ABSTRACT
Streptococcus pneumoniae is the main causative organism of acute media otitis in children and meningitis and bacterial pneumonia in the community. Since 2008 in Tuscany, central Italy, the pneumococcal conjugate vaccine (7-valent vaccine, switched to 13-valent vaccine in 2010) was actively offered free of charge to all newborns. Aim of the study is to evaluate the impact of pneumococcal pediatric vaccination in the Tuscan population on hospitalizations potentially caused by S. pneumoniae, during pre-vaccination (PVP, 2002–2007) and vaccination period (VP, 2009–2014). We analyzed hospital discharge records (HDRs) of all hospitals in Tuscany from 2002 to 2014. Hospitalizations potentially due to pneumococcal diseases were 347, 221. The general hospitalization rate was 716/100,000 inhabitants during PVP and 753/100,000 in VP, with a decrease of 29.1% in the age-group 0–9 y (“target” of the vaccination program) and an increase of 75.7% in subjects >64 y of age. During VP, admission days and hospitalization costs increased (6.2% and 24.2%, respectively), especially in patients >64 y (12.9% and 33.8%, respectively); in children <10 y decreased by 21.2% and 12.8%, respectively. The pneumococcal pediatric vaccination resulted in the decrease of hospitalizations in younger but the expected indirect effect in the elderly was not reported, justifying the Tuscan recommendation to extend the vaccination to subjects > 64 y.
Introduction
Streptococcus pneumoniae (Sp) is a capsulated Gram positive diplococcus. The capsule of Sp is formed with a complex polysaccharide that determines the serological type and contributes to the germ virulence and pathogenicity. There are over 85 types of Sp. These bacteria are commonly found as commensals of the human respiratory tract, especially in winter and early spring, and are transmitted from person to person by droplets.
S. pneumoniae is an important human pathogen causing upper and lower respiratory tract infections (otitis, sinusitis and non bacteremic pneumonia) and invasive pneumococcal diseases (IPDs), such as bacteremic pneumonia, meningitis and sepsis. The worldwide annual rates of morbidity and mortality due to Sp are very high. Children under 5 y of age and elderly people are the age groups most affected by these diseases.Citation1-2
Today, pneumococcal disease remains one of the major public health problem worldwide,Citation3 although its epidemiology has dramatically changed, particularly among young children, after the introduction of pneumococcal vaccination into national pediatric immunization.Citation4-5
Two types of vaccines are currently available worldwide: the pure polysaccharide pneumococcal vaccine (PPV), recommended for adult immunization, and the pneumococcal conjugate vaccine (PCV), initially indicated for pediatric immunization and then extended to all ages.
The heptavalent pneumococcal conjugate Vaccine (PCV7) was implemented in USA in 2000, and has been effective in reducing IPD and non-invasive infections (pneumonia and otitis media, especially complicated cases) in all age groups, with an herd immunity effect on older unvaccinated subjects.Citation6
In Europe, PCV7 vaccine was licensed by the European Medicines Agency (EMEA) in February 2001, becoming available during the summer of the same year.
In the last years PCV7 was replaced by 13-valent pneumococcal conjugate vaccine (PCV13) in many countries, in order to avoid a likely increase of non-vaccine-serotype IPD cases due to serotype replacement.Citation7 In Italy, PCV7 vaccine was officially included in the National Vaccination Plan 2005–2007 and between 2006 and 2010, several of the 21 Italian Regions included pneumococcal vaccination in their pediatric immunization schedules.Citation8 In May 2010, the Italian Ministry of Health recommended to replace PCV7 with PCV13 in all the pediatric immunization programs.Citation9
Currently, PCV13 vaccine is actively offered, free of charge, to all newborns with a 3-doses schedule at 3, 5–6, and 11–13 months of age. In addition, in the switch period, children who had already received one or more doses of PCV7, completed the immunization schedule with additional doses of PCV13.Citation10-11
In Tuscany, a Region of Central Italy, universal vaccination of infants with PCV7 was implemented since 2008, followed by the switch to PCV13 in 2010.Citation12
Aim of this study is, therefore, to assess the impact of pediatric pneumococcal vaccination in Tuscany on the entire regional population in terms of hospitalisations potentially caused by S. pneumoniae. As a matter of fact, hospitalisations suggestive of diseases potentially due to pneumococcal infections, are the most reliable data source to assess the pneumococcal burden of disease in all age groups. Hospitalisations were analyzed and compared in 2 periods: the pre-vaccination (PVP, 2002–2007) and vaccination period (VP, 2009–2014).
Results
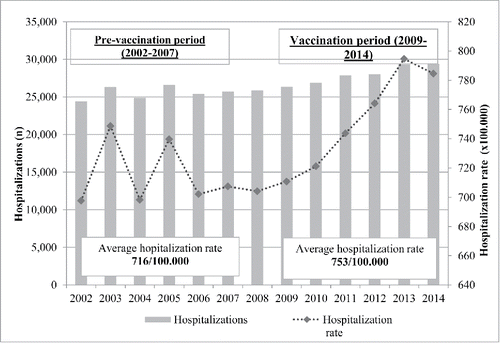
In the period 2002–2014, a total number of 347,221 hospitalizations potentially associated to pneumococcal diseases were reported in Tuscany: 153,394 hospitalizations in PVP and 167,935 hospitalizations in VP. The annual hospitalization rates throughout the whole period ranged between 697.7 hospitalizations/100,000 inhabitants in 2002 and 794.6 /100,000 in 2013, with a fluctuating trend between 2002 and 2006 and, lastly, with an increasing trend in the following years. The average hospitalization rate was 732.4/100,000 inhabitants in the overall period. In PVP the average annual rate of hospitalization was 715.6/100,000 while it was 753.1/100,000 in VP ().
Figure 1. Number of hospitalizations and hospitalization rate (x 100,000 inhabitants) potentially due to pneumococcal diseases in Tuscany (2002–2014).
In the age-group 0–9 y, the average annual hospitalization rate for the entire period was 989.2 hospitalizations/100,000 inhabitants, it shows a steady declining trend, from the highest value registered in 2002 (1,415.3/100,000) to the lowest value in 2014 (628/100,000).
In the age-group 10–64 y, the hospitalization rate remained constant in the 13-years period, with an average value of 221.7/100,000 inhabitants. In the age group > 65 y, instead, the average annual hospitalization rate was 2,134.7/100,000; it was slightly fluctuating between 2002 and 2006 and then it was steadily increasing, from 1,895.4/100,000 in 2002 to about 2,400 in 2013–2014.
These trends were also confirmed when hospitalizations were analyzed for smaller age-groups (data not shown). In particular, reductions of hospitalizations were observed in the youngest subjects, target of the immunization program, while in the adults a steady trend was reported and an increase of hospitalisations was noticed in older subjects, especially in 80–84 y and >84 y age groups.
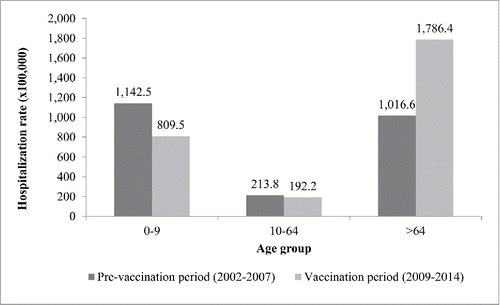
In the comparison beetween PVP and VP, the average annual hospitalization rate decreased by 29.1%, from 1,142.5/100,000 to 809.5/100,000 in the age-group 0–9 y, while in the age-group 10–64 y it decreased by 10.1%, from 213.8/100,000 to 192.2/100,000. At the same time, the average annual rate of hospitalization for the elderly was 1,016.6/100,000 in PVP and 1,786.4/100,000 in VP, resulting in a marked increase of 75.7% ().
Figure 2. Average annual rates of hospitalization potentially due to pneumococcal diseases, by age group, in PVP (2002–2007) and VP (2009–2014) in Tuscany.
As a matter of fact, in VP most hospitalizations (71.7%, corresponding to 120,406 HDR) occurred in subjects older than 64 y, while in PVP the percentage was lower (64.5%, equal to 98,896 HDR) in the same age group; 31,979 HDR (19.0%) occurred in patients aged 10–64 y versus 34,482 HDR (22.5%) in PVP and 15,550 HDR (9.3%) occurred in children aged 0–9 y vs. 20,016 HDR (13%) in PVP.
Over the years 2002–2014, an increase of hospitalizations as Ordinary admissions was observed. In the vaccination period (2009–2014), an increase of 12% was registered (from 145,311 HDR in PVP to 162,991 HDR in VP); in the same period, a decrease of 37% in DH hospitalizations was registered (5,060 in VP vs. 8,083 in PVP).
As regard to gender, the hospitalization number remained approximately stable until 2010 for both genders. Overall, during VP an increase of hospitalizations was observed in males as in females. In both periods, hospital admissions involved principally male subjects (65,165 men vs 62,491 women in the PVP; 85,074 men vs 82,861 women in VP), percentages remained stable over the years and without differences in the PVP and VP, 51% and 50.7%, respectively.
In general, most hospitalizations occurred in Italian citizens (96.4% in the whole period corresponding to 161,292 HDR in VP and 148,844 HDR in PVP). On the other hand, during VP an increase in hospital admission of foreign persons was also observed (4,862 in VP vs 3,245 in PVP, 2.9% and 2.1% respectively). Nationality was not available for a small percentage of cases (0.8% in PVP; 1.1% in VP) ().
Table 1. Type of hospitalization, gender and citizenship of hospitalized subjects in Tuscany, in PVP (2002–2007) and VP (2009–2014).
Table 2. ICD-9-CM diagnosis codes of potentially associated S. pneumoniae diseases selected for the analysis.
Trends in reduction of hospitalizations were observed in the 2002–2014 period especially for AOM, meningitis and pneumonia. In particular in 2014 the number of hospitalization due to AOM decreased by 63% compared with 2002. On the contrary, hospitalization due to septicemia showed an increasing trend in the entire period. (Data not showed)
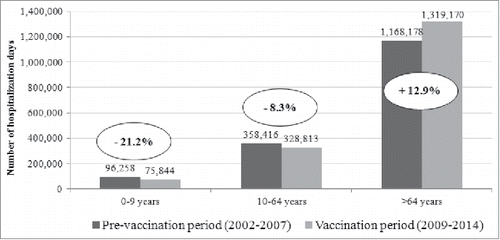
In the period 2002–2014, the total number of 347,221 hospitalizations accounted for 3,614,924 d of hospitalizations, with an average annual length of stay of 10.4 d (). In PVP, 1,622,852 d of hospitalization were recorded and in VP there were 1,723,827 d of hospitalization. No differences were observed in the LOS before and after the implementation of the pneumococcal vaccination program (10.6 d in PVP vs 10.3 d in VP). After the implementation of the pediatric pneumococcal immunization program the total number of hospitalization days increased in older subjects, meanwhile it decreased in younger age groups. Usually the length of stay of children is half the LOS of older subjects. Particularly, analyzing LOS broken down by age group, no differences were observed between the 2 periods: in particular, in the age group 0–9 y LOS was 4.8 d d vs 4.9 days, in the age group 10–64 y was 10.4 d vs 10.3 days, and in the >64 y it was 11.8 d vs 11.0 days, respectively in the PVP and VP.
Figure 3. Total days of hospitalization potentially due to pneumococcal diseases in Tuscany, by age, in PVP (2002–2007) and VP (2009–2014).
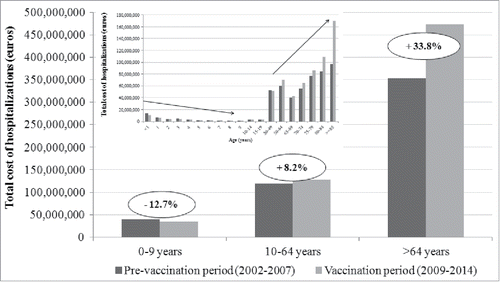
In the period 2002–2014, the total cost of hospital admissions for the included codes of hospitalization were EUR 1,239,588,279 with an average cost of EUR 3,570.03 for each hospitalization. In PVP the global costs of hospitalization was EUR 512,420,072, with a cost for each hospitalization of EUR 3340.55. In VP the total cost of hospitalization amounted to EUR 636,639,985 with a cost for each hospitalization EUR 3790.99. Our results showed an increase of hospitalization costs with aging of the patients: for subjects > 64 y the increase in cost of hospitalization was 33.8% in VP compared with PVP (from EUR 353,467,431 in PVP to EUR 473,053,447 in VP) (). Moreover, a slight increase of 8.2% in costs was observed in the age group 10–64 y (from EUR 118,729,705 in PVP to EUR 128,492,363 in VP). On the contrary, in the age group 0–9 y the total costs decreased in VP (12.7%; from EUR 40,222,936 in PVP to EUR 35,094,175).
Discussion and conclusions
Pneumococcal disease remains a major public health problem worldwide and community acquired pneumonia (CAP) is the largest cause of death from infectious disease in adults aged >65 y.
S. pneumoniae is the most frequently isolated pathogen in CAP cases in Europe (12–68% of cases) and it is also an important etiologic agent of pneumonia in residents of long-term care facilities.Citation13
Pneumococcus epidemiology has dramatically changed, particularly among young children, after the introduction of pneumococcal vaccines into national childhood immunization programs.
In Italy, PCV7 was introduced in 2005–2007 in the childhood immunization schedule and replaced in 2010 with PCV13. Since February 2012, PCV13 has been included in the list of Essential Health Interventions.Citation14 In Tuscany, universal infant vaccination with PCV7 was introduced in 2008 and replaced by PCV13 in 2010.Citation15
Literature data demonstrate the effectiveness of pediatric vaccination with PCV7 to provide a herd immunity effect in the elderly, with a concomitant reduction in hospitalization rates attributable to S. pneumoniae.Citation16-19 It is suggested that the herd immunity effect is to be related mainly to the reduction of the carriage status in the nasopharynx of children after pneumococcal vaccination. PCV7 produced a significant herd protection in unvaccinated adult population mostly because of pneumococcal carriage decrease in vaccinated children. In fact, in the UK, the use of PCV7 has mainly reduced vaccine-type (VT) related invasive pneumococcal diseases (98% in children under the age of 2 y and 81% in adults over 65 y), but an increase in non-vaccine-type (NVT) diseases was observed (68% in individuals younger than 2 y and 48% in those aged 65 y or older), giving an overall reduction in invasive pneumococcal disease of 56% in those younger than 2 y and 19% in those aged 65 y or older.Citation20-21 In Italy, serotypes causing IPD in adults are very rarely found in children nasopharyngeal swabs. Thus, pediatric vaccination with PCV13 could have had a very limited effect compared with PCV7 in creating the expected herd immunity to protect adults; currently, it seems unlikely that the vaccination of children with PCV13 could strongly influence the disease burden of adults by reducing carriage condition. Azzari et al.,Citation22-23 using the RT-PCR method implemented on naso-pharyngeal (NF) samples, found a low correlation between the serotypes responsible for IPD in adults and those in the NF of children in a recent observation period 2007–2014. The results of our study show that in children under 10 y, target of the pneumococcal immunization campaign, a reduction in related pneumococcal diseases was observed in the entire period (2002–2014), mainly due to the decrease in hospitalizations due to AOM. On the contrary, an increase in hospitalizations for potentially associated pneumococcal diseases in subjects aged >64 y was assessed in VP; in the same period, for the 10–64 age group the trend of hospitalizations have been almost stable over the years. Similarly, in VP the total number of admissions days increased (+12.9%) in patients aged >64 y and decreased (−21.2%) in the pediatric age group, mainly due to the implementation of the vaccination program. Moreover, our results demonstrated a certain impact on hospitalization costs. The only age group in which a reduction of hospitalization costs was registered was the 0–9 age group (−12.8%), while there was an increase of 33.8% and 8.2% of total costs, in patients aged >64 y and 10–64 y, respectively. Similarly to our findings, other studies, performed in Wales and England, demonstrated that few years after PCV13 implementation a reduction of 32% of VT IPD was observed, but this decrease did not generate a high impact on the general pneumococcal disease incidence (VT and NVT) due to an increase of NVT diseases.Citation20,21 The increased incidence of pneumococcal disease could be related to the rise of the hospitalization rate observed in the older age groups (adult and the elderly), also demonstrated by Amodio at al.,Citation17 for specified pneumococcal pneumonia and by Azzari e al.,Citation23 revealing a progressive increase in the incidence of IPD in adults, particularly in the elderly, aged 61–75 y. Probably, the highest incidence of the disease in the elderly population must be related to a higher prevalence of co-morbidity in this subjects rather than in younger age groups and to the influence of other factors, such as lifestyle or confounding factors (eg, smoking prevalence in the general population, influenza vaccination coverage).Citation17 According to Bechini et al.,Citation15 hospitalization rates increased with increasing age and the highest number of hospitalizations occurred in subjects aged 85 y and over. Indeed, age may be considered the first risk-factor for acquiring pneumococcal diseases. In 2012, in the USA the highest incidence rates of IPD were observed in subjects over 65 y.Citation24
It is necessary to consider that the distribution of serotypes is not constant over time but tends to change; to date, the most frequent serotypes of IPD in adults in Italy are rarely present in the NF of children.Citation23 Recent studies have shown that PCV is not able to eliminate the carriage condition probably because of the physiological decrease of antibody titers, which remain sufficiently high to prevent invasive infections but is not sufficiently high to prevent the carriage.Citation23 Pneumococcal diseases in Italy are not subject to mandatory notification, the most reliable source for assessing the burden of pneumococcal disease. As a consequence, there are few epidemiological available data on the serotype distribution of pneumococcus in Italy, in particular for the elderly, first for the poor attitude of practitioners to investigate the etiology and second for the heterogeneity of clinical presentation of pneumococcal disease.
An active surveillance system of S. pneumoniae diseases with the inclusion of bio-molecular test, as RT-PCR to be compared with results of blood cultures, is the key to assess the effectiveness of the PCV13 vaccine in the elderly. The evaluation of pneumococcal serotypes distribution in the elderly will help to establish if serotypes in this population are the same as in the pediatric population or different and to assess whether the elderly could be indirectly protected by children PCV vaccination program, implemented in Tuscany since 2008, or if they need a direct protection through a targeted vaccination program.Citation15 Limitation of our study could be traced in the uncertainty of the etiology of the diseases which required hospitalization; in this way it is difficult to attribute to pneumococcal disease the increasing trends in the hospitalization of the elderly in Tuscany. However HDR could be a reliable proxy of the circulation of S. pneumoniae in the Italian population. Currently, only few Italian Regions have implemented a specific immunization program with PCV13 for one or more cohorts of elderly people, the majority of the Regions have adopted a risk based strategy for preventing pneumococcal diseases in adults.Citation15 In Tuscany a mono-cohort vaccination strategy (65 y old subjects) was adopted in 2015 in order to directly protect the elderly.Citation12
Our results suggest the need for a direct protection with PCV13 in the elderly and confirm the recent recommendation introduced in the Tuscany Region to offer vaccination to 65 y old people, as already confirmed in other studies.Citation17 Direct protection of the elderly is the best strategy to reduce hospitalizations related to pneumococcal diseases, particularly in case of pneumonia.
Material and method
Hospital discharge records (HDRs) of all hospitalizations occurred in Tuscany in the period 2002–2014 were analyzed in order to assess the impact of the pediatric pneumococcal vaccination on hospitalizations potentially associated to S. pneumoniae. Information about HDRs, related-costs and length of stay were obtained anonymously and aggregated from the regional archives.
A selection of records was based on the appearance of specific International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes in any of the 6 discharge diagnosis fields (primary and secondary diagnoses). Hospitalizations potentially associated with S. pneumoniae were identified by selecting all HDRs in which any of the following ICD-9-CM code was reported at discharge as primary or secondary diagnosis (): pneumonia (480–487.0), meningitis (320, 320.1, 320.2, 320.7, 320.8, 320.9, 322, 322.9), otitis (382, 382.0, 382.9), acute pericarditis (420, 420.0, 420.9, 420.90, 420.91, 420.99), acute myocarditis (422, 422.0, 422.9, 422.90, 422.91, 422.92), acute bronchitis (466.0), acute pleuritis (511.0, 511.1), pneumococcal peritonitis (567.1), pyogenic arthritis (711.0), urinary tract infection unspecified (599.0); streptococcal or pneumococcal infection in other conditions (041.0, 041.2). Bacteremia was defined as a record containing a code for septicemia (038, 038.9), streptococcal or pneumococcal septicemia (038.0, 038.2) and bacteremia (790.7). Each discharge record is a single hospitalization event. Consequently, it is possible that some subjects have had multiple hospitalizations in the period of observation, according to the anonymous status.
Demographic information (age, gender), days and costs of hospitalization were collected for each observation year. Data were analyzed by age group: 0–9 y (population target of vaccination program), 10–64 y and subjects > 64 y of age. Hospitalizations in ordinary admission and in Day-Hospital were included in the analysis. The analysis on the number of hospitalizations was performed in the pre-vaccination period (PVP, 2002–2007) and the vaccination period (VP, 2009–2014), excluding 2008, year of first implementation of pneumococcal vaccination in the regional immunization program.
Total costs and length of hospital stay (LOS) by year and age group were also calculated. LOS- was defined as the time between hospital admission and discharge from the hospital or in-hospital death.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- File TM Jr., Marrie TJ. Burden of community acquired pneumonia in North American adults. Postgrad Med 2010; 122:130-4; http://dx.doi.org/10.3810/pgm.2010.03.2130
- Pneumococcal conjugate vaccine for childhood immunization-WHO position paper. Wkly Epidemiol Rec 2007; 82:93-104; PMID:17380597
- Prato R, Tafuri S, Fortunato F, Martinelli D. Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin 2010; 6:91821; http://dx.doi.org/10.4161/hv.6.11.13352
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction–eight states, 1998–2005. MMWR Morb Mortal Wkly Rep 2008; 57:144-8; PMID:18272956
- Weil-Olivier C, van der Linden M, de Schutter I, Dagan R, Mantovani L. Prevention of pneumococcal diseases in the post-seven valent vaccine era: a European perspective. BMC Infect Dis 2012; 12:207; PMID:22954038; http://dx.doi.org/10.1186/1471-2334-12-207
- Centers for Disease Control and Prevention (CDC). Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine - United States, 2007. MMWR Morb Mortal Wkly Rep 2010; 59:253-7; PMID:20224541
- Pichon B, Ladhani SN, Slack MP, Segonds-Pichon A, Andrews NJ, Waight PA, Miller E, George R. Changes in molecular epidemiology of streptococcus pneumonia causing meningitis following introduction of pneumococcal conjugate vaccination in England and Wales. J Clin Microbiol 2013; 51:8207; http://dx.doi.org/10.1128/JCM.01917-12
- Alfonsi V, D'Ancona F, Giambi C, Nacca G, Rota MC. Regional coordinators for infectious diseases and vaccinations. Current immunization policies for pneumococcal, meningococcal C, varicella and rotavirus vaccinations in Italy. Health Policy 2011; 103:176-83; PMID:22030308; http://dx.doi.org/10.1016/j.healthpol.2011.10.002
- Ministero della Salute. Italia. Indicazioni in merito alla somministrazione del vaccino antipneumococcico Prevenar 13 in età pediatrica. Circolare del 27 Maggio 2010. prot.n. 111432/72AF del 03/06/2010. [Italian] Available from: http://www.fimpcalabria.org/public/vaccinazioni/indicazioni%20in%20merito%20alla%20somministrazione%20del%20vaccino%20antipneumococcico%20prevear%2013%20in%20et%C3%A0%20pediatrica%20%282%29.pdf. Accessed 19 November 2015.
- Ministero della Salute. Piano Nazionale Prevenzione Vaccinale (PNPV) 2012–14. Gazzetta Ufficiale n. 60 del 12.03.2012 (Supplemento Ordinario n.47). [Italian]. Available from: http://www.salute.gov.it/imgs/c_17_pubblicazioni_1721_allegato.pdf
- WHO Publication. Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine 2012; 30:4717-8; PMID:22621828; http://dx.doi.org/10.1016/j.vaccine.2012.04.093
- Tuscany Region. Regional immunization schedule. [Italian] Available from: http://www301.regione.toscana.it/bancadati/atti/Contenuto.xml?id = 5099142&nomeFile = Delibera_n.571_del_27-04-2015-Allegato-1
- Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012; 67:71-9; PMID:20729232; http://dx.doi.org/10.1136/thx.2009.129502
- Martinelli D, Pedalino B, Cappelli MG, Caputi G, Sallustio A, Fortunato F, Tafuri S, Cozza V, Germinario C, Chironna M, et al. Towards the 13-valent pneumococcal conjugate universal vaccination: effectiveness in the transition era between PCV7 and PCV13 in Italy, 2010–2013. Hum Vaccin Immunother 2014; 10(1):33-9; PMID:24096297; http://dx.doi.org/10.4161/hv.26650
- Bechini A, Taddei C, Barchielli A, Levi M, Tiscione E, Santini MG, Niccolini F, Mechi MT, Panatto D, Amicizia D, et al. A retrospective analysis of hospital discharge records for S. pneumoniae diseases in the elderly population of Florence, Italy, 2010–2012. Hum Vaccin Immunother 2015; 11(1):156-65; PMID:25483529; http://dx.doi.org/10.4161/hv.34418
- Fortunato F, Martinelli D, Cappelli MG, Cozza V, Prato R. Impact of pneumococcal conjugate universal routine vaccination on pneumococcal disease in Italian children. J Immunol Res 2015; 2015:206757; PMID:26351644; http://dx.doi.org/10.1155/2015/206757
- Amodio E, Costantino C, Boccalini S, Tramuto F, Maida CM, Vitale F. Estimating the burden of hospitalization for pneumococcal pneumonia in a general population aged 50 years or older and implications for vaccination strategies. Hum Vaccin Immunother 2014; 10(5):1337-42; PMID:24577505; http://dx.doi.org/10.4161/hv.27947
- Durando P, Faust SN, Fletcher M, Krizova P, Torres A, Welte T. Experience with pneumococcal polysaccharide conjugate vaccine (conjugated to CRM197 carrier protein) in children and adults. Clin Microbiol Infect 2013; 19(Suppl 1):1-9; PMID:24083785; http://dx.doi.org/10.1111/1469-0691.12320
- Durando P, Crovari P, Ansaldi F, Sticchi L, Sticchi C, Turello V, Marensi L, Giacchino R, Timitilli A, Carloni R, et al. Collaborative Group for Pneumococcal Vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine 2009; 27:3459-62; PMID:19200823; http://dx.doi.org/10.1016/j.vaccine.2009.01.052
- Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugatevaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011 Oct; 11(10):760-8; PMID:21621466; http://dx.doi.org/10.1016/S1473-3099(11)70090-1
- Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis 2015 May; 15(5):535-43; PMID:25801458; http://dx.doi.org/10.1016/S1473-3099(15)70044-7
- Pasinato A, Indolfi G, Marchisio P, Valleriani C, Cortimiglia M, Spanevello V, Chiamenti G, Buzzetti R, Resti M, Azzari C. Italian group for the study of bacterial nasopharyngeal carriage in children. Pneumococcal serotype distribution in 1315 nasopharyngeal swabs from a highly vaccinated cohort of Italian children as detected by RT-PCR. Vaccine 2014; 32:1375-81; PMID:24486364; http://dx.doi.org/10.1016/j.vaccine.2014.01.023
- Azzari C, Cortimiglia M, Nieddu F, Moriondo M, Indolfi G, Mattei R, Zuliani M, Adriani B, Degl'Innocenti R, Consales G, et al. Pneumococcal serotype distribution in adults with invasive disease and in carrier children in Italy: Should we expect herd protection of adults through infants' vaccination? Hum Vaccin Immunother 2016; 12(2):344-50.
- Zhou F, Kyaw MH, Shefer A, Winston CA, Nuorti JP. Health care utilization for pneumonia in young children after routine pneumococcal conjugate vaccine use in the United States. Arch Pediatr Adolesc Med 2007; 161:1162-8; PMID:18056561; http://dx.doi.org/10.1001/archpedi.161.12.1162