ABSTRACT
The aim of the study was to evaluate if and how varicella prevalence has changed in Italy. In particular a seroprevalence study was performed, comparing it to similar surveys conducted in pre-immunization era. During 2013–2014, sera obtained from blood samples taken for diagnostic purposes or routine investigations were collected in collaboration with at least one laboratory/center for each region, following the approval of the Ethics Committee. Data were stratified by sex and age. All samples were processed in a national reference laboratory by an immunoassay with high sensitivity and specificity. Statutory notifications, national hospital discharge database and mortality data related to VZV infection were analyzed as well. A total of 3707 sera were collected and tested. In the studied period both incidence and hospitalization rates decreased and about 5 deaths per year have been registered. The seroprevalence decreased in the first year of life in subjects passively protected by their mother, followed by an increase in the following age classes. The overall antibody prevalence was 84%. The comparison with surveys conducted with the same methodology in 1996–1997 and 2003–2004 showed significant differences in age groups 1–19 y. The study confirms that in Italy VZV infection typically occurs in children. The impact of varicella on Italian population is changing. The comparison between studies performed in different periods shows a significant increase of seropositivity in age class 1 – 4 years, expression of vaccine interventions already adopted in some regions.
Introduction
Worldwide, varicella (chickenpox) is an endemic, highly contagious, disease caused by a Herpesvirus called varicella-zoster virus (VZV).Citation1 In temperate climates, at least 90% of the population acquires the disease within 15 y of age (95% in adulthood).Citation2 In tropical countries, chickenpox has a lower incidence; primary infection is less frequent in children and mainly involves adult age groups, as a result of climatic factors that play a role in influencing the spreading of the virus.Citation3 Primary infection usually elicits a long lasting immune response which, anyway, cannot avoid neither virus' latency nor its subsequent reactivation after many years. Virus' reactivation is linked to a decrease of VZV-specific cell-mediated response.Citation4
From the clinical point of view, varicella is often considered a benign disease. However, in 2–6% of cases some complications can occur. These latter are classified as infectious (cutaneous and soft tissue super-infections, bacterial pneumonia, etc.), neurological (cerebellitis, encephalitis, meningitis, etc.) and haematological (anemia, thrombocytopenic autoimmune purpura, etc.).Citation5
The majority of complications and hospitalizations due to chickenpox occurs in immunologically healthy children without any co-morbidity.Citation6 However, the severity of primary infection tends to increase with the age of the patient; the most severe clinical manifestations of the disease involve teenagers, adults and immunocompromised patients.Citation7 Several European studies show an incidence of hospitalization ranging between 1.3 and 4.5 for 100,000 inhabitants/year and 12.9–28.0/100,000 children ≤ 16 y of age/year, with an average length of stay ranging from 3 to 8 d.Citation8
VZV infection in a susceptible pregnant woman is particularly worrying. The incidence of VZV in pregnancy is estimated equal to 1–7/10,000 pregnancies.Citation9 The virus acquired during the last trimester may result in an increased risk of pneumonia for the pregnant woman. Besides, VZV may infect the fetus or the newborn during the intrauterine stage (congenital infection), during labor (perinatal infection) or after birth (postnatal infection). The vertical transmission of the pathogen can imply fetal death, abortion, premature birth, intrauterine growth retardation, and different defects, already evident at birth or, more frequently, occurring as sequelae.Citation10
Nowadays, chickenpox is the most widespread vaccine-preventable infectious disease. In industrialized countries with temperate climate, VZV infection in the absence of vaccination is acquired by more than 90% of subjects before adolescence and less than 5% of adults remains susceptible.Citation11 This means that each year the number of new cases approximates that of a birth cohort. According to the World Health Organization (WHO), the worldwide burden of varicella is estimated to be approximately 140 million cases with 4,200,000 severe complications that require hospitalization and 4200 deaths.Citation12
Surveillance systems in different European countries are very heterogeneous and sometimes absent; for these reasons, underestimation of cases is considerable. VZV spreads widely in Europe and in most countries the infection is acquired between 2 and 10 y of age, as demonstrated by seroprevalence studies.Citation5 The different social and educational structure of each country could explain differences, even significant, in the spread of the virus.Citation13
As a result of the healthcare and economic impact of varicella, many countries have introduced immunization in infancy, using commercially available vaccines (monovalent varicella (V) or quadrivalent Measles-Mumps-Rubella-Varicella (MMRV)). All varicella vaccines are effective, safe and imply high seroconversion rates (about 90% and 99% of immunized children develop a virus-specific protective antibody titer after the first and second dose, respectively).Citation12 Accordingly to the National Vaccination Plan (PNPV) 2012–2014, in Italy universal vaccination for varicella should have been adopted in all regions in 2015 starting from the 2014 birth cohort.Citation14 Noteworthy, due to the decentralization of the Italian Health Service, since many years 8 regions (so called “pilot” regions) already started universal varicella vaccination (UVI), administering 2 doses of vaccine, the first at 13–15 months of age and the second one at 5–6 y of age.
The European Center for Disease Prevention and Control (ECDC) recently published a document on varicella vaccination in Europe recommending to implement epidemiological surveillance. Seroepidemiological surveys are indicated as an appropriate method to monitor both the spreading of the pathogen and the impact of already implemented vaccine strategies.Citation5
The current seroepidemiological study aims, beside to update the knowledge on the spreading of the virus in Italy, to verify if and what changes have occurred over the years, taking account of similar surveys previously performed. Besides, an overview of the national burden of varicella has been evaluated taking into account data of already implemented surveillance systems.
Methods
A national cross-sectional population-based seroprevalence study of varicella antibodies was conducted.
The study was approved by the Ethics Committee of the Istituto Superiore di Sanità (ISS) in compliance with current regulations on the protection of personal data.Citation15 Anonymous unlinked samples of residual sera from routine laboratory testing, provided by a reference laboratory in each region, were collected. Samples from individuals known to have an immunosuppressive or acute infectious disease and those from individuals who had recently undergone a blood transfusion were excluded. All individuals who provided serum samples gave verbal informed consent; consent for minors was provided by parents or legal guardians.
The number of sera to be collected for each age group () was calculated using estimated antibody prevalence in that age-group obtained from a previous serology study. A minimum number of 3275 serum samples, equally distributed by gender, were to be collected.
Table 1. Estimated number of sera to collect per age-group.
Sera were stored at −20°C until tested by the laboratory of the University of Salento, Lecce, for varicella antibodies. Anti-VZV specific antibodies were detected using a commercially available enzyme linked immunoassay (ELISA) (Enzygnost anti-VZV/IgG, Siemens HealthCare Diagnostics GmbH), which according to the manufacturer has a sensitivity of 99.3% and a specificity of 100%. Sera were classified as negative if the optical density (OD) was less than 0.100 and as positive if higher than 0.200; sera with an OD reproducibly between 0.100 and 0.200 were classified as equivocal. The antibody status, expressed in mIU/ml, was based on the WHO International Standard for Varicella-Zoster Immunoglobulin (50 international units, IU).
Data were summarized as frequencies and positive antibody titres presented as geometric means along with their respective 95% confidence intervals (CI). Data were stratified by age, gender and geographic area. Differences among percentages of seropositive subjects were evaluated by Chi-square test while differences among geometric mean titers (GMTs) were performed using Student t-test on previously logarithmically transformed antibody titres. All statistical analysis were performed by Stata software version 11.2 (Stata Corporation, College Station, Texas, USA).
Varicella statutory notifications and national hospital discharge database for the period 2000–2014 were also analyzed. Varicella mortality data for the years 2001–2003 and 2006–2012 were obtained from the ISTAT database of the causes of mortality (data for the years 2004 and 2005 has never been released at national level).
Results
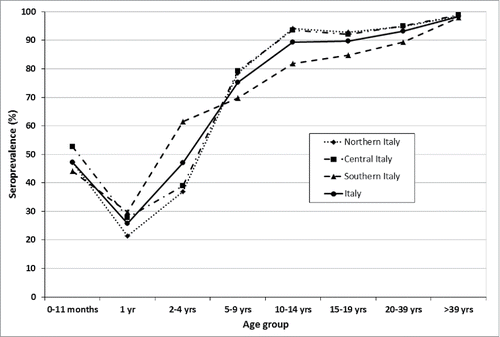
Overall, 3707 samples were collected (from January 2013 to December 2014) and analyzed; 3058 were positive and 583 negative. The remaining 66 sera, confirmed as equivocal, were excluded from the analysis. Seroprevalence showed a typical trend with a decrease in the first year of life in subjects first passively protected by their mother; then it increased in the following age classes (0–11 months: 47.2%; 1 year-old: 25.8%; 2–4 years: 47.0%; 5–9 years: 75.3%; 10–14 years: 89.4%; 15–19 years: 89.8%; 20–39 years: 93.2% and >40 years: 98.4%). Overall seroprevalence was equal to 84.0%, without any statistically significant difference by gender (p = 0.473); however, it showed a significant difference among the 3 Italian geographic areas (North 84.9%, Center 87.3%, and South 80.6%; p < 0.001). Anyway, significant differences were observed only in some age groups: in the age class 2–4 y seroprevalence was lower in Northern and Central Italy than in Southern Italy (p = 0.0003), while it was higher than in Southern Italy in the age groups 10–14 (p = 0.0001), 15–19 (p = 0.017) and 20–39 y of age (p = 0.005) ().
A significant increase in titres was observed when comparing consecutive age-groups GMTs from 1 y to 14 y of age (1 y vs 2–4 years: p = 0.011; 2–4 y vs 5–9 years: p < 0.001; 5–9 y vs 10–14 years: p < 0.015), while comparison in other age groups were not significantly different (except for 20–39 y vs >40 years: p = 0.0002). No differences in GMTs were detected between males and females. Significant differences were detected among geographic areas, being GMTs lower in Southern Italy (p < 0.001).
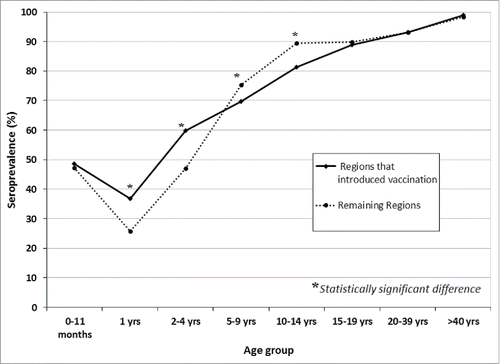
Comparing overall seroprevalence observed in regions that have not yet implemented (or have done it very recently) universal varicella immunization with seroprevalence found in the 8 “pilot” regions (Sicily, Apulia, Veneto, Tuscany, Calabria, Sardinia, Basilicata, Autonomous Province of Bolzano) that have adopted immunization since some years, no statistically significant difference was detected (p = 0.853). However, seroprevalence was significantly higher in the age classes from 1 y to 2–4 y (1 year: p = 0.045; 2–4 years: p = 0.0009;) in the “pilot” regions that first introduced universal varicella immunization, while in 5–9 and 10–14 y age groups, seroprevalence was significantly higher in the remaining regions (5–9 years: p = 0.023 and 10–14 years: p < 0.0001) ().
Figure 2. VZV seroprevalence in Italy, 2013–2014. Comparison between “pilot” vs “not pilot” Regions.
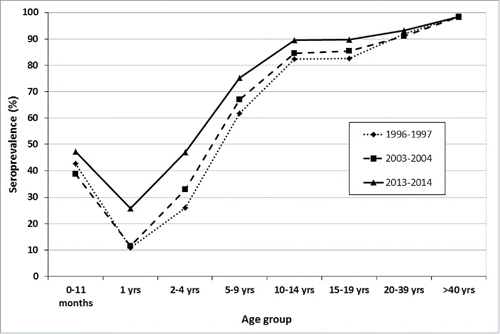
In 2013–2014 study overall seroprevalence (84.0%) was higher than those observed in 2 previous studies performed with the same methodology in 1996–1997 (73.2%) and 2003–2004 (77.8%) ().Citation16,17 More in detail, seroprevalence observed in the present study was significantly greater in comparison to the ones previously observed in the age classes from 1 to 15–19 y of age. Starting from the 20–39 age class, no statistically significant differences were detected among the 3 studies.
Figure 3. Comparison between VZV seroprevalence stratified by age class, Italy 1996–1997, 2003–2004 and 2013–2014.
The implementation of UVI in some regions had probably an impact on national epidemiology. The average annual number of cases in Italy, in the period 2003–2015, was equal to 72,854 cases (range: 34,955–123,264) with an average annual incidence of 129.4 cases per 100,000 population. The annual incidence rate reached a peak in 2004 with an incidence of 214.4 (per 100,000 population) and then steadily declined, reaching 77.3 (per 100,000 population) in 2014.
Overall, in the period 2001–2014, 24,192 hospitalizations for varicella were observed. The mean annual number of hospitalizations in Italy was 1,728 (range: 1,126–2,397) and the average annual incidence was 3 per 100,000 population, with a decreasing trend very similar to that shown by statutory notifications. Incidence of hospitalizations reached a maximum of 4.2 per 100,000 in 2002 and 2004 and a minimum of 1.9 per 100,000 in 2013 and 2014.
About 70% of the total hospitalizations due to varicella involved children (0–14 age group). Hospitalization rate was 42.9 per 100,000 population in children under one year of age, 26.6 in the 1–4 age group and 6.8 in the 5–14 age group. Mortality data showed an average of 4 and 6 deaths in the periods 2001–2010 and 2011–2012, respectively.
Discussion
Universal vaccination against varicella, already adopted in many countries during childhood with the administration of 2 doses, allows to achieve significant results in terms of reduction of the virus spreading, impact on complications and hospitalizations, protection of high-risk subjects and disease related costs' savings.Citation18
Essential prerequisite for the achievement of positive results is the early attainment and maintenance of high vaccine coverage rates to avoid indirect effects (such as the increase of the mean average age of infection and a higher risk of serious complications in older susceptible subjects that become infected).Citation19
In Italy varicella has been and still is the most relevant vaccine preventable infectious disease. For many years, notwithstanding the availability of vaccines, varicella immunization has not been recommended nor implemented at national level. The last and still ongoing National Immunization Plan 2012–2014 did not include universal varicella immunization and provided vaccination for anamnestically negative and unimmunized subjects with a 2-dose schedule. Interestingly, the same National Plan says that universal vaccination for chickenpox should have been adopted in all regions in 2015 starting from the 2014 birth cohort.Citation14 Anyway, even if the immunization for chickenpox was not planned at national level until 2015, starting from 2003, 8 regions (“pilot” regions) out of 21, have progressively offered universal varicella vaccination, administering 2 doses of vaccine in children aged 13–15 months and 5–6 y respectively. Despite the limitations of passive surveillance systems, the nationwide trend of notifications and hospitalizations from 2005 showed a progressive decrease that could be at least partly related to the impact of vaccine interventions implemented in the above mentioned regions.Citation20,21
The effectiveness of immunization has also been demonstrated in a previous research where the changes in the seroepidemiological pattern were assessed in the same 8 “pilot” regions. The increase in seroprevalence was most evident in the age groups target of immunization.Citation22
The data of the present study confirm that the impact of varicella on Italian population is changing. As a matter of fact, in comparison to data obtained in 1996–1997 and 2003–2004 the rate of seropositivity significantly increased in the age groups 1 and 2–4 y highlighting the crucial role played by immunization interventions already implemented in 8 “pilot” regions which, in terms of residents, represent the 37.2% of the Italian population. In the age group 5–19 y seroprevalence was higher in the remaining regions probably because of high circulation of varicella virus in absence of universal immunization strategy.Citation22
We must consider that the “late adopters” regions (that is, regions that have not yet started UVI) account for about 1/2 of Italian population. For this reason the active offer of varicella vaccine in all Italian regions and the catch up programmes for susceptible cohorts should be encouraged.
The continuous updating of epidemiological data, both in terms of disease impact (notification of cases, complications, hospitalizations, and deaths) as well as seroprevalence changes due to program implementation of immunization strategies, is crucial for the overall assessment of the impact of vaccination against chickenpox.
Abbreviations
| CI | = | confidence intervals |
| ECDC | = | European Center for Disease Prevention and Control |
| ELISA | = | enzyme linked immunoassay |
| GMTs | = | geometric mean titers |
| ISS | = | Istituto Superiore di Sanità |
| IU | = | international units |
| MMRV | = | quadrivalent Measles-Mumps-Rubella-Varicella vaccine |
| OD | = | optical density |
| PNPV | = | Italian National Vaccination Plan |
| UVI | = | universal varicella immunization |
| VZV | = | varicella-zoster virus |
| WHO | = | World Health Organization |
Disclosure of potential conflicts of interest
Giovanni Gabutti received grants from Sanofi Pasteur MSD, GSK Biologicals SA, Novartis, Pfizer and Sequirus for taking part to advisory boards, expert meetings, for acting as speaker and/or organizer of meetings/congresses and as principal investigator and chief of O.U. in RCTs.
Antonella De Donno, Parvanè Kuhdari, Marcello Guido, Maria Cristina Rota, Antonino Bella, Giordana Brignole, Silvia Lupi, Adele Idolo, Armando Stefanati, Martina Del Manso report no potential conflicts of interest.
Funding
The seroepidemiological part of the study was supported by the Ministry of Education, University and Research (MIUR), Project of Relevant National Interest (PRIN) 2009: Epidemiological, clinical, and pharmacoeconomic aspects of Varicella and Zoster; evaluation of the impact of vaccination against Varicella and future perspectives, no. 2009ZPM4×4_008 in the framework of “New technologies and new applications of vaccines. Impact assessment of vaccines recently introduced in prevention plans and future regional” project.
National Archive of SDO data, Ministry of Health, General Directorate of Healthcare Planning, VI Office.
References
- Whitley RJ. Varicella-zoster virus. In: Mandell L, Bennet JE, Dolin R, eds. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 7th ed. Philadelphia: Churchill Livingstone Elsevier, 2010.
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost -effectiveness of vaccination in England and Wales. Vaccine 2001; 19:3076-90; PMID:11312002; http://dx.doi.org/10.1016/S0264-410X(01)00044-5
- Nichols RA, Averbeck KT, Poulsen AG, al Bassam MM, Cabral F, Aaby P, Breuer J. Household size is critical to varicella-zoster virus transmission in the tropics despite lower viral infectivity. Epidemics 2011; 3:12-8; PMID:21420656; http://dx.doi.org/10.1016/j.epidem.2010.11.003
- Arvin A, Abendroth A. VZV: immunobiology and host response. In: Arvin A, Campadelli-Fiume G, Mocarski E, Moore P S, Roizman B, Whitleyet R, et al. eds. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge: Cambridge University Press, 2007.
- European Centre for Disease Prevention and Control. Varicella vaccine in the European Union. Stockholm: ECDC, 2014.
- Bonanni P, Breuer J, Gershon A, Gershon M, Hryniewicz W, Papaevangelou V, Rentier B, Rümke H, Sadzot-Delvaux C, Senterre J, et al. Varicella vaccination in Europe – Taking the practical approach. BMC Medicine 2009; 7:26; PMID:19476611; http://dx.doi.org/10.1186/1741-7015-7-26
- Ziebold C, Von Kries R, Lang R, Weigl J, Schmitt HJ. Severe complication of varicella in previously healthy children in Germany: 1 year survey. Pediatrics 2001; 108:E79; PMID:11694663; http://dx.doi.org/10.1542/peds.108.1.79
- Bonhoeffer J, Baer G, Muehleisen B, Aebi C, Nadal D, Schaad UB, Heininger U. Prospective surveillance of 1534 hospitalisations associated with varicella - zoster virus infections in children and adolescents. Eur J Pediatr 2005; 164:366-70; PMID:15747132; http://dx.doi.org/10.1007/s00431-005-1637-8
- Zhang HJ, Patenaude V, Abenhaim HA. Maternal outcomes in pregnancies affected by varicella zoster virus infections: population-based study on 7.7 million pregnancy admissions. J Obstet Gynaecol Res 2015; 41:62-8; PMID:25164540; http://dx.doi.org/10.1111/jog.12479
- Lamont RF, Sobel JD, Carrington D, Mazaki-Tovi S, Kusanovic JP, Vaisbuch E, Romero R. Varicella-zoster virus (chickenpox) infection in pregnancy. BJOG 2011; 118:1155-62; PMID:21585641; http://dx.doi.org/10.1111/j.1471-0528.2011.02983.x
- Centers for Disease Control and Prevention. Varicella (Yellow Book) 2016. Available from: http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/varicella-chickenpox.
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014. Weekly epidemiological record 2014; 25:265-88.
- Melegaro A, Jit M, Gay N, Zagheni E, Edmunds WJ. What types of contacts are important for the spread of infections?: using contact survey data to explore European mixing patterns. Epidemics 2011; 3:143-51; PMID:22094337; http://dx.doi.org/10.1016/j.epidem.2011.04.001
- Conferenza Permanente per i Rapporti tra lo Stato, le Regioni e le Province Autonome di Trento e Bolzano - Intesa 22 febbraio 2012 “Piano Nazionale Prevenzione Vaccinale 2012–2014.” Repertorio atti n. 54/CSR. Gazzetta Ufficiale n. 60 del 12-3-2012 - Suppl. Ordinario n. 47.
- Deliberazione n.85. Autorizzazione generale al trattamento di dati personali effettuato per scopi di ricerca scientifica–1 marzo 2012. Gazzetta Ufficiale n.72 del 26 marzo.
- Gabutti G, Penna C, Rossi M, Salmaso S, Rota MC, Bella A, Crovari P, Serological Study Group. The seroepidemiology of varicella in Italy. Epidemiol Infect 2001; 126:433-40; PMID:11467800; http://dx.doi.org/10.1017/S0950268801005398
- Gabutti G, Rota MC, Guido M, De Donno A, Bella A, Ciofi degli Atti ML, Crovari P, Seroepidemiology Group. The epidemiology of Varicella Zoster Virus infection in Italy. BMC Public Health 2008; 8:372; PMID:18954432; http://dx.doi.org/10.1186/1471-2458-8-372
- Helmuth IG, Poulsen A, Suppli CH, Mølbak K. Varicella in Europe-A review of the epidemiology and experience with vaccination. Vaccine 2015; 33:2406-13; PMID:25839105; http://dx.doi.org/10.1016/j.vaccine.2015.03.055
- Federazione Italiana di Medici Pediatri, Società Italiana di Pediatria, Società di Igiene, Medicina Preventiva e Sanità Pubblica. Documento di consenso sulla vaccinazione universale contro la varicella in Italia. Ital J Public Health 2010; 7:S1-S36.
- Trucchi C, Gabutti G, Cristina Rota M, Bella A. The burden of varicella in Italy, 2001-2010: analysis of data from multiple sources and assessment of universal vaccination impact in three pilot regions. J Med Microbiol 2015; 64:1387-94; PMID:25813818; http://dx.doi.org/10.1099/jmm.0.000061
- Bechini A, Boccalini S, Baldo V, Cocchio S, Castiglia P, Gallo T, Giuffrida S, Locuratolo F, Tafuri S, Martinelli D, et al. Impact of universal vaccination against varicella in Italy. Experiences from eight Italian regions. Hum Vaccin Immunother 2015; 11:63-71; http://dx.doi.org/10.4161/hv.34311
- Gabutti G, Rota MC, De Donno A, Guido M, Bella A, Idolo A, Lupi S, Brignole G, Gruppo di studio sulla sieroepidemiologia. Sero-epidemiology of VZV infection in Italy: impact evaluation of extensive vaccination. Epidemiol Prev 2014; 38:57-61; PMID:25759345.