ABSTRACT
Rotavirus is the leading cause of severe acute gastroenteritis in infants and young children. Most children are infected with rotavirus, and the health and economic burdens of rotavirus gastroenteritis on healthcare systems and families are considerable. In 2012 pentavalent rotavirus vaccine (RV5) and diphtheria, tetanus, acellular pertussis and inactivated poliovirus vaccine derived from Sabin strains (DTaP-sIPV) were licensed in Japan. We examined the immunogenicity and safety of DTaP-sIPV when administrated concomitantly with RV5 in Japanese infants. A total of 192 infants 6 to 11 weeks of age randomized to Group 1 (N = 96) received DTaP-sIPV and RV5 concomitantly, and Group 2 (N = 96) received DTaP-sIPV and RV5 separately. Antibody titer to diphtheria toxin, pertussis antigens (PT and FHA), tetanus toxin, and poliovirus type 1, 2, and 3 were measured at 4 to 6 weeks following 3-doses of DTaP-sIPV. Seroprotection rates for all components of DTaP-sIPV were 100% in both groups, and the geometric mean titers for DTaP-sIPV in Group 1 were comparable to Group 2. Incidence of systemic AEs (including diarrhea, vomiting, fever, and nasopharyngitis) were lower in Group 1 than in Group 2. All vaccine-related AEs were mild or moderate in intensity. There were no vaccine-related serious AEs, no deaths, and no cases of intussusception during the study. Concomitant administration of DTaP-sIPV and RV5 induced satisfactory immune responses to DTaP-sIPV and acceptable safety profile. The administration of DTaP-sIPV given concomitantly with RV5 is expected to facilitate compliance with the vaccination schedule and improve vaccine coverage in Japanese infants.
Introduction
Rotavirus is the leading cause of severe acute gastroenteritis in infants and young children worldwide.Citation1 Rotavirus gastroenteritis (RVGE) is a universal disease and nearly every child is infected with rotavirus by the age of 5 years, regardless of socioeconomic status or standards of health and sanitation.Citation2 RVGE is characterized by the sudden onset of severe diarrhea, vomiting, and fever, and these symptoms generally persist for several days. In 2008, rotavirus was estimated to account for more than 450,000 deaths per year in children less than 5 y of age in the developing countries.Citation3 While rotavirus is not associated with a high rate of mortality in Japan and the developed countries, the health and economic burden related to RVGE on healthcare systems and families are substantial.Citation4-7 To date, there is no specific therapy available against rotavirus infections and fluid replacement therapy to prevent dehydration is the primary treatment of RVGE in children.
Currently, 2 rotavirus vaccines are licensed as voluntary use for infants in Japan, the oral live pentavalent rotavirus vaccine, RV5 (RotaTeq®, Merck & Co., Inc., Kenilworth, NJ) and the oral live monovalent rotavirus vaccine (Rotarix®, GlaxoSmithKline Biologicals, Rixensart, Belgium). Administration of RV5 at 2, 3 and 4 months of age is recommended by the Japan pediatrics society. A clinical study conducted in Japan has demonstrated the effectiveness of RV5 in the prevention of RVGE caused by serotypes contained in the vaccine in Japanese infants.Citation8 Surveillance data and economic analysis indicated universal rotavirus vaccination for Japanese infants had a potential to reduce the burden of RVGE and health care utilization.Citation9-11 Rotavirus vaccines are recommended for use in infants by the World Health OrganizationCitation12 and have been introduced in the National Immunization Program (NIP) as a routine vaccine for the prevention of RVGE in many countries outside Japan.
In Japan, inactivated polio vaccine derived from Sabin strains (sIPV) was developed by the Japan Poliomyelitis Research Institute to avoid the risk of vaccine-related paralytic poliomyelitis associated with oral polio vaccine in infants.Citation13,14 sIPV was combined with diphtheria-tetanus-acellular pertussis vaccine, DTaP-sIPV (Tetrabik®, BIKEN, Osaka, Japan; Quattrovac®, Kaketsuken, Kumamoto, Japan) and introduced into the Japanese NIP for infants in November 2012.Citation15,16 Administration of DTaP-sIPV at 3, 4 and 5 months of age as a 3-dose primary vaccination and at 12–18 months after primary vaccination as a booster vaccination is recommended.
In the previous studies, concomitant administration of RV5 with licensed pediatric vaccines, including DTaP, IPV, Haemophilus influenzae type b (Hib) vaccine, recombinant hepatitis B vaccine, and 7-valent pneumococcal conjugate vaccine (PCV7) have been reported to be well tolerated and immunogenic, and immune responses to RV5 including rotavirus IgA antibody are generally similar to when RV5 is administrated alone.Citation17,18 To our knowledge, there is no report regarding immune response to DTaP-sIPV when administrated concomitantly with licensed pediatric vaccines including RV5. Therefore, present study discussed here was designed to evaluate the immunogenicity and safety of DTaP-sIPV when administered concomitantly with RV5 in Japanese infants.
Results
Participant accounting and demographics
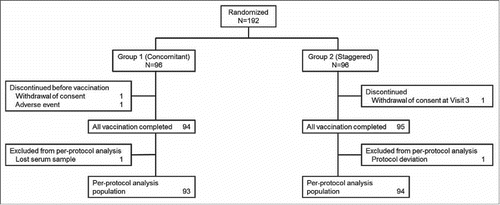
A total 192 Japanese infants aged 6 to 11 weeks were randomized 1:1 to Group 1 (concomitant vaccine administration group) or Group 2 (staggered vaccine administration group) (). There were slightly more males enrolled in each group, 51.0% and 57.3%, respectively. The mean age in weeks was comparable in both groups. The majority of subjects (94 in Group 1; 95 in Group 2) received all study doses and completed the study (). Two subjects in Group 1 were randomized but did not receive any study vaccine, and one subject in Group 2 discontinued the study due to withdrawal of consent after Visit 3. One subject in each group was excluded from the per-protocol population due to a lost serum sample following Dose 3 of DTaP-sIPV. No subjects discontinued from the study due to a clinical adverse event (AE). Overall, 93 subjects in Group 1 and 94 subjects in Group 2 were included in the immunogenicity analysis population, and 94 subjects in Group 1 and 96 subjects in Group 2 were included in the safety analysis population, respectively. At Visit 1, 3 and 5, subjects who received the other routine pediatric vaccines (e.g. 7 or 13-valent pneumococcal conjugate vaccine, recombinant hepatitis B vaccine and Hib vaccine) were higher in Group 2 (staggered group) than in Group 1 (concomitant group). 58.3% of subjects (56/96) at Visit 1, 84.4% of subjects (81/96) at Visit 3 and 64.6% of subjects (62/96) at Visit 5 in Group 2 (staggered group) received RV5 with at least one of the routine pediatric vaccines concomitantly, whereas 34.0% of subjects (32/94) at Visit 1, 3.2% of subjects (3/94) at Visit 3 and 1.1% of subjects (1/94) at Visit 5 in Group 1 (concomitant group) received the routine pediatric vaccines alone.
Table 1. Demographics of subjects at baseline.
Immunogenicity
The percentages of per-protocol subjects who achieved threshold levels (see Methods) for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA and polio virus type 1, 2, and 3 at 4 to 6 weeks following Dose 3 of DTaP-sIPV between the concomitant and staggered groups is presented in . The lower bound of the 2-sided 95% confidence interval (CI) for the group difference in seroprotection rates (concomitant group – staggered group) was above the prespecified margin of −10%, meeting the non-inferiority response for DTaP-sIPV given with RV5 concomitantly compared with staggered group.
Table 2. Seroprotection rates (SPR) for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA and polio virus type 1, 2 and 3 between concomitant and staggered group at 4 to 6 weeks following 3-dose of DTaP-sIPV.
The geometric mean titers (GMTs) of each antibody for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis filamentous hemagglutinin (FHA), and polio virus type 1, 2, and 3 at 4 to 6 weeks following Dose 3 of DTaP-sIPV were comparable between the concomitant and staggered groups ().
Table 3. Geometric Mean Titers (GMTs) of each antibody for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA and Polio virus type 1, 2 and 3 between concomitant and staggered group at 4 to 6 weeks following 3-dose of DTaP-sIPV.
Safety
Overall, 68.1% of subjects (64/94) in the concomitant group and 86.5% of subjects (83/96) in the staggered group reported 1 or more AEs during Day 1 to Day 14 following any scheduled visits (). The most common AEs (≥ 10% in any group) were diarrhea (25.5%, Group 1; 46.9%, Group 2), vomiting (8.5%, Group 1; 16.7%, Group 2), fever (10.6%, Group 1, 22.9%, Group 2), nasopharyngitis (7.4%, Group 1; 20.8%, Group 2), upper respiratory tract inflammation (9.6%, Group 1; 13.5%, Group 2), and upper respiratory tract infection (9.6%, Group 1; 12.5%, Group 2). Frequency in diarrhea and fever from Day 1 to Day 7 following any scheduled visits were significantly lower in the concomitant group than in the staggered group (diarrhea: 24.5% in Group 1, 44.8% in Group 2, P = 0.003; fever: 10.6% in Group 1, 21.9% in Group 2, P = 0.037) however, there were no significant difference in the frequency of other systemic AEs and injection-site AEs between the 2 groups. Vaccine-related AEs were reported by 19.1% of subjects (18/94) in the concomitant group and 45.8% of subjects (44/96) in the staggered group during Day 1 to Day 14 following any scheduled visit. All of the vaccine-related AEs including injection-site reactions were reported as mild or moderate in intensity and were resolved without discontinuing the study.
Table 4. Adverse Events Summary (1 to 14 d following any scheduled visitsFootnote†).
There were 2 subjects in the staggered group that reported having a serious AE during Day 1 to Day 14 following any scheduled visit; both were assessed as not related to the study vaccine by the study investigator. One subject reported an upper respiratory tract inflammation with onset 8 d after receiving the second dose of DTaP-sIPV, and; one subject reported a respiratory syncytial viral pneumonia with onset 14 d after receiving the second dose of RV5. No serious AEs were reported in the concomitant group during Day 1 to Day 14 following any scheduled visit. There were no reports of serious vaccine-related AEs, deaths, and/or cases of intussusception throughout the duration of the study.
Discussion
To prevent serious diseases (e.g., diphtheria, tetanus, pertussis, poliomyelitis, Streptococcus pneumoniae, Haemophilus influenzae type b), infants are required to receive their routine vaccines in accordance with the recommended vaccination schedule. With a growing number of new pediatric vaccines being developed, the need for concomitant administration of vaccines has been increasing to maintain an acceptable vaccination schedule. To support the use of concomitant administration of multiple vaccines during a single patient visit, it is crucial to demonstrate that there is no significant interference with the immunogenicity or changes to the safety profiles of these vaccines when administered concomitantly compared with when they are administered sequentially.
This is the first study to assess the immunogenicity and safety of DTaP-sIPV when administered concomitantly with RV5. The immunogenicity data demonstrated that the seroprotection rates for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA, and polio virus type 1, 2, and 3 at 4 to 6 weeks following Dose 3 of DTaP-sIPV in the concomitant group were 100% for all antigens and non-inferior to those in the staggered group. The GMTs of each antibody for DTaP-sIPV at 4 to 6 weeks following Dose 3 of DTaP-sIPV in the staggered group showed a tendency to be numerically higher than those in the concomitant group. However, this difference is not significant because in each case, the 95% CIs overlap. Furthermore, the GMTs for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA, and polio virus type 1, 2, and 3 in both groups were well above the threshold levels that are considered protective for these diseases.
In the clinical trials conducted in the United Stated and EU, the concomitant administration of RV5 with other licensed vaccines including DTaP, IPV, Hib vaccine, hepatitis B vaccine, and PCV7 have been shown to be safe and well tolerated and antibody responses to the licensed vaccines were similar to when the licensed vaccines were administered alone.Citation17,18 This is consistent with the observation of our concomitant study of DTaP-sIPV with RV5 conducted in Japan.
This study observed that the frequency of AEs differed between the 2 groups. A lower incidence of systemic AEs (including diarrhea, vomiting, fever, and nasopharyngitis) was observed in the concomitant group compared with the staggered group. At Visit 1, 3 and 5, most of subjects received RV5 with other routine pediatric vaccines concomitantly in the staggered group, whereas few subjects received other routine pediatric vaccines in the concomitant group. The different frequency of AEs between 2 groups might be confounded by the possible AEs related to other routine pediatric vaccines. This observation was possibly due to the study design since AEs (diarrhea, vomiting and fever) were solicited for 7 d after each study visit, regardless of whether RV5 was administered alone or concomitantly with other routine pediatric vaccines.
The type of AEs observed in both the concomitant and staggered groups were similar to those in the prior Japan Phase III studyCitation8 and most AEs reported in this study were mild or moderate in intensity. There were no vaccine-related serious AEs, no deaths, and no cases of intussusception during the study and the safety profile of RV5 when given concomitantly with DTaP-sIPV is consistent with those observed in the previous clinical trials.Citation17,18 Therefore, DTaP-sIPV was shown to be well tolerated when administered concomitantly with RV5.
The administration of DTaP-sIPV given concomitantly with RV5 is expected to simplify the Japanese vaccination schedule for infants, thereby facilitating better compliance, improved vaccine coverage, and timeliness of vaccine administration in Japanese infants. The data represented in this report provide evidence that the 3-dose regimen of the 0.5 mL/dose of Japanese manufactured DTaP-sIPV derived from Sabin strains given as a subcutaneous injection can be administered concomitantly with the 3-dose regimen of the 2 mL/dose orally administrated RV5.
The results from this study had some limitations, including that this was open-label study, other routine pediatric vaccines were allowed to be administered concomitantly with RV5 in the staggered group, and it is unclear whether concomitant administration of DTaP-sIPV with RV5 interfere with immunogenicity of RV5 in Japanese infants.
Conclusion
This study demonstrated that the concomitant administration of DTaP-sIPV and RV5 in Japanese infants could elicit immune responses to DTaP-sIPV comparable to those observed in the administration of DTaP-sIPV alone. DTaP-sIPV is well tolerated when administrated concomitantly with RV5 in Japanese infants. These findings support the concomitant administration of DTaP-sIPV and RV5 to facilitate the vaccination schedule for infants in Japan.
Materials and methods
Study design
From September 2013 to June 2014, this open-label, multicenter, randomized, clinical trial was conducted across 7 sites in Japan. This study evaluated the immunogenicity, safety, and tolerability of concomitant versus staggered administration of RV5 and DTaP-sIPV vaccines in Japanese infants. Subjects between 6 and 11 weeks of age (≥ 42 d to ≤ 76 d from date of birth) were randomly assigned in a 1:1 ratio to one of 2 groups.
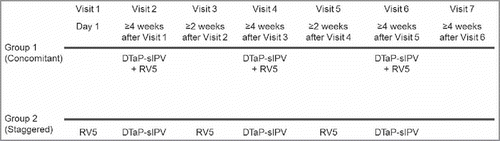
Subjects in Group 1 (concomitant group) received 3 concomitant doses of RV5 and DTaP-sIPV at Visit 2, Visit 4, Visit 6; while subjects in Group 2 (staggered group) received RV5 at Visit 1, Visit 3, Visit 5 and DTaP-sIPV at Visit 2, Visit 4, Visit 6, separately (). To keep the safety follow-up period consistent between Group 1 and Group 2, subjects in Group 1 maintained a study visit at Visit 1, Visit 3 and Visit 5 where there was no study vaccination and safety follow-up was collected for 14 d. The first dose of RV5 was given by age 14 weeks and 6 d and the third dose of RV5 should not be given after 32 weeks of age in accordance with Japanese product circular. Concomitant administration of DTaP-sIPV with other routine pediatric vaccines was prohibited in this study to exclude the influence of other routine pediatric vaccines on the immune responses to DTaP-sIPV, with the exception of RV5. However, to avoid the loss of opportunity to provide other routine pediatric vaccines (e.g., 7 or 13-valent pneumococcal conjugate vaccine, hepatitis B vaccine and Hib vaccine), concomitant administration of other routine pediatric vaccines with RV5 at Visit 1, Visit 3 and Visit 5 were allowed only in the staggered group. Serum samples (3 mL) were collected before the first vaccination at Visit 1 (baseline) and post vaccination with DTaP-sIPV at Visit 7 (4 to 6 weeks following Dose 3 of DTaP-sIPV/RV5 or DTaP-sIPV) to measure the antibody responses to DTaP-sIPV.
Study objectives
The primary immunogenicity objective was to demonstrate that the immunogenicity of a 3-dose vaccination series of DTaP-sIPV in Group 1 (concomitant group) is non-inferior to that in Group 2 (staggered group). GMTs of diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA, and polio virus type 1, 2, and 3 at baseline and at 4 to 6 weeks after Dose 3 of DTaP-sIPV in Group 1 and Group 2 were evaluated. The safety objective was to evaluate the safety and tolerability of the concomitant administration of DTaP-sIPV and RV5 through the study.
Study population
Japanese infants between 6 and 11 weeks of age were eligible for the study. Subjects were excluded if they: had a gastrointestinal disorder; demonstrated growth retardation; showed a failure to thrive; had a history of intussusception; had impairment of immunological function; had underlying diseases (such as cardiovascular, renal, liver, or blood); had a history of convulsions; were undergoing immunosuppressive therapy; received blood products; were previously vaccinated with rotavirus vaccine and/or DTaP-sIPV (including its components); received a live vaccine within 28 or an inactivated vaccine within 7 d of enrollment; participated in another interventional study within 14 d of enrollment or at any time during the study; were at high risk for tuberculosis exposure; or had a known hypersensitivity to any of the vaccines components. The study was conducted in accordance with principles of Good Clinical Practice, approved by the Institutional Review Board of each participating site, and written informed consent was obtained from parent/legal guardian of subject before study entry. This study is registered with ClinicalTrials.gov, number NCT01926015.
Vaccine descriptions
RV5 (RotaTeq®, lot WL00052861; Merck & Co., Inc., Kenilworth, NJ) is a ready-to-use solution of live reassortant rotaviruses, containing G1, G2, G3, G4, and P1A[8] which contains a minimum of 2.0 – 2.8 × 106 infectious units (IU) per individual reassortant dose, depending on the serotype, and not greater than 116 × 106 IU per aggregate dose. Each dose is supplied in a container consisting of a squeezable plastic dosing tube with a twist-off cap, allowing for direct oral administration.
DTaP-sIPV (Tetrabik®, lot JM-0021; BIKEN, Japan) is approved in Japan for the prevention of diphtheria, pertussis, tetanus, and acute poliomyelitis and used in this study. The DTaP-sIPV vaccine is supplied in 0.5 mL pre-filled syringes contained ≥ 14 IU of diphtheria toxoid, ≥ 9 IU of tetanus toxoid, ≥ 4 U (pertussis protective unit) of pertussis protective antigen, 1.5 D-antigen units (DU) of poliovirus type 1 (Sabin strains), 50 DU of poliovirus type 2 (Sabin strains), and 50 DU of poliovirus type 3 (Sabin strains) for subcutaneous injection.
Measures
Immunogenicity
Serum samples were stored at −20°C until used for testing. Antibody titer to diphtheria toxin and poliovirus type 1, 2, and 3 were determined by serum neutralization assay and tetanus toxin titer was determined by passive hemagglutination. Antibody titers to pertussis toxoid and pertussis FHA were determined by enzyme-linked immunosorbent assay. Assay for antibody titers of DTaP-sIPV were performed at BIKEN (Kan-onji, Kagawa, Japan). For the primary objective, the end point was the percentage of subjects who achieved threshold levels for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA, and polio virus type 1, 2, and 3 at 4 to 6 weeks following Dose 3 of DTaP-sIPV. The seroprotection rates for DTaP-sIPV were defined as: ≥ 0.1 IU/mL for diphtheria toxin; ≥ 0.01 IU/mL for tetanus toxin; ≥ 10 EU/mL for PT and FHA; and neutralizing antibody titers ≥ 1:8 for polio types 1, 2, and 3.Citation19-22
Safety
All subjects were followed for safety for 14 d after each scheduled study visit. This safety period was consistent with RV5 clinical study in Japan.Citation8 Solicited AEs included axillary body temperatures, vomiting and diarrhea. The parent or legal guardian was instructed to record axillary body temperatures and episodes of vomiting and diarrhea from Day 1 through Day 7 on a vaccination report card following each study visit. All AEs including local and systemic AEs, and serious and non-serious AEs were also recorded from Day 1 through Day 14, and all vaccine-related serious AEs, deaths, and cases of intussusception that occurred at any time were to be reported throughout the study period.
Statistical analysis
Immunogenicity
The primary immunogenicity analyses were based on a per–protocol population, defined as subjects who received the scheduled 3 doses of DTaP-sIPV and adhered to guidelines for the administration of the study vaccines. The immunogenicity of DTaP-sIPV when administered concomitantly with RV5 (Group 1) in a 3-dose regimen was considered non-inferior to DTaP-sIPV and RV5 when administered alternately (Group 2) if the lower bound of the 2-sided 95% CI of the between-treatment difference (concomitant group and staggered group) of the percentage of subjects who achieved the seroprotection rates for diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA, and polio virus type 1, 2, and 3 following Dose 3 of DTaP-sIPV are no lower than −10%. The Miettinen and Nurminen method was used to provide the 95% CI of the between-treatment difference [concomitant group and staggered group] of the seroprotection rate.Citation23 No multiplicity adjustment was planned as the non-inferiority is admitted only when all lower bounds of 95% CI on the 7 primary endpoints are larger than the relevant non-inferiority margins (−10%). With 190 subjects (85 evaluable subjects per group after accounting for a possible 10% dropout), there is about 78% power to declare that the immunogenicity of DTaP-sIPV in the concomitant group is non-inferior to that in the staggered group. As for the measurements, response rates will be used for evaluation of diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA and Polio virus type 1, 2 and 3 based on well-established protective antibody levels. The power calculation is based on the assumed true percentage of responders for diphtheria toxin: 98.5%, tetanus toxin: 97.8%, pertussis toxin: 98.5%, pertussis FHA: 98.5% and Polio virus type 1, 2 and 3: 98.5% based on the data of Tetrabik®.
In addition, the proportion of subjects who achieved the threshold response criteria, as well as the GMTs of antibody responses to diphtheria toxin, tetanus toxin, pertussis toxin, pertussis FHA, and polio virus type 1, 2, and 3 (at baseline and post Dose 3 of DTaP-sIPV) were to be summarized for each group. The corresponding 2-sided 95% CI on the proportion and GMTs are provided for each vaccination group.
Safety
All randomized subjects who received at least 1 dose of study vaccine and had safety follow-up were included in the safety analysis (All Subjects as Vaccinated). The frequencies and percentages of AEs were summarized. For AEs reported with a proportion >0% in any vaccination group, a comparison of the percentage of subjects reporting AEs between the 2 vaccination groups was performed. The frequencies were tabulated for both vaccination groups, with risk differences between the 2 vaccination groups using point estimates and a 2-sided 95% CI, using the Miettinen and Nurminen method.Citation23 AEs of special interest included fever, vomiting, diarrhea, and injection-site reactions. Frequencies and percentages were tabulated for both vaccination groups for these AEs of special interest with risk differences between the 2 vaccination groups using point estimates, 2-sided 95% CI and p-value. P≤ 0.05 is considered statistically significant for this study.
Disclosure of potential conflicts of interest
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc. (sponsor). In conjunction with the external investigators, this study was designed, executed, and analyzed by the sponsor. The sponsor formally reviewed a penultimate draft. All co-authors approved the final version of the manuscript. All authors are employees of Merck Sharp & Dohme Corp. and may hold stock and/or stock options in the company.
Acknowledgments
The authors would like to thank all the infants and their parents or guardians for participating in this trial. Also, the authors are deeply grateful to Yasunori Ishihara (Fukui Aiiku Hospital), Hiroshi Sakiyama (Sakiyama Children's Clinic), Haruo Kuroki (Sotobo Children's Clinic), Akiyoshi Sasamoto (Seijyo Sasamoto Pediatric), Hiroji Okawa (Okawa Children and Family Clinic), Shigeru Mori (Momotarou Clinic) and Yoshihiro Umezawa (Denenchohu Family Clinic) the great effort of subject enrollment. Finally we would like to express our deepest appreciation to Hironori Yoshii and Yasuyuki Gomi (BIKEN) who had committed to assay the antibody titers for DTaP-sIPV.
References
- Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. Global mortality associated with rotavirus disease among children in 2004. J Infect Dis 2009; 200:S9-15; https://doi.org/10.1086/605025
- Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis 2003; 9(5):565-72; PMID:12737740; https://doi.org/10.3201/eid0905.020562
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. WHO-coordinated Global Rotavirus Surveillance Network. 2008 e stimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:136-41; https://doi.org/10.1016/S1473-3099(11)70253-5
- Ito H, Otabe O, Katsumi Y, Matsui F, Kidowaki S, Mibayashi A, Nakagomi T, Nakagomi O. The incidence and direct medical cost of hospitalization due to rotavirus gastroenteritis in Kyoto, Japan, as estimated from a retrospective hospital study. Vaccine 2011; 29:7807-10; PMID:21821087; https://doi.org/10.1016/j.vaccine.2011.07.105
- Kawai K, O'Brien MA, Goveia MG, Mast TC, El Khoury AC. Burden of rotavirus gastroenteritis and distribution of rotavirus strains in Asia: a systematic review. Vaccine 2012; 30:1244-54; PMID:22212128; https://doi.org/10.1016/j.vaccine.2011.12.092
- Nakagomi T, Kato K, Tsutsumi H, Nakagomi O. The burden of rotavirus gastroenteritis among Japanese children during its peak months: an internet survey. Jpn J Infect Dis 2013; 66:269-75; PMID:23883835; https://doi.org/10.7883/yoken.66.269
- Mast TC, DeMuro-Mercon C, Kelly CM, Floyd LE, Walter EB. The impact of rotavirus gastroenteritis on the family. BMC Pediatr 2009; 9:11; PMID:19200366; https://doi.org/10.1186/1471-2431-9-11
- Iwata S, Nakata S, Ukae S, Koizumi Y, Morita Y, Kuroki H, Tanaka Y, Shizuya T, Schödel F, Brown ML, et al. Efficacy and safety of pentavalent rotavirus vaccine in Japan: a randomized, double-blind, placebo-controlled, multicenter trial. Hum Vaccin Immunother 2013; 9:1626-33; PMID:23732903; https://doi.org/10.4161/hv.24846
- Sato T, Nakagomi T, Nakagomi O. Cost-effectiveness analysis of a universal rotavirus immunization program in Japan. Jpn J Infect Dis 2011; 64:277-83. PMID:21788701
- Itzler R, O'Brien MA, Yamabe K, Abe M, Dhankhar P. Cost-effectiveness of a pentavalent rotavirus vaccine in Japan. J Med Econ. 2013; 16:1216-27; PMID:23919721; https://doi.org/10.3111/13696998.2013.831869
- Hashizume M, Nakagomi T, Nakagomi O. An early detection of decline in rotavirus cases during the 2013/2014 season in Japan as revealed by time-series analysis of national surveillance data. Trop Med Health. 2015; 43:177-81; PMID:26543393; https://doi.org/10.2149/tmh.2015-23
- World Health Organization. Rotavirus Vaccines WHO Position Paper – January 2013. Wkly Epidemiol Rec 2013; 88:49-64. PMID:23424730
- Simizu B, Abe S, Yamamoto H, Tano Y, Ota Y, Miyazawa M, Horie H, Satoh K, Wakabayashi K. Development of inactivated poliovirus vaccine derived from Sabin strains. Biologicals 2006; 34:151-4; PMID:16679028; https://doi.org/10.1016/j.biologicals.2006.02.010
- Shimizu H. Development and introduction of inactivated poliovirus vaccines derived from Sabin strains in Japan. Vaccine 2016; 34:1975-85; PMID:25448090; https://doi.org/10.1016/j.vaccine.2014.11.015
- Ogawa H. Efficacy and safety of absorbed acellular pertussis-diphtheria-tetanus-sabin strains derived inactivated poliovirus combined vaccine. BIO Clinica 2013; 28:72-79 [in Japanese]
- Okada K, Miyazaki C, Kino Y, Ozaki T, Hirose M, Ueda K. Phase II and III clinical studies of diphtheria-tetanus-acellular pertussis vaccine containing inactivated polio vaccine derived from sabin strains (DTaP-sIPV). J Infect Dis. 2013; 208:275-83; PMID:23568174; https://doi.org/10.1093/infdis/jit155
- Rodriguez ZM, Goveia MG, Stek JE, Dallas MJ, Boslego JW, DiNubile MJ, Heaton PM. Concomitant use of an oral live pentavalent human-bovine reassortant rotavirus vaccine with licensed parenteral pediatric vaccines in the United States. Pediatr Infect Dis J 2007; 26:221-7; PMID:17484218; https://doi.org/10.1097/01.inf.0000254391.71103.e8
- Ciarlet M, He S, Lai S, Petrecz M, Yuan G, Liu GF, Mikviman E, Heaton PM, Panzer F, Rose T, et al. Concomitant use of the 3-dose oral pentavalent rotavirus vaccine with a 3-dose primary vaccination course of a diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type b vaccine: immunogenicity and reactogenicity. Pediatr Infect Dis J 2009; 28:177-81; PMID:19209092; https://doi.org/10.1097/INF.0b013e31818c0161
- Vitek CR, Wharton M. Diphtheria Toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Saunders Elsevier 2008; 139-56
- Wassilak SGF, Roper MH, Kretsinger K, Orenstein WA. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Saunders Elsevier 2008; 805-39
- Kato T. DTaP. J Pediatr Practice 1990; 53:2275-81 [in Japanese]
- Plotkin SA, Vidor E. Poliovirus vaccine-inactivated. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines. 5th ed. Saunders Elsevier 2008; 605-29
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985; 4:213-26; PMID:4023479; https://doi.org/10.1002/sim.4780040211