovirus type 11 was the genotype most frequently identified in this study; similarly, echovirus type 11 was detected in 31.5% of wastewater samples in Tehran and echovirus type 11, 3 and 6 represented 2-third of NPEVs detected in ES in Poland.Citation12
In Guangdong province of China, the role of echovirus type 30 in an outbreak of aseptic meningitis has been assessed through continuing ESCitation35; in Wisconsin (US) an association between NPEVs detected in environmental samples and those affecting the population has been documented.Citation36 These pieces of evidence emphasize that ES is able to provide comprehensive epidemiological information about EVs circulation and phylogenetic study can be used to assess links of NPEV dynamics and transmission route in a population.
To date, no systematic NPEV hospital- and community-based surveillance systems are ongoing in Italy, contrary to other countries (i.e. France, Spain, and Australia); information on NPEV circulation in Italy derived exclusively from occasional studies in particular population, especially in children affected by severe pathological conditions.Citation37-42
In conclusion, AFPS proved to be successful especially after the PHEIC declaration and ES revealed a wide NPEVs circulation, mainly supported by echovirus type 11, 6 and 3.
Keeping strong and encouraging these surveillance activities all over the world is crucial to ensure the PV will not return unnoticed in polio-free countries and, as a final point, to achieve the global polio eradication goal.
Materials and methods
This study was conducted from January, 1st 2012, to December, 31st 2015, in Lombardy region (Northern Italy) by the Department of Biomedical Sciences for Health, University of Milan, which is one of the 6 Italian Sub-National Reference Laboratories (SNRLs) performing virological and serological investigations on samples collected in the operational framework of AFPS and ES.Citation24 SNRLs strictly complies with WHO guidelines, methods and recommendations for conducting AFPS and ES activities.Citation43,44 SNRLs are regularly evaluated by the National Polio Reference Laboratory (NPRL) for proficiency in isolation and typing of EVs.Citation24
SNRLs share the results of AFPS and ES activities obtained at regional level with the NPRL and the Italian Ministry of Health.
Surveillance systems
AFPS – According to WHO case definition of AFP, all children <15 y with a clinically evident AFP of one or more limbs with deceased or absent deep tendon reflex with sudden onset or with a bulbar paralysis were enrolled by collaborating hospitals of the surveillance network. AFP cases were notified by sending an ‘AFP case report’ to the reference SNRL twice a month, according to the “zero reporting” methodology. Prompt collection of i) 2 stool specimens taken 24–48 hours apart within 14 d since the onset of paralysis, and ii) paired serum samples, one acute and one convalescent, from each AFP case was requested for virological and serological tests, respectively. Epidemiological (i.e., type of PV vaccine received and date of administration), demographical (i.e., age, gender, country of origin), and clinical (i.e., symptoms and date of onset) data were collected by means of an ‘ad hoc’ questionnaire by physicians or healthcare workers.Citation43 After case notification, another ‘ad hoc’ questionnaire with more comprehensive medical information about the case should be filled to disclose the final outcome of AFP case (i.e., residual paralysis or death 60–90 d after the onset of paralysis) and to clarify the conclusive diagnosis.Citation43 Patients' privacy and confidentiality issues were managed in full agreement with national legislations.
To evaluate AFPS performance, a number of indicators were assessed.Citation43 In particular: i) the incidence index (i.e., at least one AFP case detected per 100′000 children <15 y annually); ii) completeness of case investigation (i.e., virological analysis of 2 stool samples collected 24–48 hours apart, within 14 d after the onset of paralysis, in at least 80% of AFP cases); and iii) completeness of follow-up (i.e., clinical follow-up 60–90 d after the onset of paralysis completed in at least 80% of AFP cases).
ES – The sample size representative for study population is defined according to the WHO guidelines.Citation44 Wastewater samples (1000 ml) were collected twice a month at the intel of 3 WWTPs, placed in high-density urban setting in Milan, serving a population of about 1 million inhabitants. Wastewater samples were collected during 24h-automated flow at the WWTPs and shipped frozen to the reference SNRL for PV and NPEVs identification and molecular characterization. Half volume (500 ml) of wastewater sample was used for virological investigation; the left volume was kept frozen (−80 °C) until virological assessment.Citation44
PV and NPEVs identification and molecular characterization
For viral detection, 10% of stool sample collected from each AFP case was prepared in phosphate-buffered saline and clarified by micro-tube centrifugation (13,000 rpm for 15 min). Wastewater samples were treated using the 2-phase (poly-ethylene-glycol and dextran) separation method, as previously described, in order to obtain a 50-fold volume concentration.Citation44
Once processed, stool and wastewater samples were analyzed to detect EVs by virus isolation in RD (human rhabdomyosarcoma cells, permissive to EVs) and L20B (murine transgenic L cells expressing the gene for the human cellular receptor for PV, sensitive to PV and few other NPEVs) cell cultures,Citation44 according to WHO algorithm.Citation5 For rapid virus identification, the lysate of cell cultures that showed cytopathic effect were tested by RT-nested-PCR specific for the 5′ noncoding region (5′NCR) (nucleotide [nt.] 179 to 575) common to all EVs.Citation45 The molecular characterization of EVs was performed by sequence analysis of the VP1 gene (nt. 2′602–2′977).Citation46 The nucleotide sequences were obtained by automated DNA sequencing (Macrogen Inc., Korea) and nucleotide sequence alignments were obtained using BioEdit software.Citation47
Detection of anti-PV antibodies in serum samples
The presence of anti-PV antibodies was assessed in paired serum samples collected from each AFP case by microneutralization assay.Citation43 According to WHO guidelines, an antibody titer ≥1:8 was considered protective; seroconversion was defined as the 4-fold or greater increase in antibody titer between the paired serum samples.Citation48
Abbreviations
| AFP | = | Acute Flaccid Paralysis |
| AFPS | = | AFP Surveillance |
| CISID | = | Centralized Information System for Infectious Diseases |
| ES | = | Environmental Surveillance |
| EV | = | Enterovirus |
| GBS | = | Guillain-Barré Syndrome |
| IPV | = | Inactivated Polio Vaccine |
| NPEV | = | Non-Polio Enterovirus |
| NPRL | = | National Polio Reference Laboratory |
| OPV | = | Oral Polio Vaccine |
| PHEIC | = | Public Health Emergency of International Concern |
| Polio | = | Poliomyelitis |
| PV | = | Poliovirus |
| SNRL | = | Sub-National Reference Laboratory |
| VDPV | = | Vaccine Derived Poliovirus |
| WHO | = | World Health Organization |
| WWTP | = | Waste-Water Treatment Plant |
Disclosure of potential conflicts of interest
The authors have no competing interest with respect to this manuscript.
Acknowledgments
The authors would like to thank the physicians and healthcare professionals involved in AFP surveillance, all children enrolled in this study and their parents, and wastewater treatment plants workers in charge to collect samples for the environmental surveillance.
References
- Eichner M, Brockmann SO. Polio emergence in Syria and Israel endangers Europe. Lancet 2013; 382:1777; PMID:24211043; http://dx.doi.org/10.1016/S0140-6736(13)62220-5
- Eurosurveillance editorial team. Note from the editors: WHO declares international spread of wild poliovirus a public health emergency of international concern. Euro Surveill 2014; 19(18):pii=20798. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20798
- (WHO) WHO. Certification of Poliomyelitis Eradication: European Region declared ‘polio-free’. Fifteenth meeting of European regional Certification Commission. Copenhagen, 19-21 June 2002.
- (WHO) WHO. Field Guide: For Supplementary Activities aimed at achieving polio eradication-1996 revision. 1996.
- (WHO) WHO. The Polio Eradication & Endgame Strategic Plan 2013-2018 (WHO/POLIO/13.02). 2012.
- El Bassioni L, Barakat I, Nasr E, de Gourville EM, Hovi T, Blomqvist S, Burns C, Stenvik M, Gary H, Kew OM, et al. Prolonged detection of indigenous wild polioviruses in sewage from communities in Egypt. Am J Epidemiol 2003; 158:807-15; PMID:14561671; http://dx.doi.org/10.1093/aje/kwg202
- Manor Y, Handsher R, Halmut T, Neuman M, Bobrov A, Rudich H, Vonsover A, Shulman L, Kew O, Mendelson E. Detection of poliovirus circulation by environmental surveillance in the absence of clinical cases in Israel and the Palestinian authority. J Clin Microbiol 1999; 37:1670-5; PMID:10325305
- Shulman LM, Mendelson E, Anis E, Bassal R, Gdalevich M, Hindiyeh M, Kaliner E, Kopel E, Manor Y, Moran-Gilad J, et al. Laboratory challenges in response to silent introduction and sustained transmission of wild poliovirus type 1 in Israel during 2013. J Infect Dis 2014; 210 Suppl 1:S304-14.
- Hovi T, Shulman LM, van der Avoort H, Deshpande J, Roivainen M, DE Gourville EM. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol Infect 2012; 140:1-13; PMID:21849095; http://dx.doi.org/10.1017/S095026881000316X
- Battistone A, Buttinelli G, Fiore S, Amato C, Bonomo P, Patti AM, Vulcano A, Barbi M, Binda S, Pellegrinelli L, et al. Sporadic isolation of Sabin-like polioviruses and high detection of non-polio enteroviruses during sewage surveillance in seven Italian cities, after several years of inactivated polio vaccination. Appl Environ Microbiol 2014; 80:4491-501; PMID:24814793; http://dx.doi.org/10.1128/AEM.00108-14
- Pellegrinelli L, Binda S, Chiaramonte I, Primache V, Fiore L, Battistone A, Fiore S, Gambino M, Bubba L, Barbi M. Detection and distribution of culturable Human Enteroviruses through environmental surveillance in Milan, Italy. J Appl Microbiol 2013; 115:1231-9; PMID:23910458; http://dx.doi.org/10.1111/jam.12321
- Wieczorek M, Ciąćka A, Witek A, Kuryk Ł, Żuk-Wasek A. Environmental Surveillance of Non-polio Enteroviruses in Poland, 2011. Food Environ Virol 2015; 7:224-31 ; http://dx.doi.org/10.1007/s12560-015-9195-3
- Ndiaye AK, Diop PA, Diop OM. Environmental surveillance of poliovirus and non-polio enterovirus in urban sewage in Dakar, Senegal (2007-2013). Pan Afr Med J 2014; 19:243; PMID:25848458; http://dx.doi.org/10.11604/pamj.2014.19.243.3538
- Kargar M, Sadeghipour S, Nategh R. Environmental surveillance of Non-Polio Enteroviruses in Iran. Virol J 2009; 6:149; PMID:19781063; http://dx.doi.org/10.1186/1743-422X-6-149</bib>
- Pellegrinelli L, Primache V, Fiore L, Amato C, Fiore S, Bubba L, Pariani E, Amendola A, Barbi M, Binda S. Surveillance of acute flaccid paralysis (AFP) in Lombardy, Northern Italy, from 1997 to 2011 in the context of the national AFP surveillance system. Hum Vaccin Immunother 2015; 11:277-81; PMID:25483546; http://dx.doi.org/10.4161/hv.36164
- WHO. WHO. Centralized Information for Infectious Disease (CIDIS). Database Acute Flaccid Paralysis 2016.
- Fiore L, Buttinelli G, Fiore S, Donati V, Di Lonardo A. [Surveillance of acute flaccid paralysis in Italy, 1997-2001]. Ann Ig 2002; 14:73-80; PMID:12389295
- Prato R, Labianca M, Calvario A, Bozzi A, Rizzo C, Fiore L, Vellucci L, Buttinelli G, Donati V, Lopalco PL, et al. [Evaluation of the Surveillance System of Acute Flaccid Paralysis in Puglia: 5 years of work]. Ann Ig 2002; 14:487-94; PMID:12638352
- Angelillo IF, Pavone L, Rito D. Acute flaccid paralysis surveillance in Southern Italy. Public Health 2001; 115:130-2; PMID:11406778; http://dx.doi.org/10.1016/S0033-3506(01)00431-0
- Patti AM, Santi AL, Ciapetti C, Fiore L, Novello F, Vellucci L, De Stefano F, Fara GM, Paralysis GdSdAAF. [Flaccid paralysis surveillance in the Latium Region]. Ann Ig 2000; 12:333-8; PMID:11140100
- D'Errico MM, Barbadoro P, Bacelli S, Esposto E, Moroni V, Scaccia F, Tantucci L, Prospero E, Group AS. Surveillance of acute flaccid paralysis in the Marches region (Italy): 1997-2007. BMC Infect Dis 2008; 8:135; http://dx.doi.org/10.1186/1471-2334-8-135
- Poorolajal J, Ghasemi S, Farahani LN, Hosseini AS, Bathaei SJ, Zahiri A. Evaluation of acute flaccid paralysis in hamadan, iran from 2002 to 2009. Epidemiol Health 2011; 33:e2011011; PMID:22111031
- Marx A, Glass JD, Sutter RW. Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiol Rev 2000; 22:298-316; PMID:11218380; http://dx.doi.org/10.1093/oxfordjournals.epirev.a018041
- Fiore L, Novello F, Simeoni P, Amato C, Vellucci L, De Stefano D, Grandolfo ME, Luzzi I. Surveillance of acute flaccid paralysis in Italy: 1996-1997. AFP Study Group. Acute flaccid paralysis. Eur J Epidemiol 1999; 15:757-63.
- Khuzwayo LS, Kuonza LR, Ngcobo NJ. Evaluating the acute flaccid paralysis surveillance system in South Africa, 2005-2009–an analysis of secondary data. Pan Afr Med J 2013; 14:86; PMID:23646222; http://dx.doi.org/10.11604/pamj.2013.14.86.2032
- Lam RM, Tsang TH, Chan KY, Lau YL, Lim WL, Lam TH, Leung NK, Eradication NCftCoWP. Surveillance of acute flaccid paralysis in Hong Kong: 1997 to 2002. Hong Kong Med J 2005; 11:164-73; PMID:15951581
- Hanumaiah H, Raut CG, Sinha DP, Yergolkar PN. Non-polio Enteroviruses in Karnataka, India: Virological surveillance of acute flaccid paralysis cases (July 1997-2013). Indian J Med Microbiol 2016; 34:22-6.
- Messacar K, Schreiner TL, Maloney JA, Wallace A, Ludke J, Oberste MS, Nix WA, Robinson CC, Glodé MP, Abzug MJ, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet 2015; 385:1662-71; PMID:25638662; http://dx.doi.org/10.1016/S0140-6736(14)62457-0
- Hu Y, Jiang L, Peng HL. Clinical Analysis of 134 Children with Nervous System Damage Caused by Enterovirus 71 Infection. Pediatr Infect Dis J 2015; 34:718-23; PMID:25860536; http://dx.doi.org/10.1097/INF.0000000000000711
- Iannazzo S, Rizzuto E, Pompa MG. [Polio vaccination failure in Italy, years 2006-2010]. Epidemiol Prev 2014; 38:98-102; PMID:25759353
- Hird TR, Grassly NC. Systematic review of mucosal immunity induced by oral and inactivated poliovirus vaccines against virus shedding following oral poliovirus challenge. PLoS Pathog 2012; 8:e1002599; PMID:22532797; http://dx.doi.org/10.1371/journal.ppat.1002599
- Diop OM, Burns CC, Sutter RW, Wassilak SG, Kew OM. (CDC) CfDCaP. Update on Vaccine-Derived Polioviruses - Worldwide, January 2014-March 2015. MMWR Morb Mortal Wkly Rep 2015; 64:640-6; PMID:26086635
- Patti AM, Santi AL, Fiore L, Vellucci L, De Stefano D, Bellelli E, Barbuti S, Fara GM. Environmental surveillance of poliovirus in Italy: pilot study. Ann Ig 2003; 15:97-105; PMID:12838824
- Cesari C, Colucci ME, Veronesi L, Giordano R, Paganuzzi F, Affanni P, Bracchi MT, Capobianco E, Ferrari G, Tanzi ML. Detection of enteroviruses from urban sewage in Parma. Acta Biomed 2010; 81:40-6; PMID:20857852
- Lu J, Zheng H, Guo X, Zhang Y, Li H, Liu L, Zeng H, Fang L, Mo Y, Yoshida H, et al. Elucidation of echovirus 30s origin and transmission during the 2012 aseptic meningitis outbreak in Guangdong, China, through continuing environmental surveillance. Appl Environ Microbiol 2015; 81:2311-9 ; http://dx.doi.org/10.1128/AEM.03200-14
- Sedmak G, Bina D, MacDonald J. Assessment of an enterovirus sewage surveillance system by comparison of clinical isolates with sewage isolates from milwaukee, wisconsin, collected august 1994 to december 2002. Appl Environ Microbiol 2003; 69:7181-7; PMID:14660364; http://dx.doi.org/10.1128/AEM.69.12.7181-7187.2003
- Parisi SG, Basso M, Del Vecchio C, Andreis S, Franchin E, Bello FD, Pagni S, Biasolo MA, Manganelli R, Barzon L, et al. Virological testing of cerebrospinal fluid in children aged less than 14 years with a suspected central nervous system infection: A retrospective study on 304 consecutive children from January 2012 to May 2015. Eur J Paediatr Neurol 2016; 20:588-96; PMID:27129875; http://dx.doi.org/10.1016/j.ejpn.2016.04.002
- Piralla A, Mariani B, Stronati M, Marone P, Baldanti F. Human enterovirus and parechovirus infections in newborns with sepsis-like illness and neurological disorders. Early Hum Dev 2014; 90 Suppl 1:S75-7; http://dx.doi.org/10.1016/S0378-3782(14)70023-4
- Arbustini E, Porcu E, Bellini O, Grasso M, Pilotto A, Dal Bello B, Morbini P, Diegoli M, Gavazzi A, Specchia G, et al. Enteroviral infection causing fatal myocarditis and subclinical myopathy. Heart 2000; 83:86-90; PMID:10618342; http://dx.doi.org/10.1136/heart.83.1.86
- Neri I, Dondi A, Wollenberg A, Ricci L, Ricci G, Piccirilli G, Lazzarotto T, Patrizi A. Atypical Forms of Hand, Foot, and Mouth Disease: A Prospective Study of 47 Italian Children. Pediatr Dermatol 2016; 33:429-37; PMID:27292085; http://dx.doi.org/10.1111/pde.12871
- Bubba L, Pellegrinelli L, Pariani E, Primache V, Amendola A, Binda S. A novel multiplex one-step real-time RT-PCR assay for the simultaneous identification of enterovirus and parechovirus in clinical fecal samples. J Prev Med Hyg 2015; 56:E57-60; PMID:26789989
- Esposito S, Daleno C, Prunotto G, Scala A, Tagliabue C, Borzani I, Fossali E, Pelucchi C, Principi N. Impact of viral infections in children with community-acquired pneumonia: results of a study of 17 respiratory viruses. Influenza Other Respir Viruses 2013; 7:18-26; PMID:22329841; http://dx.doi.org/10.1111/j.1750-2659.2012.00340.x
- W.H.O.. WHO. Polio Laboratory Manual. WHO/IVB/04.10. 2004.
- W.H.O.. WHO. Guidelines for environmantal surveillance of poliovirus circulation. WHO/V&B/03.03. 2003.
- Severini GM, Mestroni L, Falaschi A, Camerini F, Giacca M. Nested polymerase chain reaction for high-sensitivity detection of enteroviral RNA in biological samples. J Clin Microbiol 1993; 31:1345-9; PMID:8388893
- Nix WA, Oberste MS, Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 2006; 44:2698-704; PMID:16891480; http://dx.doi.org/10.1128/JCM.00542-06
- T. A. Hall. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 1999; 41:95-98; PMID:10780396
- WHO Immunization, Vaccines and Biologicals. 2014.