ABSTRACT
In order to estimate the burden of influenza and to describe the genetic evolutionary pattern and antigenic variability of type B viral strains, data deriving from 3 surveillance systems active in Liguria region, Northern Italy, were described. Since the re-emergence of the Victoria lineage in 2001, the clinical-epidemiological and syndromic surveillances demonstrated the heavy burden of influenza like illness (ILI) syndrome. Focusing on type B influenza virus, it predominated or played a relevant epidemic role in the 50% of the evaluated influenza seasons. Furthermore, the virologic surveillance demonstrated the frequent co-circulation of both lineages an heterogeneous circulation of different influenza B strains, determining a partial or complete mismatch in at least 6 influenza seasons. The undemonstrated cross-reactivity between lineages and the unpredictability of predominant lineage arose the scientific debate about the opportunity to include the quadrivalent influenza vaccine among the preventive tools to improve the protection against type B viruses. The integration of different surveillance systems highly contribute to estimate the poorly evaluated burden of type B influenza virus and help to find variants to include in the vaccine formulation.
Introduction
Seasonal influenza epidemics are associated with a heavy health and societal burden worldwide, being responsible for 3–5 million cases of severe illness and about 250–500,000 estimated deaths each year.Citation1 Moreover, their large economic impact includes not only direct medical costs, but also reduced quality of life and loss of work productivity.Citation2-5
Until recent times, influenza A conventionally remained the primary focus of influenza control, because of its pandemic potential and its predominance in most seasonal influenza epidemics.Citation6-8 Nevertheless, during interpandemic periods, influenza B could account for a relevant proportion of cases. Citation9-14
Influenza B viruses evolutionary pattern extremely differs from that of type A viruses. In particular, humans are the only host of epidemiological relevance for influenza B viruses. The main consequence of this fact is that no antigenic shift has been observed in this type of influenza viruses.Citation15
Moreover, influenza B viruses have a lower genetic diversity than type A and they are not classified into antigenically distinct subtypes based on the membrane glycoproteins, that consist of single hemagglutinin (HA) and neuraminidase (NA) type.Citation16
Finally, even though influenza B viruses undergo antigenic drift, the evolutionary rates of HA gene is slower than those of influenza A strains.Citation17
Despite the low rate of antigenic changes, since 1983 influenza B viruses evolved into 2 antigenically and genetically different lineages, named Victoria and Yamagata,Citation18,19 The Victoria-lineage was isolated in the majority of influenza B cases during the 1980s, while the Yamagata-lineage prevailed in the most part of the world in the late 1990s.Citation20
According to epidemiological surveillance data, in 2001 the Victoria-lineage strains re-emerged in Europe and United States, and since then the 2 lineages and respective sublineages have widespread co-circulated.Citation21,22
The comprehension of evolutionary mechanisms through efficient surveillance systems allows to improve the vaccine composition and to assess the matching between circulating and vaccine strains, that contribute to the effectiveness of influenza vaccines.Citation23-25
In order to define the burden and the nature of evolution among influenza B viruses in Liguria region, Northern Italy, in the 15 y following the re-emergence of Victoria-lineage during the 2001–2002 influenza season, we integrated data deriving from 3 data sources: the epidemiological, virological and syndromic surveillance systems.
Results
Clinical-epidemiological surveillance
The results of ILI clinical-epidemiological surveillance from 2001 to 2016 are shown in .
Figure 1. Incidence of Inluenza-like illness (ILI) in Liguria Region, Italy, during influenza seasons from 2001/2002 to 2015/2016.
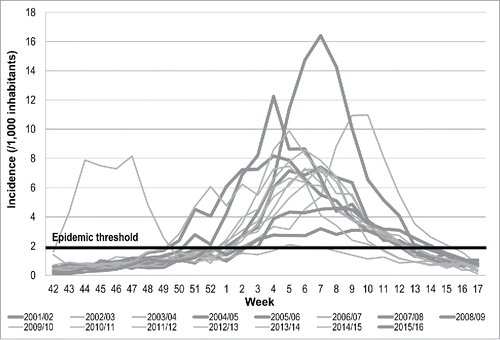
Considering the entire study period, it can be estimated that a mean of 56,641 cases of ILI occurred in Liguria region from October to April of each influenza season. However, excluding the 2009 H1N1v pandemic, the number of cases was nearly 53,000. The mean incidence was 2.7 ILI cases per 1,000 inhabitants, ranging from 1.6/1,000 in the season 2005/2006 to 4.1/1,000 in the season 2004/2005.
Among the epidemic periods that were usually observed between the beginning of January and the end of March, the highest and the lowest incidence peaks were 16.4 and 3.2 per 1,000 inhabitants, and they were observed in the weeks 7th/2005 and 7th/2006, respectively. On average, the length of the epidemic was 11 weeks and ranged from 7 to 16 weeks.
The epidemic period, its length in weeks and the estimated number of cases, broken down by age-class, for each influenza season were reported in Supplementary file 1.
Comparing the influenza seasons when the type B influenza virus prevailed with the epidemic when type A was prevalent, the mean incidences of ILI were 2.6 ( ± 2.5SD) and 2.7 per 1,000 inhabitants ( ± 2.7SD) (p = 0.86).
The mean incidence observed when a type B virus mismatch occurred was significantly higher than the incidence estimated when a circulating strains matched with vaccine strains, both not including and including the pandemic season (2.8 vs 2.6 per 1,000 inhabitants, p < 0.001).
The median epidemic period did not differ between the seasons when the vaccine mismatch and when no mismatch was observed.
Considering the stratification by age-class, the highest peaks of morbidity were observed in the pediatric age (0–14 y until 2002/2003 and 0–4 and 5–14 subsequently). The incidence rates were usually lowest in the elderly, being appreciable only in 2001/2002 (peak value of about 2.6 per 1,000), when the type B viruses were prevalent and in 2004/2005 (peak value of about 1.7 per 1,000), when A/H3N2 predominated.
Considering the influenza seasons when a type B vaccine mismatch was observed, the highest peak incidences in the pediatric age were observed during the 2001/2002 and the 2004/2005 season (26 and 9.2 per 1,000 inhabitants). During the other seasons the highest incidence rates were 8.9 and 8.7 per 1,000 inhabitants in 2002/2003 and 2010/2011, respectively. Also the highest incidence rates observed in adults and the elderly were observed when a type B vaccine mismatch was observed, in particular during the 2001/2002 season (6.7 and 2.6 per 1,000, respectively).
Syndromic surveillance
The syndromic surveillance system is active from the season 2006/2007, when no type B influenza virus was detected in Liguria region (). In children, the mean of the epidemic activity indicators in the whole period was 1.65 ( ± 0.75SD), with a median duration of 69 d (25–75p: 59–74). The epidemic threshold were commonly exceeded from the end of December to the end of March, with the exception of the pandemic season, when the breakthrough was observed earlier, from the end of September to end of November, 2009. The mean of the activity indicators above the cut-off observed during the seasons when a type B mismatch was observed was 1.5 ( ± 0.44SD), lasting a median of 62 d each season. During the seasons when a type B vaccine matching was observed, the mean of the activity indicator was 1.7 ( ± 0.85SD), with a median of epidemic period of 71 d for each season (p<0.001). The highest mean activity indicators were observed during the 2007/2008 influenza season, when type B partial mismatch was observed and type A/H3N2 predominated and during the pandemic and the 2010/2011 influenza season, when the A/H1N1 virus prevailed.
Figure 2. Sindromic surveillance activity indicator for Influenza-like illness (LI) in children (A) and adults (B) in Liguria Region, Italy, by season.
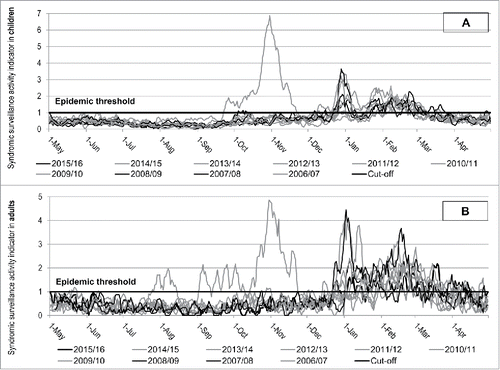
As regards the ED accesses of adults for ILIs (), the mean of the epidemic activity indicators in the whole period was 1.66 ( ± 0.64SD), with a mean duration of 79 d (25–75p: 58–94). The epidemic thresholds were commonly exceeded from the end of December or early January to March–April, with the exception of the 2009/2010 pandemic season, that was characterized by an early and prolonged breakthrough, from August to April. Considering the influenza seasons when a mismatch occurred, the mean of the activity indicators above the cut-off was 1.63 ( ± 0.63 SD) and lasted a median of 92 d each season. When a vaccine matching was observed and excluding the pandemic season, the mean of the activity indicator was 1.65 ( ± 0.54 SD), with a median of epidemic period of 58 d for each season (p = 0.66). As observed in the ED accesses of children, the highest mean activity indicators were observed during the pandemic and the 2010/2011 epidemic seasons, when the A/H1N1 virus predominated.
Virological surveillance
The seasonal distribution of the number of Ligurian influenza virus positive samples detected from the 2001/2002 to the 2015/2016 influenza seasons is reported in .
Figure 3. Seasonal distribution of the Ligurian influenza virus positive samples detected from the 2001/2002 to the 2015/2016 influenza seasons.
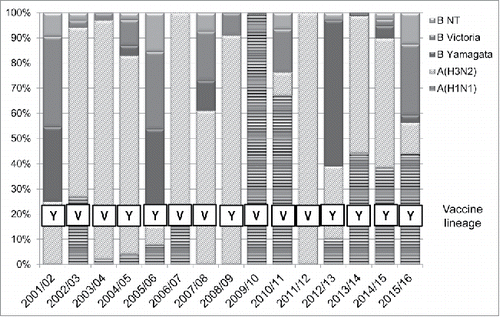
From the 2001/2002 to the 2015/2016 seasons 1850 influenza viruses positive samples were detected. Among these, 235 (12.7%) were type B viruses. The proportion of isolated type B compare with type A influenza viruses positive samples widely ranged from 0% to 85%. When the lineage was evaluated, the co-circulation of both Yamagata and Victoria lineages was observed in about 40% of the evaluated influenza seasons. Furthermore, during 9 (60%) seasons, the Victoria lineage predominated. A type B vaccine mismatch between circulating and vaccine lineage was observed in 7 (47%) seasons. In particular, during the 2001/2002, 2004/2005, 2005/2006, 2007/2008 seasons the mismatch was partial (between 20% and 59% of the isolated strains belonged to a lineage not included in the trivalent seasonal influenza vaccine) and during the 2009/2010 and the 2015/2016 seasons the vaccine mismatch was complete (> 60% of the isolated strains belonged to a lineage not included in the trivalent seasonal influenza vaccine).
As observed in Tramuto F et al.Citation15 a lineage swap from the predominance of Victoria to that of Yamagata lineage occurred between 2010/2011 and 2012/2013 seasons.
Antigenic and molecular analysis of influenza B isolates
Antigenic relationships between influenza B virus isolated during the surveilled period and investigated by the HI and/or the NT tests and comparative analyses of the amino acid sequences of the HA1 domains are shown in Supplementary files 2 and 3.
Most of the HAs of the B isolates of the 2001/2002 season cluster in distinct clades of the Victoria- and the Yamagata-lineages. HAs within the Yamagata-lineage showed high identity to the HA of the B/Sichuan/379/99 and B/Harbin/7/94 strains and differed by 2 amino acid changes (L58F and N126D). HAs of viruses belonging to the Victoria lineage were similar to the vaccine strain B/HongKong/330/2001, with the exception of the amino acid substitution T199I.
Isolates Victoria-like of the 2002/2003 and 2004/2005 seasons showed highly similar NT titres and amino acidic sequences compare with B/Shandong/07/1997. Considering the Yamagata-like strains that circulated during the 2004/2005, 2005/2006, 2007/2008 seasons, high matching was found with the B/Jangsu/10/2003 virus, with the exception of 4 amino acidic changes.
From 2005 to 2010, circulating viruses Victoria-like showed the same sequence amino acidic of the vaccine strain B/Malaysia/2506/2004, with the exception of the 2008/2009 influenza season, when viruses B/Brisbane/60/2008-like were found both from a serologic and molecular point of view. Viruses belonging to the same lineage and circulating in the following seasons were B/Brisbane/60/2008-like, too and only one amino acidic substitution was found (I146V).
As shown in the phylogenetic tree (), the Yamagata-like viruses isolated during the 2012/2013 season were B/Wisconsin/01/10-like, even if 8 amino acidic substitutions were found. The following seasons were characterized by viruses B/Massachusetts/02/12-like and B/Phuket/3073/2013-like. Of note, the virus B/Genoa/01/2014 showed distinguished antigenic and molecular pattern.
Figure 4. Phylogenetic analysis of the subunit 1 of the Hemoagglutin (HA1) nucleotide sequences from influenza B Yamagata-land Victoria-lineages isolated in Liguria region, Italy, from 2001/02 to 2015/16 influenza seasons.
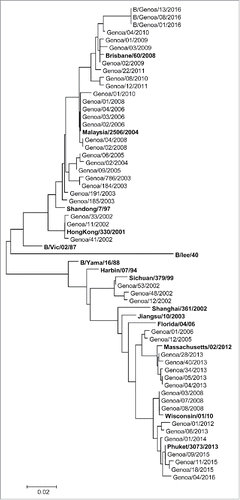
Finally, the phylogenetic tree is noticeably composed of 2 main lineages, the Victoria- and the Yamagata-lineages. A gradual drift was observed in both lineages, allowing to identify furthers subgroups into each one.
The Victoria-lineage include B/HongKong/330/0, B/Shandong/7/97, B/Malaysia/2506/2004 and B/Brisbane/60/2008 genetic clades, whereas within the Yamagata-lineage there are 4 branches represented by B/Sichuan/379/99, B/Massachusetts/02/2012 B/Wisconsin/01/10 and B/Phuket/3073/2013.
Discussion
The estimation of public health impact of the influenza epidemics is challenging. Etiologic agents of ILIs are numerous, the probability of contact with GPs varies by age,Citation26 and even integrated surveillance systems such as those active in Liguria region don't allow conclusive evaluations except for particular epidemiologic conditions.
However, this 15-years study allowed to better define the epidemiology and the clinical burden of influenza viruses, with a particular focus on type B viruses, in the Liguria Region of Italy.
From the epidemiologic standpoint, both clinical-epidemiological and syndromic surveillances highlighted the heavy burden of ILI syndrome particularly among children: as reported, the age group with the highest peak incidence in all seasons was that of subjects aged < 14 y. The incidence was relevant also among adults and elderly, even though the pronounced variability observed among seasons.
Syndromic surveillance further confirmed the impact of ILI in terms of ED accesses, revealing breaking through the epidemic threshold during all influenza seasons and particularly in the period from the end of December to the end of March characterized by sustained circulation of influenza viruses.
In this context, the virologic surveillance evidenced the heavy impact of influenza B viruses that resulted the predominant cause of influenza in 3 of 15 seasons and determined a relevant number of influenza cases in further 4 seasons.
Moreover, based on sequence analysis data, we assisted to an heterogeneous circulation of different influenza B strains during the surveillance period.
In particular, the genetically characterization of the influenza B viruses demonstrated the co-circulation of both B lineages in the majority of the evaluated seasons and allowed to recognize a partial or complete mismatch with type B vaccine strain in at least 6 seasons. In particular, we registered a complete vaccine mismatch in 2008/2009 and 2015/2016 seasons, because of the circulation of viruses belonging to the Victoria-lineage, while the vaccine strains belonged to the Yamagata-lineage. The co-circulation of both lineage and the subsequent partial mismatch was observed during 4 influenza seasons (namely 2001/2002, 2004/2005, 2005/2006 and in 2007/2008).
This regional virologic scenario was coherent with the Italian one,Citation27 substantially differing from that observed in other European countries and in US where a complete type B mismatch was registered more frequently (Supplementary file 4).Citation28
During 2001/2002 season, Victoria-lineage, Hong Kong-like viruses appeared on Italian and global scene, with an heavy burden on Italian population, in which an high rate of susceptible individuals was present, due to the lack of circulation of this lineage during the 1990s.Citation29 However, Yamagata-lineage viruses were not replaced by Victoria-like viruses, determining a co-circulation of both lineages, event already observed during previous seasons.Citation30,31
Since 2002/2003 influenza season, reassortant B viruses, possessing a Victoria-lineage HA and Yamagata-lineage NA, were isolated more and more frequently, but they showed not to be the result of reassortment between the co-circulating strains during 2001/2002 season in Italy, as described previously by Puzelli et al.Citation23 These mixed strains were the results of previous recombinant event between co-circulating B viruses occurred outside Italy.Citation32,33
After a prevalent circulation of Victoria-lineage viruses in the period 2009–2012, since 2012–2013 Yamagata-lineage viruses predominated over Victoria-lineage strains.Citation15 During the 2015/16 season, Victoria-lineage Brisbane/08-like viruses prevailed again both at global and local level. This picture led to the new recommendations for the influenza trivalent vaccine composition for the season 2016/2017 including Victoria-lineage Brisbane/08 virus in replacement of Yamagata-lineage Phuket/13 virus.
Noteworthy, the partial or complete mismatch between circulating and vaccine type B strains resulted associated with a higher incidence of ILI in all age groups and with a higher incidence of ED accesses as well as with a longer epidemic period among children and adolescents. The predominant role of influenza B viruses among these latter age-groups is in line with other international data.Citation34
The antigenic analysis confirmed the high variability of circulating influenza B virus and allowed to estimate the antigenic distance between the vaccine candidates and the circulating strains of influenza virus. Our results indicated a frequent co-circulation of influenza B viruses closely related to the vaccine strains and the appreciable proportion of viruses presenting lower reactivity with the reference serum. Importantly, HI assay results highlighted the high antigenic distance between viruses belonging to the 2 lineages.
Combined serologic and molecular analyses are useful to reveal the major changes in antigenicity and to better evaluate the characteristics of any new or re-emerging influenza B viruses, in order to allow the best choice of the viral strain to include in the vaccine composition.
The high proportion of type B isolates observed during the last 15 y in the majority of European and Extra-European countries and the high frequency of vaccine mismatch, that potentially impaired the vaccine effectiveness, arose the scientific debate about the opportunity to include both lineages of type B influenza virus in the seasonal influenza vaccine to improve the protection against type B viruses.Citation35
The study had some major limits regarding the methodology of epidemiologic surveillance that varied during the study period, with the introduction of syndromic surveillance since 2006/2007 season. A further limit was represented by the lack of demographic and clinic data about the patients affected by ILI.
Moreover, the number of samples collected for the virologic surveillance and their sources were heterogeneous and varied among seasons; antigenic and molecular analysis were performed only on a small proportion of samples, even though representative of the circulating strains.
In conclusion, Influenza viruses type B cause significant burden in terms of morbidity and ED accesses, with variable incidence in the considered period.
In the majority of the observed influenza seasons, the co-circulation of 2 antigenically distinct lineages was observed and no cross-reactivity between lineages has been detected.
Both the unpredictability of predominant lineage and the subsequent mismatch between circulating and vaccine strains and the heterogeneity of circulating sublineage may determine reduced vaccine effectiveness.
The integration of epidemiological, syndromic and virological (molecular and serological) surveillance systems allow to monitor the burden of influenza and to follow the genetic evolutionary pattern and antigenic variability of viral strains and to find variants that will probably circulate in the coming season and include them in the vaccine formulation.
The recent introduction of quadrivalent influenza vaccine would more accurately reflect the current epidemiology of influenza and would improve vaccine effectiveness, optimizing the control of this public health threat.
Materials and methods
The clinical-epidemiological surveillance system
The Liguria region contributes to the national surveillance of influenza-like illness (ILI) instituted by the National Institute of Health (NIH). In particular, the clinical and epidemiological surveillance system is performed by a network of sentinel general practitioners (GPs) who cover 2% of the regional population (meanly 50,443 patients per year) and notify cases of ILIs from week 42th to week 17th of each influenza season.
ILIs cases are defined as acute onset of fever together with respiratory symptoms and one systemic symptom such as headache, general discomfort, asthenia, myalgia, according to the Italian surveillance network guideline.Citation36 A standardized report form, including demographic and clinical information is used for notification.Citation36
The reports of ILIs cases diagnosed in Liguria region are sent to the inter-University Center for Research on Influenza and other Transmissible Infections (CIRI-IT), Genoa (Italy), that is one of the 2 reference centers of the Italian epidemiological surveillance system of influenza (Influnet). Regional data are weekly sent to the NIH, that collects and elaborates epidemiological information at the national level.Citation36,37
The syndromic surveillance system
The ILI syndromes were monitored through the Syndromic surveillance system (SSS) based on Emergency Department (ED) accesses. This SSS is active since 2006 and evaluate data collected at the Ligurian reference university hospital for adults “San Martino,” that covers approximately 55% of all catchment area in Genoa, the regional capital city.Citation38,39
Since 2007 the regional SSS collects data from the EDs of another main hospital for adults and the regional reference hospital for children in Genoa, allowing to cover 72% and 100% of all urban area ED accesses for adults and children, respectively.
Syndrome coding, data capture, transmission and processing, statistical analysis to assess indicators of disease activity and alert thresholds and signal response were operatively described in Ansaldi et al.Citation38
The number of ED accesses and incidence of accesses for ILIs in the considered period were stratified by age and influenza season. Further analysis about the incidence of ILIs during seasons when vaccine matching and mismatching occurred were conducted.
The virological surveillance system
Nasopharyngeal swabs were collected using Virocult swabs (MWE, Medical Wire, Corsham, UK) from patients with ILI syndrome who accessed to the abovementioned hospitals or visited by the sentinel GPs during the influenza epidemic season and then sent to the Regional Reference Laboratory for influenza surveillance at the Department of Health Sciences, University of Genoa for influenza virus characterization.
The characterization was also conducted by the National Influenza Center at the NIH and/or by the World Health Organization (WHO) Influenza Collaborating Center in London (UK), which participate in the WHO Influenza Surveillance Network.
All influenza positive samples were tested for type A and B influenza viruses,Citation19 and a representative subset was further subjected to an antigenic and/or genetic characterization.
The antigenic characterization was performed by hemagglutination inhibition (HI) and/or microneutralisation (NT) tests, in order to identify the antigenic variants circulating in human populations during the winter season.
The HI test was performed using whole viruses and hyperimmune sheep serum or post-infection ferret sera to reference viruses (provided by WHO Influenza Collaborating Center, London, UK) as described by Puzelli et al.,Citation23 and by Ansaldi et al.Citation29
The microneutralisation assay was performed as reported by Ansaldi et al.Citation40
Furthermore, since the antigenic variability of an influenza virus HA mainly occurs in the HA1 domain,Citation41 we determined the nucleotide sequences encoding the HA1 subunit of selected influenza B viruses circulating in Liguria during the last 15 influenza seasons, as previously described.Citation29
Finally, the construction of phylogenetic trees was performed applying the Kimura-2 distance method and the Neighbor-Joining algorithm with 1000 bootstrap replicates using the MEGA v5.05 software package.Citation42
Abbreviations
| ILI | = | Influenza-like illness |
| HA | = | Hemagglutinin |
| NA | = | Neuraminidase |
| NIH | = | National Institute of Health |
| GP | = | General Practitioner |
| CIRI-IT | = | Inter-University Center for Research on Influenza and other Transmissible Infections |
| Influnet | = | Italian epidemiological surveillance system of influenza |
| SSS | = | Syndromic surveillance system |
| ED | = | Emergency Department |
| WHO | = | World Health Organization |
| HI | = | Hemagglutination inhibition |
| NT | = | Microneutralization. |
Disclosure of potential conflicts of interest
Filippo Ansaldi, Giancarlo Icardi and Laura Sticchi have previously participated at speaker's bureaus and advisory board meetings sponsored by GSK, Pfizer, Novartis and Sanofi Pasteur. Cecilia Trucchi, Cristiano Alicino, Chiara Paganino and Andrea Orsi Ilaria Barberis, Federico Grammatico, Paola Canepa, Emanuela Rappazzo, Bianca Bruzzonedeclare that they have no conflict of interest.
1264779_Supplemental_Material.zip
Download Zip (83.4 KB)Funding
Filippo Ansaldi, Giancarlo Icardi and Laura Sticchi received research funding as investigator or principal investigator from GSK, Pfizer, Novartis and Sanofi Pasteur MSD.
References
- Cromer D, van Hoek AJ, Jit M, Edmunds WJ, Fleming D, Miller E. The burden of influenza in England by age and clinical risk group: a statistical analysis to inform vaccine policy. J Infect 2014; 68(4):363-71; PMID:24291062; http://dx.doi.org/10.1016/j.jinf.2013.11.013
- Nair H, Brooks WA, Katz M, Roca A, Berkley JA, Madhi SA, Simmerman JM, Gordon A, Sato M, Howie S, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet 2011; 378:1917-30; PMID:22078723; http://dx.doi.org/10.1016/S0140-6736(11)61051-9
- World Health Organization. Media Center. Influenza (seasonal). Fact Sheet no 211. March 2014
- Keech M, Beardsworth P. The impact of influenza on working days lost: a review of the literature. Pharmacoeconomics 2008; 26:911-24; PMID:18850761; http://dx.doi.org/10.2165/00019053-200826110-00004
- Tafalla M, Buijssen M, Geets R, Vonk Noordegraaf-Schouten M. A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Hum Vaccin Immunother 2016; 12(4):993-1002; PMID:26890005; http://dx.doi.org/10.1080/21645515.2015.1111494
- Finkelman BS, Viboud C, Koelle K, Ferrari MJ, Bharti N, Grenfell BT. Global patterns in seasonal activity of influenza A/H3N2, A/H1N1,and B from 1997 to 2005: viral coexistence and latitudinal gradients. PLoS ONE 2007; 2:e1296; PMID:18074020; http://dx.doi.org/10.1371/journal.pone.0001296
- Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179-186; PMID:12517228; http://dx.doi.org/10.1001/jama.289.2.179
- Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333-1340; http://dx.doi.org/10.1001/jama.292.11.1333
- Kilbourne ED. Influenza pandemics of the 20th century. Emerg Infect Dis 2006; 12:9-14; PMID:16494710; http://dx.doi.org/10.3201/eid1201.051254
- Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health 2013; 103:e43-e51; PMID:23327249; http://dx.doi.org/10.2105/AJPH.2012.301137
- Camilloni B, Neri M, Lepri E, Basileo M, Sigismondi N, Puzelli S, Donatelli I, Iorio AM. An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. Vaccine 2010; 28(47):7536-41; PMID:20846530; http://dx.doi.org/10.1016/j.vaccine.2010.08.064
- Grant KA, Carville K, Fielding JE, Barr IG, Riddell MA, Tran T, Kelly HA. High proportion of influenza B characterizes the 2008 influenza season in Victoria. Communicable Diseases Intelligence Quarterly Report 2009; 33(3):328-36; PMID:20043604
- Yin-Coggrave M, Kadri Z. Type B influenza in Singapore. Singapore Medical J 1965; 6(2):71-4
- Burnet FM, Stone JD, Anderson SG. An epidemic of influenza B in Australia. Lancet 1946; 247(6405):807-11
- Tramuto F, Orsi A, Maida CM, Costantino C, Trucchi C, Alicino C, Vitale F, Ansaldi F. The Molecular Epidemiology and Evolutionary Dynamics of Influenza B Virus in Two Italian Regions during 2010–2015: The Experience of Sicily and Liguria. Int J Mol Sci 2016; 17(4):549; PMID:27089319; http://dx.doi.org/10.3390/ijms17040549
- Matsuzaki Y, Sugawara K, Takashita E, Muraki Y, Hongo S, Katsushima N, Mizuta K, Nishimura H. Genetic diversity of influenza B virus: The frequent reassortment and cocirculation of the genetically distinct reassortant viruses in a community. J Med Virol 2004; 74:132-140; PMID:15258979; http://dx.doi.org/10.1002/jmv.20156
- Bedford T, Riley S, Barr IG, Broor S, Chadha M, Cox NJ, Daniels RS, Gunasekaran CP, Hurt AC, Kelso A, et al. Global circulation patterns of seasonal influenza viruses vary with antigenic drift. Nature 2015; 523(7559):217-20; PMID:26053121; http://dx.doi.org/10.1038/nature14460
- Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, Belongia EA. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004–2005 through 2007–2008. Influenza Other Respir Viruses 2012; 6:37-43; PMID:21668663; http://dx.doi.org/10.1111/j.1750-2659.2011.00263.x
- Biere B, Bauer B, Schweiger B. Differentiation of influenza B virus lineages Yamagata and Victoria by real-time PCR. J Clin Microbiol 2010; 48:1425-1427; PMID:20107085; http://dx.doi.org/10.1128/JCM.02116-09
- Moa AM, Muscatello DJ, Turner RM, MacIntyre CR. Epidemiology of Influenza B in Australia: 2001–2014 Influenza Seasons. Influenza Other Respir Viruses 2016 (in press); PMID:27650482; http://dx.doi.org/10.1111/irv.12432
- Belshe RB, Coelingh K, Ambrose CS, Woo JC, Wu X. Efficacy of live attenuated influenza vaccine in children against influenza B viruses by lineage and antigenic similarity. Vaccine 2010; 28:2149-56; PMID:20003926; http://dx.doi.org/10.1016/j.vaccine.2009.11.068
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28(Suppl 4):D45-53; PMID:20713260; http://dx.doi.org/10.1016/j.vaccine.2010.08.028
- Puzelli S, Frezza F, Fabiani C, Ansaldi F, Campitelli L, Lin YP, Gregory V, Bennett M, D'Agaro P, Campello C, et al. Changes in the hemagglutinins and neuraminidases of human influenza B viruses isolated in Italy during the 2001–2002, 2002–2003, and 2003–2004 seasons. J Med Virol 2004; 74:29-40; PMID:15258965; http://dx.doi.org/10.1002/jmv.20225
- Skowronski DM, Janjua NZ, Sabaiduc S, de Serres G, Winter AL, Gubbay JB, Dickinson JA, Fonseca K, Charest H, Bastien N, et al. Influenza A/subtype and B/lineage effectiveness estimates for the 2011–2012 trivalent vaccine: Cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126-137; PMID:24446529; http://dx.doi.org/10.1093/infdis/jiu048
- Lo YC, Chuang JH, Kuo HW, Huang WT, Hsu YF, Liu MT, Chen CH, Huang HH, Chang CH, Chou JH, et al. Surveillance and vaccine effectiveness of an influenza epidemic predominated by vaccine-mismatched influenza B/Yamagata-lineage viruses in Taiwan, 2011–2012 season. PLoS One 2013; 8:e58222; PMID:23472161; http://dx.doi.org/10.1371/journal.pone.0058222
- Trucchi C, Paganino C, Orsi A, De Florentiis D, Ansaldi F. Influenza vaccination in the elderly: why are the overall benefits still hotly debated? J Prev Med Hyg 2015; 56(1):E37-43
- Rizzo C, Bella A. The impact of influenza virus B in Italy: myth or reality? J Prev Med Hyg 2016; 57(1):E23-7
- Caini S, Huang QS, Ciblak MA, Kusznierz G, Owen R, Wangchuk S, Henriques CM, Njouom R, Fasce RA, Yu H, et al. Global Influenza B Study. Epidemiological and virological characteristics of influenza B: results of the Global Influenza B Study. Influenza Other Respir Viruses 2015; 9(Suppl 1):3-12; PMID:26256290
- Ansaldi F, D'Agaro P, De Florentiis D, Puzelli S, Lin YP, Gregory V, Bennett M, Donatelli I, Gasparini R, Crovari P, et al. Molecular characterization of influenza B viruses circulating in northern Italy during the 2001–2002 epidemic season. J Med Virol 2003; 70(3):463-9; PMID:12767012; http://dx.doi.org/10.1002/jmv.10418
- Kanegae Y, Sugita S, Endo A, Ishida M, Senya S, Osako K, Nerome K, Oya A. Evolutionary pattern of the hemagglutinin gene of influenza B viruses isolated in Japan: cocirculating lineages in the same epidemic season. J Virol 1990; 64(6):2860-5; PMID:2335820
- Rota PA, Wallis TR, Harmon MW, Rota JS, Kendal AP, Nerome K. Cocirculation of two distinct evolutionary lineages of influenza type B virus since 1983. Virology 1990; 175(1):59-68; Rota PA, Hemphill ML, Whistler T, Regnery HL, Kendal AP. Antigenic and genetic characterization of the haemagglutinins of recent cocirculating strains of influenza B virus. J Gen Virol 1992; 73(Pt 10):2737-42; PMID:2309452; http://dx.doi.org/10.1016/0042-6822(90)90186-U
- Lindstrom SE, Hiromoto Y, Nishimura H, Saito T, Nerome R, Nerome K. Comparative analysis of evolutionary mechanisms of the hemagglutinin and three internal protein genes of influenza B virus: multiple cocirculating lineages and frequent reassortment of the NP, M, and NS genes. J Virol 1999; 73(5):4413-26; PMID:10196339
- Shaw MW, Xu X, Li Y, Normand S, Ueki RT, Kunimoto GY, Hall H, Klimov A, Cox NJ, Subbarao K. Reappearance and global spread of variants of influenza B/Victoria/2/87 lineage viruses in the 2000–2001 and 2001–2002 seasons. Virology 2002; 303(1):1-8; PMID:12482653; http://dx.doi.org/10.1006/viro.2002.1719
- World Health Organization FluNet website. Available at: http://www.who.int/influenza/gisrs_laboratory/flunet/en/.
- Tisa V, Barberis I, Faccio V, Paganino C, Trucchi C, Martini M, Ansaldi F. Quadrivalent influenza vaccine: a new opportunity to reduce the influenza burden. J Prev Med Hyg. 2016; 57(1):E28-33; PMID:27346937
- Italian Ministry of Health. Operative protocol - Influenza season. 2015–2016. Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2418_allegato.pdf. Last access: 21/10/2016
- Gasparini R, Bonanni P, Amicizia D, Bella A, Donatelli I, Cristina ML, Panatto D, Lai PL. Influenza epidemiology in Italy two years after the 2009–2010 pandemic: need to improve vaccination coverage. Hum Vaccin Immunother 2013; 9(3):561-7; PMID:23292210; http://dx.doi.org/10.4161/hv.23235
- Ansaldi F, Orsi A, Altomonte F, Bertone G, Parodi V, Carloni R, Moscatelli P, Pasero E, Oreste P, Icardi G. Emergency department syndromic surveillance system for early detection of 5 syndromes: a pilot project in a reference teaching hospital in Genoa, Italy. J Prev Med Hyg 2008; 49(4):131-5; PMID:19350960
- Ansaldi F, Orsi A, Trucchi C, De Florentiis D, Ceravolo A, Coppelli M, Schiaffino S, Turello V, Rosselli R, Carloni R, et al. Potential effect of PCV13 introduction on Emergency Department accesses for lower respiratory tract infections in elderly and at risk adults. Hum Vaccin Immunother 2015; 11(1):166-7; PMID:25483530; http://dx.doi.org/10.4161/hv.34419
- Ansaldi F, Bacilieri S, Amicizia D, Valle L, Banfi F, Durando P, Sticchi L, Gasparini R, Icardi G, Crovari P. Antigenic characterisation of influenza B virus with a new microneutralisation assay: comparison to haemagglutination and sequence analysis. J Med Virol 2004; 74(1):141-6; PMID:15258980; http://dx.doi.org/10.1002/jmv.20157
- Wiley DC, Skehel JJ. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem 1987; 56:365-94; PMID:3304138; http://dx.doi.org/10.1146/annurev.bi.56.070187.002053
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 30(12):2725-9; PMID:24132122; http://dx.doi.org/10.1093/molbev/mst197
