ABSTRACT
[Purpose] Multi-drug resistant (MDR), Mycobacterium tuberculosis (TB) is a big problem in the world. We have developed novel TB therapeutic vaccine (HVJ-E/HSP65 DNA +IL-12 DNA).
[Methods and Results] DNA vaccine expressing TB heat shock protein 65 and IL-12 was delivered by the hemagglutinating virus of Japan (HVJ)-envelope. This vaccine provided remarkable protective efficacy and strong therapeutic efficacy against MDR-TB and XDR-TB in murine models. Furthermore, this vaccine provided therapeutic efficacy of prolongation of survival time of TB infected monkeys and augmented the immune responses.
Therefore, the preclinical tests were studied for clinical trial. The injection of 100 μg of the vaccine /mouse i.m. three times in two weeks induced significantly strong production of IFN-γ and IL-2. 100 μg and 200 μg DNA vaccine/mouse i.m. augmented the production of these cytokines compared with 25 μg DNA vaccine/mouse i.m.. The ratio of 100 μg pDNA to 1AU HVJ-E enhanced the production of IFN-γ and IL-2. The decrease in the number of M. tuberculosis in liver of mice was observed by the vaccination of 100μg pDNA. By using these conditions, safety pharmacology study and toxicology test is being studied in monkeys administered by GMP level DNA vaccines. By the toxicology test using monkeys, high dose GMP level vaccine/ monkey is administrated. Safety pharmacological study of repeated administration is also being investigated in GLP level.
Furthermore, we have planned to do clinical phase I trial. Targets are human patients with MDR-TB. The safety and tolerability of the vaccine will be evaluated.
[Conclusion and recommendations] These data indicate that our novel vaccine might be useful against tuberculosis including XDR-TB and MDR-TB for human therapeutic clinical applications.
Introduction
Tuberculosis is a major global threat to human health, with about 1.5 million people dying every year from Mycobacterium tuberculosis (TB) infection. The only tuberculosis vaccine currently available is an attenuated strain of Mycobacterium bovis BCG (BCG), although its efficacy against adult TB disease remains controversial. Furthermore, multi-drug resistant tuberculosis (MDR-TB) and extremely drug resistant TB (XDR-TB) are becoming big problems in the world.Citation1 About 500,000 of people around the world are affected by MDR-TB every year. However, there are a small number of effective drugs against MDR-TB. In such circumstances, the development of therapeutic vaccine against TB as well as prophylactic vaccine against TB is required. Therefore, we have developed a novel TB vaccine, a DNA vaccine expressing mycobacterial heat shock protein 65 (HSP65) and interleukin-12 (IL-12) delivered by the hemagglutinating virus of Japan (HVJ)-envelope (E).Citation2-7 This vaccine was more efficient than BCG in the murine TB-prophylactic model on the basis of the elimination of M. tuberculosis mediated by the induction of CTL and production of IFN-γ, as several reports demonstrated that CTL, Th1 cell and IFN-γ played a very important role of TB vaccine.Citation2-4,8-19 Furthermore, in our previous study, (1) HVJ-E/pcDNA3.1 HSP65 DNA + IL-12 DNA vaccine showed therapeutic efficacy against MDR-TB in mice. (2) Significant prolongation of survival was observed in the XDR-TB infected mice by the treatment with this vaccine. (3) Therapeutic efficacy of this vaccine on chronic TB disease models using mice infected with TB in the aerosol chamber was demonstrated.Citation9,10 A nonhuman primate model of TB will provide information for vaccine development.Citation4,5,20 In fact, in the previous study, we evaluated the therapeutic and protective efficacy of HSP65 DNA + IL-12 DNA vaccine in the cynomolgus monkey model, which is an excellent model of human tuberculosis.Citation3,4,9,10 HVJ-E/pVAX1-HSP65 DNA + IL-12 DNA vaccine exerted the therapeutic activity in the cynomolgus therapeutic model [prolongation of survival, augmentation of proliferation of PBL and improvement of erythrocyte sedimentation rate (ESR)].Citation9,10 It is very important to evaluate the long-term survival in a monkey models, as human TB is a chronic infection disease.Citation4,Citation9,10 Thus, we are taking advantage of the availability of multiple animal models and are accumulating essential data on the HVJ-E/HSP65 DNA + IL-12 DNA vaccine in anticipation of initiating a phase I clinical study.Citation10
Therefore, in this study, we evaluated the preclinical study of pharmacological efficacy using animal models and discussed the toxicity, pharmacological safety and toxicokinetics (TK) for human clinical trial.
Results
Construction of DNA vaccine for preclinical study.
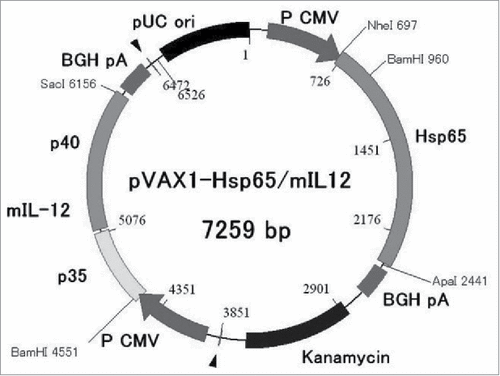
HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine was constructed for preclinical study. The vaccine contains two kinds of DNA in one plasmid vector (pVAX1) (). Combination of cDNA-IgHsp65 derived from M. tuberculosis and cDNA-mouse(m)IL-12 p40p35-F was constructed in pVAX1 plasmid and encapsulated into HVJ-Envelope.Citation2,10
Administration route of the vaccine.
Figure 1. Construction of DNA vaccine for preclinical trial. HVJ-Envelope/HSP65 DNA+mouse IL-12 DNA vaccine was constructed for preclinical vaccine which contains two kinds of DNA in one plasmid vector (pVAX1).
Efficacy of vaccine of intramuscular administration (i.m.) was compared with the efficacy of intradermal administration (i.d.). BALB/c mice and C57BL/6 mice were administered with the vaccine from one time to 6 times during two weeks by i.m. or i.d.. IFN-γ from spleen cells of BALB/c mice was more significantly produced, when the vaccine was administered intramuscularly compared with intradermal administration. IL-2 was also strongly produced by the administration of vaccine i.m..(data not shown)
Administration dose and times of HVJ-E/pVAX-HSP65 DNA+IL-12 DNA vaccine.
In order to investigate the effective dose and times of the vaccine, 1∼6 times administration of 25∼200 μg DNA vaccine was i.m. injected into C57BL/6 mice in two weeks as described in Material and Methods, and the method of previous report.Citation10,21
After 4 weeks, spleen cells obtained were cultured in vitro by the stimulation with recombinant HSP 65 antigen, PPD, or killed H37Ra TB antigens for 1 day or two days. IFN-γ, IL-2, and IL-6 in the culture supernatants were analyzed using ELISA.
As shown in and , the injection of 100 μg of the vaccine/mouse i.m. three times in two weeks induced significantly (p<0.01) high production of IL-2 by the stimulation with HSP65, PPD and killed H37Ra TB antigens (). On the other hand, IFN-γ production was augmented by the stimulation with HSP65 (p<0.01) and PPD (p < 0.05) in the vaccinated group. IL-6 production in the 1 day culture supernatants was enhanced by the vaccination with 100 μg of DNA compared with control. (data not shown)
Figure 2. Three times administration of 100 μg DNA vaccine (HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine)(■) was i.m. injected into C57BL/6 mice in two weeks. 100 μg pDNA (HSP65 DNA + IL-12 DNA) + 1AU HVJ-E were used as vaccine. After 4 weeks, spleen cells were cultured in vitro by the stimulation with 10μg/ml of recombinant HSP65 antigen (derived from H37Rv TB), 20μg/ml of PPD, or 20μg/ml of killed H37Ra TB antigen for 1 day. Five % of trehalose phosphate buffer was used for control(□). IL-2 in the culture supernatants was analyzed using ELISA. Results are expressed as the mean ± S.D. of pg of IL-2. The statistical significance of differences between individual groups in the pg of IL-2 was determined by Dunnett's test; ** p < 0.01: the statistical significance of differences (p < 0.01).
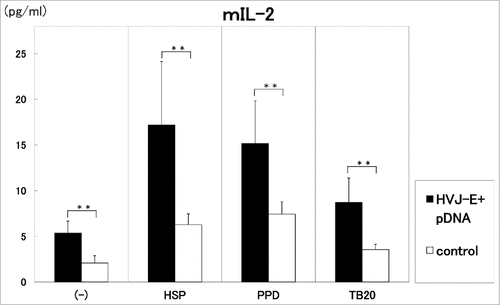
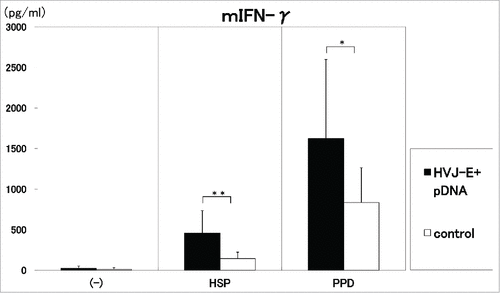
Figure 3. Three times administration of 100 μg DNA vaccine (HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine) (■) was i.m. injected into C57BL/6 mice in two weeks. (□)control (5% trehalose phosphate buffer). After 4 weeks, spleen cells were cultured in vitro by the stimulation with 10μg/ml of recombinant HSP65 antigen (derived from H37Rv TB) and 20μg/ml of PPD for 1 day. IFN-γ in the culture supernatants was analyzed using ELISA.** p<0.01: the statistical significance of differences (p<0.01). *p<0.05 (Dunnett's test).
As shown in , 100 μg and 200 μg of DNA vaccine/mouse i.m. augmented the production of IL-2 cytokine by the stimulation with HSP65 compared with control group. (p<0.05) 25 μg of DNA vaccine/mouse i.m. did not exert the enhancement of IL-2 production significantly in comparison to control group.
Figure 4. 25 μg, 100μg or 200μg DNA vaccine/mouse was i.m. administrated into C57BL/6 mice in two weeks. (
)25μg pDNA (HSP65 DNA+IL-12 DNA)+HVJ-E 0.25AU/mouse, (▪) 100μg pDNA+HVJ-E 1.0AU/mouse, (
 ) 200μg pDNA+HVJ-E 2.0AU/mouse. (□) control (5% trehalose phosphate buffer). After 4 weeks, spleen cells were cultured in vitro by the stimulation with 10 μg/ml of recombinant HSP65 antigen and 20 μg/ml of PPD for 1 day. IL-2 in the culture supernatants was analyzed using ELISA. * p<0.05: the statistical significance of differences (p<0.05) by Dunnett's test.
) 200μg pDNA+HVJ-E 2.0AU/mouse. (□) control (5% trehalose phosphate buffer). After 4 weeks, spleen cells were cultured in vitro by the stimulation with 10 μg/ml of recombinant HSP65 antigen and 20 μg/ml of PPD for 1 day. IL-2 in the culture supernatants was analyzed using ELISA. * p<0.05: the statistical significance of differences (p<0.05) by Dunnett's test.
The production of IL-2 by the vaccination using 200 μg DNA was same level of IL-2 production by the vaccination using 100 μg DNA. Therefore, 100 μg DNA was administered for the vaccine against TB hereafter ().
Three times immunization with this vaccine in two weeks induced significantly (p<0.05) strong production of IL-2 in comparison to control (). The thrice vaccination in two weeks also augmented strong IFN-γ production. (data not shown)
Figure 5. Analysis of administration times of DNA vaccine. One time (
), three times (▪) and six times (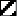
 ) administration of 100 μg DNA vaccine (HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine) into the BALB/c mice were done i.m. in two weeks. Five % trehalose phosphate buffer was used as control (□). After 4 weeks, spleen cells were cultured in vitro by the stimulation with 10 μg/ml of recombinant HSP65 antigen and 20 μg/ml of PPD for 1 day. IL-2 in the culture supernatants was analyzed using ELISA. * p<0.05: the statistical significance of differences (p<0.05) by Dunnett's test.
) administration of 100 μg DNA vaccine (HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine) into the BALB/c mice were done i.m. in two weeks. Five % trehalose phosphate buffer was used as control (□). After 4 weeks, spleen cells were cultured in vitro by the stimulation with 10 μg/ml of recombinant HSP65 antigen and 20 μg/ml of PPD for 1 day. IL-2 in the culture supernatants was analyzed using ELISA. * p<0.05: the statistical significance of differences (p<0.05) by Dunnett's test.
Six times vaccination in BALB/c mice was not significantly stronger than 3 times vaccination for the production of cytokine, IL-2 (). Using C57BL/6 mice, it was shown that six times vaccination was not also significantly higher than 3 times vaccination for the production of cytokines (IFN-γ, IL-2), contributing tuberculosis immunity (data not shown).
The ratio of pDNA to HVJ-E.
Furthermore, we investigated the ratio of pDNA to HVJ-E for the induction of the cytokines(IFN-γ and IL-2). The ratio of 100 μg pDNA to 1AU HVJ-E induced significantly high IL-2 production by the stimulation with 20 μg/ml of PPD antigen, compared with control (). The same ratio of pDNA to HVJ-E also exert the enhancement of IFN-γ production. (data not shown)
Therapeutic efficacy of pVAX-HSP65 DNA+IL-12 DNA vaccine.
Figure 6. The ratio of pDNA to HVJ-E. The ratio of pDNA to HVJ-E was investigated for the induction of the cytokine (IL-2). The ratio of 100 μg pDNA to 0.2∼2AU HVJ-E was administrated into C57BL/6 mice. HSP65 DNA + IL-12 DNA: pDNA 100μg + HVJ-E 0.2AU (
), pDNA 100μg + HVJ-E 1AU (▪), pDNA 100μg + HVJ-E 2AU (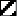
 ), control (5% trehalose phosphate buffer) (□). IL-2 production by the stimulation with 10 μg/ml of HSP65 protein or 20 μg/ml PPD antigen was analyzed by ELISA. * p<0.05: statistical significance (Student t).
), control (5% trehalose phosphate buffer) (□). IL-2 production by the stimulation with 10 μg/ml of HSP65 protein or 20 μg/ml PPD antigen was analyzed by ELISA. * p<0.05: statistical significance (Student t).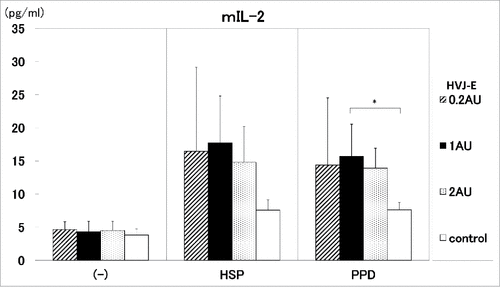
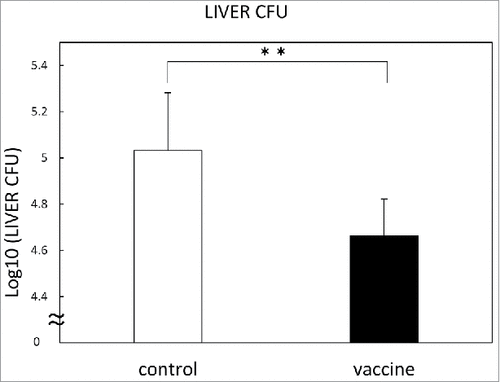
Therapeutic efficacy of pVAX-HSP65 DNA+IL-12 DNA vaccine on the TB infection in the C57BL/6 mice was investigated. Groups of mice were challenged by intravenous injection with 5 × 104 CFU TB, and then treated three times with the 100 μg of pVAX-HSP65 DNA + IL-12 DNA vaccine in two weeks. Three weeks after the challenge of TB infection, therapeutic efficacy was measured by enumerating the bacterial loads (CFU) in the liver. Therapeutic efficacy of the pVAX-HSP65 DNA+IL-12 DNA vaccine was significantly demonstrated (p<0.01) in the liver ().
Figure 7. Therapeutic efficacy of pVAX1-HSP65 DNA+IL-12 DNA vaccine on the TB infection in the C57BL/6 mice. Groups of mice were challenged by intravenous injection with 5 × 104 CFU TB, and then treated three times with the vaccine, as described in Material and Methods. Three weeks after challenge, therapeutic efficacy was measured by enumerating the bacterial loads (CFU) in the liver. Results are expressed as the mean log10 ± S.D. of CFU. The statistical significance of difference between vaccinated group (■) and control group (□) was determined by student's test (n = 10). * p<0.01: the statistical significance of differences of vaccinated group to control group.
Discussion
In the present preclinical study, pVAX-HSP65 DNA+IL-12 DNA which contains two kinds of DNA in one plasmid vector for clinical therapeutic trial, exerted a significant therapeutic effect against TB, as same efficacy was reported previously using pcDNA 3.1 vector (pcDNA-HSP65 DNA+pcDNA-IL-12 DNA) which contains one DNA in each plasmid vector.Citation9,10 The decrease in the CFU of TB in the liver of mice was demonstrated by the vaccination of pVAX-HSP65 DNA+IL-12 DNA. Therefore, HVJ-E/pVAX-HSP65 DNA+mIL-12 DNA was used for preclinical study in animals.
In the present preclinical study, this vaccine showed strong induction of immune responses against M.TB, as indicated by(1) significant augmentation of IL-2 and IFN-γ production from mice vaccinated with 100μg of pDNA/mouse i.m. (2) remarkable induction of these cytokines from mice vaccinated three times, and (3) suitable production of these cytokines by the ratio of 100μg pDNA to 1AU HVJ-E.
Furthermore, by using these experimental conditions, safety pharmacology study and toxicology test of preclinical study for human clinical trial is being studied in monkeys administered by GMP level-DNA vaccines (–).
Single-dose toxicity test using monkey will be examined by subcutaneous administration of high dose of this vaccine. Toxicity will be evaluated by the general state, food consumption, body weight, blood examination and blood biochemistry ().
Repeated-dose toxicity test of GLP-level has been planned, using HVJ-E/pVAX-HSP65 DNA+humanIL-12 DNA vaccine (GMP level) for clinical trial, as shown in . The vaccine will be repeatedly i.m. administered into cynomolgus monkeys. Repeated-dose toxicity is planned to include evaluation test for acute toxicity, local irritability, toxicokinetics, and central nervous system at GLP level ().
Table 1. Toxicity test and safety pharmacology study using monkey.
Table 2. Repeated dose toxicity test (GLP). [Evaluation for ① acute toxicity ② local irritability ③ toxicokinetics ④ central nervous system].
Toxicokinetics(TK) will be studied by the concentration of human IL-12 in the blood of monkeys vaccinated with HVJ-E/pVAX-HSP65 DNA+humanIL-12 DNA vaccine (GMP level) for clinical trial. Human IL-12 will be examined by the ELISA ().
Safety pharmacology study of GLP-level is planned to include cardiovascular system, respiratory system and body temperature. Blood pressure, heart rate, EKG (PR, QRS etc.), respiratory function (respiratory rate, ventilation volume and body temperature in the vaccinated monkeys) will be evaluated ().
Table 3. Safety pharmacology study (GLP). [Cardiovascular system, body temperature and respiratory system.]
MDR-TB and XDR-TB are becoming big problems around the world. About 500,000 new patients with MDR-TB are shown every year. However, the effective drugs against MDR-TB are few.Citation1,22
In our previous study, the HVJ-E/pcDNA3.1-HSP65 DNA+pcDNA3.1-IL-12 DNA vaccine exerted the therapeutic activity even against XDR-TB which is resistant to RFP, INH, SM, EB, EVM, TH, PAS, LVFX, PZA and only sensitive to CS.Citation9 Furthermore, pVAX-HSP65 DNA+IL-12 DNA vaccine which contains these two kinds of DNA in one plasmid vector (pVAX) demonstrated the therapeutic efficacy against TB in the monkey model.Citation9,10 HSP65 DNA+IL-12 DNA vaccine exerted strong therapeutic efficacy (100% survival at 19weeks after challenge and augmentation of immune responses) in the TB-infected monkeys.Citation9,10
On the other hand, CTL and type I helper T cells are very important for the protective and therapeutic efficacy against TB including MDR-TB and XDR-TB, as reported using several animal models and infectious diseases.Citation8-11,18,21,23,24
DNA vaccine is a relatively new approach to immunization for infectious diseases.Citation2,3,4,12-16
A hemagglutinating virus of Japan envelope (HVJ-Envelope) using inactivated Sendai virus has been developed, as a nonviral vector for drug delivery.Citation3,4,6,7 It can efficiently deliver DNAs, siRNAs, proteins and anti-cancer drugs into cells both in vitro and in vivo. Therefore, HVJ-Envelope was used as an efficient and safe vector for DNA vaccine against TB in the present study.
It will be a high priority for the clinical development programs to evaluate the current vaccines for post-exposure vaccine which prevents reactivation of TB in the large proportion of the global population latently infected with TB.
According to our knowledge, only a few therapeutic vaccine against TB has been reported.Citation18,19
Using animal models (monkey), preclinical studies of toxicology and safety pharmacology were investigated for human clinical trials of several novel prophylactic vaccines for TB, such as M72 vaccine, MVA 85A vaccine and AERAS-402.Citation25-27
Thus, taken together, our results with the HVJ-Envelope/HSP65 DNA+IL-12 DNA vaccine in the present murine preclinical study and monkey preclinical study plan (safety pharmacology, toxicokinetic study and toxicology test) should provide a significant rationale for moving this vaccine into clinical trial.
Plan for clinical trial of this vaccine against the patients with MDR-TB (RFP resistant and INH resistant) is shown in . Principal evaluation for clinical trial is safety and approval. Secondary evaluation for clinical trial is anti-tuberculosis efficacy (decrease in the number of TB in the sputum of patients with MDR-TB) and immune responses against TB. The trial will be studied in the National Hospital Organization Hospitals in Japan.
Table 4. Plan for clinical trial of HVJ-E/HSP65 DNA+IL-12 DNA therapeutic vaccine for the patients with MDR-TB.
Multidrug- resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are an increasing public health threat.Citation1 Delamanid and bedaquiline are two drugs that were recently approved by the Food and Drug Administration for treatment of MDR-TB and XDR-TB.Citation28 However, acquired resistance to delamanid and bedaquiline in therapy for tuberculosis is recently described.29-31The stepwise amplification of drug resistance of bedaquiline and delamanid in a patient with XDR-TB was observed. It serves as a warning for the future appearance of resistant TB against new antituberculosis drugs. Thus, it emphasized the need for the novel therapeutic vaccines which are different from antituberculosis chemical drugs.
In conclusion, these data indicate that HVJ-Envelope/pVAX-HSP65 DNA+IL-12 DNA vaccine might be useful against TB including XDR-TB and MDR-TB for human therapeutic clinical trials.
Material & methods
(Material and Methods for preclinical study of the vaccine using mice.: Test for Pharmacological Efficacy)
Animals
Mice (female BALB/c CrSlc and female C57BL/6 N CrSlc) were purchased from Japan SLC (Shizuoka, Japan). Mice were maintained in isolator cages and manipulated in laminar flow hoods. All animal experiments were approved by the National Hospital Organization Kinki-chuo Chest Medical Center Animal Care and Use Committee. All vaccinations, TB administration and experiments on isolate tissue of animal were done under anesthetic state with sevoflurane.Citation10 Infected animals were housed in individual micro-isolator cages in a Biosafety Level (BL) 3 animal facility of the NHO Kinki-Chuo Chest Medical Center.
Plasmid construction and HVJ-E
pVAX1-HSP65 DNA + mouse IL-12 DNA (two kinds DNA in one plasmid vector) was originally constructed as described previously.Citation9,10 The pDNA was made in Oriental Yeast (Shiga, Japan).Citation9,10 Five % of trehalose phosphate buffer was used as control.
The hemagglutinating virus of Japan (HVJ)-envelope (HVJ-E GenomOne-Neo, lot 602032) was purchased from Ishihara (Osaka, Japan). and used as previously described.Citation4,9,10 One hundred μg of pDNA: pVAX-HSP65 DNA + IL-12 DNA and 1AU of HVJ-E were used as vaccine, usually.
Bacteria
M. tuberculosis strain H37Rv was kindly provided by Dr. I. Sugawara (JATA, Tokyo, Japan).Citation2,10,11
Vaccination
Administration times and route of the vaccine: The effective administration routes of vaccines were investigated using these mice.The intramuscular (i.m.) administration route was compared with the intradermal (i.d.) administration using IL-2, IFN-γ and IL-6 production activity of spleen cells.Mice were administered with the vaccine from one time to six times during two weeks by i.m. or i.d.. After 4 weeks, spleen cells obtained were cultured in RPMI1640 medium contained 10% FCS(Hyclone:lot NoANTG19241) + SM + PC + 2ME.Citation32 Spleen cells were stimulated with recombinant HSP65 antigen (10μg/ml or 20μg/ml)(Oriental Yeast), 20μg of PPD (Japan BCG Laboratory: lotT369), and killed H37Ra TB antigens (Difco: lot3262499) for 1 day or two days. IFN-γ, IL-2, and IL-6 in the culture supernatants were analyzed using ELISA (BD Biosciens:BD opt EIA).
Administration doses of the vaccine 25μg, 100μg, or 200μg of pDNA (pVAX1-HSP65 DNA + IL-12 DNA) was immunized i.m. into C57BL/6 mice 3 times in two weeks. After 4 weeks spleen cells were stimulated with 10μg/ml of HSP65 protein, 20μg/ml of PPD or 20μg/ml of killed H37Ra TB antigen for 1 day. IFN-γ, IL-2 and IL-6 in the culture supernatants were analyzed using ELISA.
Ratio of pDNA to HVJ-E to induce cytokines (IL-2, IFN-γ and IL-6) was also investigated.
Production of cytokines (IL-2, IFN-γ, and IL-6):
Mouse cytokines were measured in quantitative ELISAs for IL-2, IFN-γ, and IL-6 as described previously.Citation2,33
Therapeutic efficacy of pVAX1-HSP65 DNA+IL-12 DNA vaccine on the TB infection in the C57BL/6 mice. Groups of mice were challenged by intravenous injection with 5 × 104 CFU TB, and then treated three times with the vaccine without HVJ-E. Human virulent H37Rv M. tuberculosis was used as TB.Citation2 Three weeks after challenge, therapeutic efficacy was measured by enumerating the bacterial loads (CFU) in the liver, spleen and lung.Citation9-11
Statistical analysis
Dunnett's tests (multiple comparisons) were used to compare the cytokines production between groups in the ELISA assay. A P-value of < 0.05 was considered significant.Citation8
Abbreviations
| TB | = | tuberculosis |
| MDR-TB | = | multi-drug resistant tuberculosis |
| HSP65 | = | heat shock protein 65 kDa from M. TB |
| IL-12 | = | Interleukin 12 |
| HVJ | = | hemagglutinating virus of Japan |
| HVJ-E | = | HVJ-Envelope |
| CTL | = | Cytotoxic T lymphocytes |
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
This research was also supported by Health and Labor Science Research Grants from MHLW, Japan.
Acknowledgments
The authors thank Dr. Y. Inoue, Dr. K. Tsuyuguchi, Dr. T. Nakajima, Dr. Y. Kaneda, and Dr. K. Tomono for their helpful support.
Funding
This research was supported by the Research Program on Emerging and Re-emerging Infectious Diseases from Japan Agency for Medical Research and Development, AMED.
References
- Dye C, Floyd K, Uplekar M. WHO report. World Health Organization; Geneva, 2008.
- Yoshida S, Tanaka T, Kita Y, Kuwayama S, Kanamaru N, Muraki Y, Hashimoto S, Inoue Y, Sakatani M, Kobayashi E, et al. DNA vaccine using hemagglutinating virus of Japan-liposome encapsulating combination encoding mycobacterial heat shock protein 65 and interleukin-12 confers protection against Mycobacterium tuberculosis by T cell activation. Vaccine 2006; 24:1191-1204; PMID:16216394; http://dx.doi.org/10.1016/j.vaccine.2005.08.103
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, McMurray DN, Dela Cruz EC, Tan EV, Gelber R, et al. Evaluation of a novel vaccine (HVJ-liposome/ HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomologus monkey model of TB. Vaccine 2007; 25(16):2990-3; PMID:17280753; http://dx.doi.org/10.1016/j.vaccine.2007.01.014
- Okada M, Kita Y. Tuberculosis vaccine development; The development of novel (preclinical) DNA vaccine. Human Vaccines 2010; 6(4):297-308; PMID:20372079; http://dx.doi.org/10.4161/hv.6.4.10172
- Kita Y, Tanaka T, Yoshida S, Ohara N, Kaneda Y, Kuwayama S, Muraki Y, Kanamaru N, Hashimoto S, Takai H, et al. Novel recombinant BCG and DNA-vaccination against tuberculosis in a cynomolgus monkey model. Vaccine 2005; 23:2132-2135; PMID:15755583; http://dx.doi.org/10.1016/j.vaccine.2005.01.057
- Kaneda Y, Nakajima T, Nishikawa T, Yamamoto S, Ikegami H, Suzuki N. Hemagglutinating virus of Japan (HVJ) envelope vector as a versatile gene delivery system. Mol Ther 2002; 6:219-226; PMID:12161188; http://dx.doi.org/10.1006/mthe.2002.0647
- Mima H, Yamamoto S, Ito M, Tomoshige R, Tabata Y, Tamai K, Kaneda Y. Targeted chemotherapy against intraperitoneally disseminated colon carcinoma using a cationized gelatin-conjugated HVJ envelope vector. Mol Cancer Ther 2006; 5:1021-1028; PMID:16648574; http://dx.doi.org/10.1158/1535-7163.MCT-05-0352
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Nakatani H, et al. Novel prophylactic vaccine using a prime-boost method and hemagglutinating virus of Japan-envelope against tuberculosis. Clin Dev Immunol 2011; 2011:Article ID:549281; http://dx.doi.org/10.1155/2011/549281
- Okada M Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, McMurray DN, Dela Cruz EC, Tan EV, Saunderson P, et al. Novel prophylactic and therapeutic vaccine against tuberculosis. Vaccine 2009; 27:3267-70; PMID:19200841; http://dx.doi.org/10.1016/j.vaccine.2009.01.064
- Okada M, Kita Y, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Nakatani H, et al. Novel therapeutic vaccine: Granulysin and new DNA vaccine against Tuberculosis. Human Vaccines 2011; 7:60-7; PMID:21546794; http://dx.doi.org/10.4161/hv.7.0.14563
- Okada M, Kita Y, Nakajima T, Hashimoto S, Nakatani H, Nishimatsu S, Nishida Y, Kanamaru N, Kaneda Y, Takamori Y, et al. The study of novel DNA vaccines against tuberculosis: Induction of pathogen-specific CTL in the mouse and monkey models of tuberculosis. Human Vaccines and Immunotherapeutics 2013; 9(3):515-25; PMID:23249543; http://dx.doi.org/10.4161/hv.23229
- Kita Y, Okada M, Nakajima T, Kanamaru N, Hashimoto S, Nagasawa T, Kaneda Y, Yoshida S, Nishida Y, Nakatani H, et al. Development of therapeutic and prophylactic vaccine against tuberculosis using monkey and granulysin transgenic mice models. Human Vaccines 2011; 7:108-14; PMID:21263229; http://dx.doi.org/10.4161/hv.7.0.14571
- Huygen K. DNA vaccines: application to tuberculosis. Int J Tuberc Lung Dis 1998; 2(12):971-8; PMID:9869111
- Lowrie DB. DNA vaccines against tuberculosis. Curr Opin Mol Ther 1999; 1(1):30-3; PMID:11249680
- Hoft D. Tuberculosis vaccine development:goals, immunological design, and evaluation. Lancet 2008; 372:164-75
- Gupta UD, Katoch VM, McMurray DN. Current status of TB vaccines. Vaccine 2007; 25:3742-51; PMID:17321015; http://dx.doi.org/10.1016/j.vaccine.2007.01.112
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol 2001; 19:93-129; PMID:11244032; http://dx.doi.org/10.1146/annurev.immunol.19.1.93
- Rook G.A.W, Lowrie D.B., Hernandez-Pando R. Immunotherapeutics for Tuberculosis on experimental animals: Is there a common pathway activated by effective protocols? J Inf Dis 2007; 196:191–198; http://dx.doi.org/10.1086/518937
- Kaufmann S.H.E, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. Lancet Respir Med 2014; 2(4):301-20; PMID:24717627; http://dx.doi.org/10.1016/S2213-2600(14)70033-5
- Walsh GP, Tan EV, dela Cruz EC, Abalos RM, Villahermosa LG, Young LJ. The Philippine cynomolgus monkey provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med 1996; 2(4):430-6; PMID:8597953; http://dx.doi.org/10.1038/nm0496-430
- Kita Y, Hashimoto S, Nakajima T, Nakatani H, Nishimatsu S, Nishida Y, Kanamaru N, Kaneda Y, Takamori Y, McMurray D, et al. Novel therapeutic vaccines [(Hsp65+IL-12)DNA-, granulysin- and Ksp37-vaccine] against tuberculosis and synergistic effects in the combination with chemotherapy., Human Vaccines and immunotherapeutics 2013; 9(3):526-33; http://dx.doi.org/10.4161/hv.23230
- Global tuberculosis control-surveillance, planning, financing. Geneva, World Health Organization, 2014.
- Okada M, Okuno Y, Hashimoto S, Kita Y, Kanamaru N, Nishida Y, Peiris JS, Chen PJ, Yamamoto N, Tashiro M, et al. Development of vaccines and passive immunotherapy against SARS corona virus using SCID-PBL/hu mouse models. Vaccine 2007; 25:3038-3040; PMID:17289225; http://dx.doi.org/10.1016/j.vaccine.2007.01.032
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol 2001; 19:93-129; PMID:11244032; http://dx.doi.org/10.1146/annurev.immunol.19.1.93
- Day CL, Tameris M, Mansoor N, van Rooyen M, de Kock M, Geldenhuys H, Erasmus M, Makhethe L, Hughes EJ, Gelderbloem S, et al. Induction and regulation of T-cell immunity by the novel tuberculosis vaccine M72/AS01 in South African adults. Am J Respir Crit Care Med 2013; 88(4):492-502; http://dx.doi.org/10.1164/rccm.201208-1385OC
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 2013; 381(9871):1021-8; PMID:23391465; http://dx.doi.org/10.1016/S0140-6736(13)60177-4
- Kagina BM, Tameris MD, Geldenhuys H, Hatherill M, Abel B, Hussey GD, Scriba TJ, Mahomed H, Sadoff JC, Hanekom WA, et al.: The novel tuberculosis vaccine, AERAS-402, is safe in healthy infants previously vaccinated with BCG, and induces dose-dependent CD4 and CD8T cell responses. Vaccine 2014; 32(45):5908-17; PMID:25218194; http://dx.doi.org/10.1016/j.vaccine.2014.09.001
- Zumla A, Memish ZA, Maeurer M, Bates M, Mwaba P, Al-Tawfi q, Denning DW, Hayden HG, Hui DS. Emerging novel and antimicrobial- resistant respiratory treat infections: new drug development and therapeutic options. Lancet Infect Dis 2014; 14:1136-49; PMID:25189352; http://dx.doi.org/10.1016/S1473-3099(14)70828-X
- Bloemberg GV, Keller PM, Stucki D, Trauner A, Borrell S, Latshang T, Coscolla M, Rothe T, Hömke R, Ritter C, et al. Acquired resistance to bedaquiline to delamanid in therapy for tuberculosis. N Eng. J. Med 2015; 373(20):1986-1988; http://dx.doi.org/10.1056/NEJMc1505196
- Tadolini M, Lingtsang RD Tiberi S Enwerem M D'Ambrosio L Sadutshang TD Centis R Migliori GB. First case of extensively drug-resistant tuberculosis treated with both delamanidand bedaquiline. The European Respiratory Society 2016 Jun 10; Pii:ERJ-00637-2016.
- Hoffmann H, Kohl TA, Hofmann-Thiel S, Merker M, Beckert P, Jaton K, Nedialkova L, Sahalchyk E, Rothe T, Keller PM, et al. Delamanid and Bedaquiline Resistance in Mycobacterium tuberculosis Ancestral Beijing Genotype Causing Extensively Drug-Resistant Tuberculosis in a Tibetan Refugee. Am J Respir Crit Care Med 2016; 193(3):337-40; PMID:26829425; http://dx.doi.org/10.1164/rccm.201502-0372LE
- Okada M, Yoshimura N, Kaieda T, Yamamura Y, Kishimoto T. Establishment and characterization of human T hybrid cells secreting immunoregulatory molecules. Proc Natl Acad Sci U S A 1981; 78(12):7717-21; PMID:6801660; http://dx.doi.org/10.1073/pnas.78.12.7717
- Okada M, Kita Y, Kanamaru N, Hashimoto S, Uchiyama Y, Mihara M, Inoue Y, Ohsugi Y, Kishimoto T, Sakatani M. Anti-IL-6 receptor antibody causes less promotion of tuberculosis infection than anti-TNF-α antibody in mice. Clin Dev Immunol 2011; 2011: Article ID: 404929; http://dx.doi.org/10.1155/2011/404929
