ABSTRACT
This study was aimed at assessing the anti-HBs persistence and immune memory 18–19 y after vaccination against hepatitis B in healthy individuals primed as infants or adolescents. We enrolled 405 teenagers (Group A) vaccinated as infants, and 409 young adults (Group B) vaccinated as adolescents. All vaccinees were tested for anti-HBs and anti-HBc antibodies; those found anti-HBc positive were further tested for HBsAg and HBV DNA. Eight individuals belonging to Group B were positive for anti-HBc alone, and were excluded from analysis. Individuals with anti-HBs concentration ≥ 10 mIU/ml were considered protected while those with anti-HBs concentration <10 mIU/ml were offered a booster dose and re-tested 2 weeks later. Overall, 67.9% individuals showed anti-HBs concentrations ≥ 10 mIU/ml (48.9% in Group A vs 87.0% in Group B, p < 0.001). The antibody geometric mean concentration (GMC) was higher in Group B than in Group A (102.5 mIU/ml vs 6.9 mIU/ml; p < 0.001). When boosted, 94.2% of vaccinees with anti-HBs <10 mIU/ml belonging to Group A and 94.7% to Group B showed an anamnestic response. Post-booster GMCs were similar in both groups (477.9 mIU/ml for Group A vs 710.0 mIU/ml for Group B, p = n.s.). Strong immunological memory persists for at least 18–19 y after immunization of infants or adolescents with a primary course of vaccination. Thus, booster doses are not needed at this time, but additional follow up is required to assess the long-life longevity of protection.
Introduction
Hepatitis type B is still a problem of worldwide major concern being a leading cause of acute and chronic hepatitis including cirrhosis and hepatocellular carcinoma. Safe and effective vaccines have been available since the early ‘80s and, at present, 184 countries have implemented a universal infant hepatitis B vaccination program offering the opportunity to prevent and control the acute disease, the development of carrier state and the HBV-related mortality on global scale.Citation1 As previously reported, in Italy a selective vaccination against hepatitis B targeted to high-risk groups was at first introduced in 1983 and then became mandatory for all infants and 12-year-old adolescents in 1991.Citation2 Vaccination of the latter cohort was restricted to the first 12 y of application of the law and thus in 2004, when the first cohort vaccinated in 1991 reached the age (12 years) of adolescent immunization, vaccination of such individuals was stopped and that of infants was maintained. Thanks to this policy of vaccination and the high coverage rate (steadily over 95%) achieved since the beginning of the introduction of our program of vaccination, over 20 million individuals (approximately 1/3 of the resident Italian population) or 35 age cohorts have been currently vaccinated with success.Citation3 Several published studies show that hepatitis B vaccination is highly immunogenic being able to confer seroprotection (anti-HBs antibody concentration over or equal to 10 mIU/ml as measured at 1–3 months after primary immunization with a 3-dose vaccination series) in over 90–95% of healthy children and adults.Citation4-6 However, the anti-HBs antibody concentrations achieved after primary vaccination tend to decline over time, and 15–50% of vaccinated children have low or undetectable concentrations of antibody 5–15 y after vaccination.Citation7 A part from specific anti-HBs, evidence shows that cell immune memory can persist beyond the loss of circulating antibody providing long-term protection. Thus, despite the loss of antibody many vaccinated individuals maintain active immune memory and show a strong anamnestic response following a booster dose of vaccine (the so-called boostability) given up to 20–25 y.Citation8-12 However other studies, mainly conducted in Asiatic countries, indicate that immune memory starts to wane during the second decade after vaccination still arising the question whether there is a need of a booster dose to maintain long-term immunity.Citation13,14
The aim of this study was to assess the duration of anti-HBs antibody and immune memory in a cohort of teenagers vaccinated as infants and in a cohort of young adults vaccinated as adolescents, 18–19 y earlier.
Results
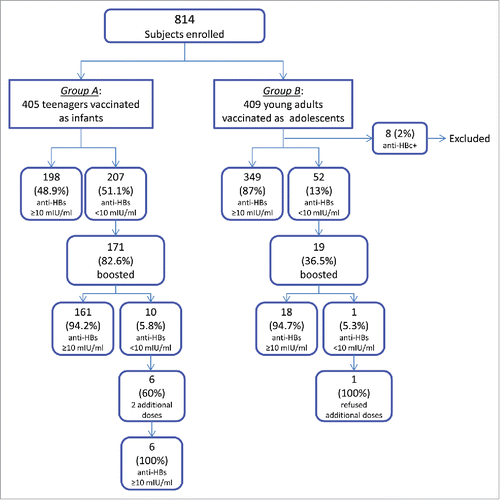
As shown in , the population enrolled in this study (n = 814) consisted of a cohort of 405 teenagers (Group A: median age 19 years, range 18–20 years; 44% males, 56% females) vaccinated as infants and a cohort of 409 adults (Group B: median age 29 years, range 27–31 years; 62% males, 38% females) vaccinated as adolescents. Eight vaccines, all belonging to Group B (8/409 or 2.0%), were found anti-HBc antibody positive but negative for both HBsAg and HBV DNA and were excluded from further data analysis; all but one had anti-HBs ≥ 10 mIU/ml.
The proportion of vaccinees showing protective concentration of anti-HBs (≥ 10 mIU/ml) 18–19 y after primary vaccination was higher among Group B than among Group A (349/401 or 87.0% vs 198/405 or 48.9%, p < 0.001) (). In the same way the antibody GMC was significantly higher in young adults compared with teenagers (102.5 mIU/ml vs 6.9 mIU/ml, p < 0.001). The proportion of those with antibodies below the detection limit (< 2 mIU/ml) was higher among Group A than among Group B (102/405 or 25.2% vs 16/401 or 4.0%, p <0.001).
Table 1. Anti-HBs concentration (mIU/ml) in Group A and Group B, 18–19 y after the primary course of vaccination.
One hundred seventy one (82.6%) of the vaccine recipients with antibody below 10 mIU/ml belonging to Group A and 19 (36.5%) of those belonging to Group B had a challenge dose of vaccine, while 36 (17.4%) of the former group and 33 (63.5%) of the latter declined participation (). The proportion of vaccinees who had an anamnestic response to booster was similar in the 2 groups (161/171 or 94.2% in Group A vs 18/19 or 94.7% in Group B, p = n.s.). As shown in and , post-booster anti-HBs antibody GMCs also did not differ significantly between the 2 groups (Group A: 477.9 mIU/ml vs Group B: 710 mIU/ml, p = n.s.). In both groups, post-booster antibody concentrations were higher among those with pre-booster antibody within 2 mIU/ml and 9.9 mIU/ml than among those with values below 2 mIU/ml (detection limit) ( and ).
Table 2. Anti-HBs concentration after booster vaccination in teenagers (Group A) with non-protective level of antibodies (< 10 mIU/ml) at enrollment.
Table 3. Anti-HBs concentration after booster vaccination in young adults (Group B) with non-protective level of antibodies (< 10 mIU/ml) at enrollment.
No serious adverse events were reported after boosting.
Six of 10 (60.0%) vaccinees of Group A who maintained anti-HBs below 10 mIU/ml after booster agreed to have 2 additional doses of vaccine, while 4 (40.0%) refused further vaccination. The same decision was taken by the only one vaccinee of Group B who did not show a post-booster anamnestic response. One-three months after completion of their second cycle of vaccination, 4 (66.7%) vaccinees developed antibody concentrations greater than 100 mIU/ml and 2 (33.3%) vaccinees developed antibody ranging between 10 and 100 mIU/ml ().
Table 4. Anti-HBs response after completion of a second course of vaccination in 6 teenagers.
Discussion
This study shows that at 18–19 y distance from primary vaccination, the proportion of vaccinees with seroprotective concentrations (≥ 10 mIU/ml) of anti-HBs antibody as well as the antibody GMCs are significantly higher among those vaccinated as adolescents than among those vaccinated as infants. This finding confirms and extends previously reported data from a study performed in Italy showing a more rapid anti-HBs antibody decline in children vaccinated in the first year of age compared with Air Force recruits vaccinated at 12 y of age over 10 y after primary vaccination series.Citation15 A body of evidence indicates that the kinetics of antibody decay and thus the persistence of antibody over time strongly depends on the magnitude of the peak antibody level achieved after primary immunization. In other words, the higher is the antibody concentration developed after a primary vaccination course, the longer is the serological duration of antibody. Other factors which may play a crucial role in favoring high post-vaccination antibody concentrations and thus its retention over time include host genetic factors, age at vaccination, and vaccine dosage.Citation16,17 Therefore, vaccination during the first year of life when the immune system is still on the path of its maturation and the administration of lower pediatric dosages compare with the higher adult dosages given to the adolescents, may explain the differences in the long-term persistence of antibody over the level considered protective between the 2 groups of vaccinees. Possible development of natural booster responses (i.e. increases in anti-HBs concentration without revaccination and no appearance of anti-HBc), that are supposed to be more frequent during the adulthood when risk of exposure to HBV becomes higher, might be one of the causes of the more prolonged persistence of anti-HBs in Group B than in Group A. In this context, the 8 benign breakthrough infections (anti-HBc positivity without HBsAg and/or HBV DNA), all detected among vaccinees of Group B seem to support, at least to a certain degree, this hypothesis. A limitation of this study is the lack of serological data concerning the post-priming antibody concentrations. However, since there is strong evidence that the post-vaccination rates of seroconversion in healthy children and young adults are close to 100%, we can infer that the absence of antibody in a proportion of vaccinees is much more likely due to its loss over time rather than to a non-responsiveness to the primary series of vaccination.
Of importance, most of vaccinated individuals (either of Group A or Group B) with anti-HBs antibody below 10 mIU/ml who were given a booster dose of vaccine maintained immune memory showing a strong anamnestic response when boosted with a dose of vaccine given 18–19 y after priming, with similar proportion of responders and GMCs between groups.
This study, in agreement with data from other observational studies, shows that the immunological memory for HBsAg can persist beyond the time at which anti-HBs antibody disappears, and thus booster doses are not needed at this time to retain protection against acute disease and the development of the HBsAg carrier rate.Citation18-20 Recently Bruce et al.Citation21 showed a post-booster anamnestic response of 88% in vaccine recipients who lost anti-HBs antibody 30 y after primary vaccination. However, other studies, mainly performed in Asiatic countries, indicate that the immune memory tends to rapidly wane after the second decade post-vaccination.Citation22-26
Further long-term follow up studies are still needed to understand the meaning of the loss of post-booster anamnestic response in terms of reacquired susceptibility to HBV.
Material and methods
Study participants
Enrollment took place from 2010 to 2011. The study population consisted of a cohort of teenagers vaccinated as infants (Group A; median post-priming follow up: 19 years) and of a cohort of young adults vaccinated against hepatitis B as 12-year-old adolescents (Group B; median post-priming follow up: 18 years). According to the Italian schedules of vaccination, infants received 3 pediatric doses of monovalent recombinant hepatitis B vaccine (either Engerix-B or Recombivax HB) given at 3, 5 and 11 months of age, while adolescents were given 3 adult doses of the same 2 vaccines at 0, 1 and 6 months. In this study no comparison between vaccines was done since they were considered similarly safe and immunogenic and both recommended by our Ministry of Health.
For each participant, proper completion of the vaccination schedules was assessed by checking the vaccination registries or the individual vaccination certificates. At enrollment, all participants were in good health, none reported a history or presented signs or symptoms of hepatitis and none was born to an HBsAg positive mother. Exclusion criteria included congenital or acquired immune disorders, allergy to any component of the vaccine used for boosting and having received extra doses of hepatitis B vaccine after priming. This study was approved by the Ethic Committee of the University of Milan.
Procedures and definition
At enrollment, all vaccinees were tested for anti-HBs antibody concentration and the presence of antibody to hepatitis B core antigen (anti-HBc) using commercially available assays (AxSYM AUSAB and CORE, Abbott Park, IL, USA). Vaccinees found anti-HBc positive were further tested for hepatitis B surface antigen (HBsAg AxSYM), and for HBV DNA using the TaqMan HBV test (Roche, Branchburg, NJ, USA). As reported by the manufacturer's instructions, the quantitative measurement of AxSYM AUSAB assay was ranging between 2–1000 mIU/ml. Thus, to obtain the final concentration, samples with anti-HBs >1000 mIU/ml were diluted. According to an algorithm previously reported, individuals with anti-HBs ≥ 10 mIU/ml were considered immune, whereas those with antibody <10 mIU/ml were given an adult booster dose of vaccine (either Engerix B or HBVAXPRO) and retested 2 weeks later.Citation15 A rise in anti-HBs concentration to at least 10 mIU/ml was regarded as a positive anamnestic response. Vaccinees whose antibody level remained below 10 mIU/ml were offered 2 additional doses of vaccine at 1 and 6 months after booster, and retested 1–3 months after the third dose.
In this study, a benign breakthrough infection was defined as the presence of anti-HBc in absence of HBsAg and HBV DNA.
Statistical analysis
Anti-HBs concentrations were compared between groups by using the non-parametric Mann-Whitney U-test. In the case of undetectable concentration, an arbitrary value of 0.05 mIU/ml was assigned to allow the calculation of geometric mean concentrations (GMCs). Differences in frequency were detected with the chi-square test or Fisher exact test when necessary, and regarded p < 0.05 as significant. Ninety-five percent confidence intervals (95% CI) were also calculated if appropriate. All statistical analysis was conducted using STATA statistical software version 3.1.
Study Group
Enea Spada, Valeria Alfonsi (Istituto Superiore di Sanità, Roma); Anna Sallustio, Rossella Procacci (Dipartimento di Scienze Mediche e Oncologia Umana, Università di Bari); Giuseppina Masia, Angelo Meloni (Dipartimento di Sanità Pubblica, Medicina Clinica e Molecolare, Università di Cagliari); Silvana Lo Grande, Cantone Filippo, Vito Gonfalone (ASP Catania); Rosa Maria Consagra, Angela Russotto, Domenico Frangapani (AUSL1 Agrigento, Unità Operativa di Licata); Giovanna Ulivieri, Alessandra Nini (AUSL Cesena); Morena Maldini, Giuseppe Cafarelli, Antonio Giambersio (ASP Potenza); Rosa Alfieri, Milena Scotto di Santolo (ASL Napoli 2 Nord); Emanuela Zamparo (AAS5 Friuli Occidentale, Pordenone); Elena Cacello (Dipartimento di Scienze della Sanità Pubblica e Pediatriche, Università di Torino); Domenico Montu’ (ASL Cuneo 1, Saluzzo); Annamaria Belloni (ATS Milano Città Metropolitana, Lodi).
Abbreviations
| Anti-HBc | = | antibody to hepatitis B core antigen |
| Anti-HBs | = | antibody to hepatitis B surface antigen |
| GMC | = | geometric mean concentration |
| HBsAg | = | hepatitis B surface antigen |
| HBV | = | hepatitis B virus |
| HBV DNA | = | hepatitis B viral DNA |
| 95% CI | = | ninety-five percent confidence intervals |
Disclosure of potential conflicts of interest
All authors declare no potential conflict of interest relevant to this article.
Author contributions
All authors contributed to the collection, analysis, interpretation of data, and critical revision of the article. LR, MET, ARZ designed the study and wrote the final version of this paper.
Acknowledgments
The authors are grateful to all participants enrolled in the study.
Funding
This study was supported by the Italian Ministry of Health (Programma CCM 2010; Fasc. ISS 1M56) and by the Italian Ministry of Education, University and Research (PRIN 2008; prot: 20088HNWSP).
References
- World Health Organization (WHO). Available from http://www.who.int/mediacentre/factsheets/fs204/en/ (last access March 7, 2017).
- Zanetti AR, Tanzi E, Romanò L, Grappasonni I. Vaccination against hepatitis B: the Italian strategy. Vaccine 1993; 11:521-4; PMID:8488702; http://dx.doi.org/10.1016/0264-410X(93)90222-J
- Ministero della Salute. Available from http://www.salute.gov.it/imgs/C_17_tavole_20_allegati_iitemAllegati_0_fileAllegati_itemFile_3_file.pdf (last access March 7, 2017).
- Assad S, Francis A. Over a decade of experience with a yeast recombinant hepatitis B vaccine. Vaccine 1999; 18:57-67; PMID:10501235; http://dx.doi.org/10.1016/S0264-410X(99)00179-6
- Venters C, Graham W, Cassidy W. Recombivax-HB: perspectives past, present and future. Expert Rev Vaccines 2004; 3:119-29; PMID:15056038; http://dx.doi.org/10.1586/14760584.3.2.119
- Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine 2008; 26:6266-73; PMID:18848855; http://dx.doi.org/10.1016/j.vaccine.2008.09.056
- Van Damme P. Long-term protection after hepatitis B vaccine. J Infect Dis 2016; 214:1-3; PMID:26802140; http://dx.doi.org/10.1093/infdis/jiv750
- Lu JJ, Chen CC, Chou SM, Hor CB, Yang YC, Wang HL. Hepatitis B immunity in adolescents and necessity for boost vaccination: 23 years after nationwide hepatitis B virus vaccination program in Taiwan. Vaccine 2009; 27:6613-8; PMID:19698812; http://dx.doi.org/10.1016/j.vaccine.2009.08.007
- Mendy M, Peterson I, Hossin S, Peto T, Jobarteh ML, Jeng-Barry A, Sidibeh M, Jatta A, Moore SE, Hall AJ, et al. Observational study of vaccine efficacy 24 years after the start of hepatitis B vaccination in two Gambian villages: no need for a booster dose. PloS One 2013; 8:e58029; PMID:23533578; http://dx.doi.org/10.1371/journal.pone.0058029
- Ni YH, Chang MH, Wu JF, Hsu HY, Chen HL, Chen DS. Minimization of hepatitis B infection by a 25 year universal immunization program. J Hepatol 2012; 57:730-5; PMID:22668640; http://dx.doi.org/10.1016/j.jhep.2012.05.021
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Crasta PD, Messier M, Hardt K. Long-term anti-HBs antibody persistence following infant vaccination against hepatitis B and evaluation of anamnestic response: a 20-year follow-up study in Thailand. Hum Vaccin Immunother 2013; 9:1679-84; PMID:23732904; http://dx.doi.org/10.4161/hv.24844
- Spada E, Romanò L, Tosti ME, Zuccaro O, Paladini S, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, et al. Hepatitis B immunity in teenagers vaccinated as infants: an Italian 17-year follow-up study. Clin Microbiol Infect 2014; 20:1040-6; http://dx.doi.org/10.1111/1469-0691.12591
- Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis 2008; 197:1419-26; PMID:18444799; http://dx.doi.org/10.1086/587695
- Lin CC, Yang CY, Shih CT, Chen BH, Huang YL. Waning immunity and booster responses in nursing and medical technology students who had received plasma-derived or recombinant hepatitis B vaccine during infancy. Am J Infect Control 2011; 39:408-14; PMID:21255876; http://dx.doi.org/10.1016/j.ajic.2010.07.010
- Zanetti AR, Mariano A, Romanò L, D'Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS, et al. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet 2005; 366:1379-84; PMID:16226616; http://dx.doi.org/10.1016/S0140-6736(05)67568-X
- Hollinger FB. Factors influencing the immune response to hepatitis B vaccine, booster dose guidelines, and vaccine protocol recommendations. Am J Med 1989; 87:36S-40S; PMID:2528297; http://dx.doi.org/10.1016/0002-9343(89)90530-5
- Ryckman KK, Fielding K, Hill AV, Mendy M, Rayco-Solon P, Sirugo G, van der Sande MA, Waight P, Whittle HC, Hall AJ, et al. Host genetic factors and vaccine-induced immunity to HBV infection: haplotype analysis. PLoS ONE 2010; 5:e12273; PMID:20806065; http://dx.doi.org/10.1371/journal.pone.0012273
- But DY, Lai CL, Lim WL, Fung J, Wong DK, Yuen MF. Twenty-two years follow-up of a prospective randomized trial of hepatitis B vaccines without booster dose in children: final report. Vaccine 2008; 26:6587-91; PMID:18835318; http://dx.doi.org/10.1016/j.vaccine.2008.09.034
- McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis 2009; 200:1390-6; PMID:19785526; http://dx.doi.org/10.1086/606119
- Poovorawan Y, Chongsrisawat V, Theamboonlers A, Bock HL, Leyssen M, Jacquet JM. Persistence of antibodies and immune memory to hepatitis B vaccine 20 years after infant vaccination in Thailand. Vaccine 2010; 28:730-6; PMID:19892043; http://dx.doi.org/10.1016/j.vaccine.2009.10.074
- Bruce MG, Bruden D, Hurlburt D, Zanis C, Thompson G, Rea L, Toomey M, Townshend-Bulson L, Rudolph K, Bulkow L, et al. Antibody levels and protection after hepatitis B vaccine: results of a 30-year follow-up study and response to a booster dose. J Infect Dis 2016; 214:16-22; PMID:26802139; http://dx.doi.org/10.1093/infdis/jiv748
- Su FH, Cheng SH, Li CY, Chen JD, Hsiao CY, Chien CC, Yang YC, Hung HH, Chu FY. Hepatitis B seroprevalence and anamnestic response amongst Taiwanese young adults with full vaccination in infancy, 20 years subsequent to national hepatitis B vaccination. Vaccine 2007; 25:8085-8090; PMID:17920732; http://dx.doi.org/10.1016/j.vaccine.2007.09.013
- Bialek SR, Bower WA, Novak R, Helgenberger L, Auerbach SB, Williams IT, Bell BP. Persistence of protection against hepatitis B virus infection among adolescents vaccinated with recombinant hepatitis B vaccine beginning at birth: a 15-year follow-up study. Pediatr Infect Dis J 2008; 27:881-5; PMID:18756185; http://dx.doi.org/10.1097/INF.0b013e31817702ba
- Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology 2010; 51:1547-1554; PMID:20209603; http://dx.doi.org/10.1002/hep.23543
- Wu Q, Zhuang GH, Wang XL, Wang LR, Li N, Zhang M. Antibody levels and immune memory 23 years after primary plasma-derived hepatitis B vaccination: results of a randomized placebo-controlled trial cohort from China where endemicity is high. Vaccine 2011; 29:2302-7; PMID:21277403; http://dx.doi.org/10.1016/j.vaccine.2011.01.025
- Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology 2013; 57:37-45; PMID:22858989; http://dx.doi.org/10.1002/hep.25988