ABSTRACT
The regulatory effect of allergic responses induced by IgG antibodies on human intra-thymic cells has not been reported in the literature. The aim of this study was to evaluate the possible differential effect of purified IgG from atopic and non-atopic individuals on human intra-thymic αβT cell cytokine production. Thymic tissues were obtained from 14 patients who were less than 7 d old. Additionally, blood samples were collected from atopic and non-atopic volunteers. Thymocytes and peripheral blood mononuclear cells were cultured with purified atopic or non-atopic IgG, and intracellular cytokine production was assessed. Purified IgG did not influence the frequency or viability of human intra-thymic αβT cells. Purified non-atopic IgG induced greater IFN-γ production by intra-thymic CD4+CD8+ T cells than did the mock treatment and atopic IgG. A similar effect of purified non-atopic IgG on TCD8 cells was observed compared with the mock treatment. Atopic IgG inhibited IFN-γ and TGF-β production by intra-thymic TCD4 cells. Treatment with intravenous immunoglobulin resulted in intermediate levels of IFN-γ and TGF-β in intra-thymic TCD4 cells compared with treatment with atopic and non-atopic IgG. Peripheral TCD4 cells from non-atopic individuals produced IFN-γ only in response to atopic IgG. This report describes novel evidence revealing that IgG from atopic individuals may influence intracellular IFN-γ production by intra-thymic αβT cells in a manner that may favor allergy development.
Introduction
Our group has been investigating adoptive maternal-foetal immune interactions in the context of allergy regulation.Citation1-10 The potential of IgG antibodies as allergy regulators has been discussed for decades. In the early 80s, direct evidence that maternal IgG can suppress IgE production in offspring was obtained in a murine model of ovalbumin (OVA) immunization.Citation11 Several years later, the transfer of maternal IgG-recognizing allergens, including pollen, cat epithelium,Citation12 or dietary antigens such as OVA,Citation13 was shown to be related to allergy inhibition in children during the first years of life. It was also shown that the passive transfer of purified IgG during pregnancy in mice can modulate the B cell phenotype of the offspring.Citation14
The IgG repertoire and the transfer of IgG molecules to offspring during pregnancy and breast-feeding can differ between atopic and non-atopic individuals. Atopic mothers have been shown to be capable of transferring higher levels of anti-Dermatophagoides pteronyssinus IgG via breast milk than non-atopic mothers.Citation15 Another finding regarding IgG is that its reactivity to IgE can play a pivotal role in the mechanism by which non-atopic individuals produce IgE without a response to allergen exposure.Citation16 Human atopic children have also been shown to exhibit higher serum levels of anti-OVA IgG than non-atopic children at age 2.Citation17
The precise mechanisms by which passively transferred maternal IgG can influence the immune status of offspring are incompletely understood. Recently, we hypothesized a novel mechanism for allergen-specific maternal IgG antibodies to mediate allergy inhibition by interacting with immature cells in the thymus,Citation18 which could be mediated directly by IgG molecules.Citation19 The thymus can mature diverse populations of lymphocytes with modulatory and regulatory potential, but especially T cells that express αβ T cell receptors (> 90% of all T cells), including TCD4 and TCD8 cells.
The observation that IgG can reach primary lymphoid organs was described decades ago,Citation20 but no study has yet examined the direct effect of IgG on intra-thymic cells during the maturation process. In humans, several previous studies have reported that purified IgG used as an in vivo human therapy (intravenous immunoglobulin, IVIg) can modulate the in vitro production of cytokines, including interferon (IFN)-γ, interleukin (IL)-10 and IL-12, by peripheral blood mononuclear cells (PBMCs) and umbilical cord cells.Citation21-23 The interactions that may be responsible for this modulatory effect appear to stimulate peripheral αβT cells via T cell receptor activation.Citation24 Recently, it was also demonstrated that human IgG can directly permeate the cell membrane of various cell types, resulting in intracellular interactions that are incompletely understood.Citation25 This evidence expands the possible mechanisms of IgG-mediated regulation via its interactions with αβT cells.
Taken together, these findings strongly suggest that IgG can interact in the membrane or the cytoplasm with human αβT cells undergoing maturation and that this process can result in the functional modulation of these cells.
Based on the above evidence, the aim of this study was to evaluate the possible differential effects of purified IgG from atopic and non-atopic individuals on cytokine production by human intra-thymic αβT cells, especially IFN-γ production. Because the modulatory potential of IVIg has been well described in the literature, we further assessed the effect of IVIg on intra-thymic αβT cells. Finally, we examined whether mature αβT cells exhibit a similar profile in response to atopic and non-atopic IgG.
Results
Purified IgG did not influence the frequency or viability of human intra-thymic αβT cells in vitro
To evaluate the in vitro effect of purified IgG, thymocytes were evaluated at time 0 or cultured in the presence of purified IgG for 3, 7, 10 or 14 d. We found that T double-positive (TDP) cells represented almost 50% of all thymocytes after thawing, and a similar percentage of TDP cells remained until 10 d in culture (). Approximately 40% of this population was viable at time 0. However, this value was not sustained beyond 3 days, and the percentage of viable TDP cells gradually decreased until 10 d in culture (). TCD4 cells represented approximately 30% of all thymocytes at time 0, and this value gradually decreased to approximately 15% at 14 d in culture (). Approximately 80% of all TCD4 cells were viable at time 0. However, this value gradually decreased beyond 3 days, and at 14 d in culture, the TCD4 cell viability rate was approximately 20% (). TCD8 cells represent approximately 10% of all thymocytes from time 0 to 14 d in culture (), and their viability rate was approximately 90% during this period ().
Figure 1. Time course of cell frequency and viability and of the effect of purified IgG on human intra-thymic αβT cells. Thymocytes from children less than 7 d old (n = 14) were evaluated at time 0 or after 3, 7, 10 and 14 d in culture in RPMI medium supplemented with FBS. At each time point, the frequency and viability of TDP (A-B), TCD4 (C-D) and TCD8 cells (E-F) were evaluated by flow cytometry. Thymocytes were also cultured for 3 or 7 d in RPMI medium supplemented with FBS in the absence (mock) or presence of 100 µg/mL IgG purified from atopic or non-atopic individuals. At each time point, the frequency and viability of TDP (G and J), TCD4 (H and K) and TCD8 cells (I and L) were evaluated by flow cytometry. The symbols represent the means with standard error. The results are illustrated by box and whiskers graphs with 25th percentiles, and the Tukey method was used to plot outliers.
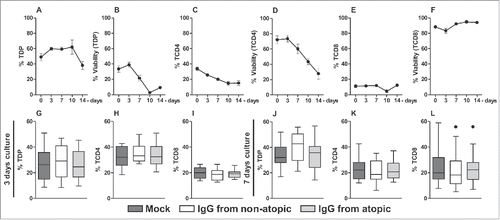
Based on the cell frequency and viability results, we decided to evaluate cytokine production by αβT cells after 3 and 7 d in culture. Therefore, we assessed whether IgG from atopic or non-atopic individuals could influence these parameters of the examined cell populations after culturing these cells for the indicated period. As shown in , IgG from both groups did not influence the frequency or viability of any evaluated cell population after 3 () or 7 d in culture ().
Purified IgG can modulate cytokine production by intra-thymic αβT cells
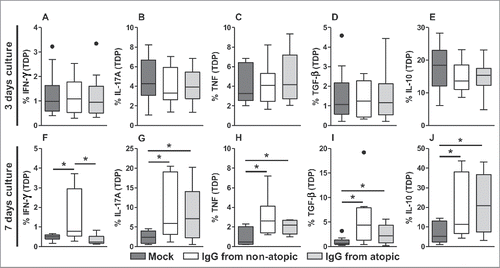
After evaluating cell frequency and viability, we analyzed cytokine production in αβT cells. In TDP cells, no influence of IgG on any evaluated cytokine was observed after 3 d in culture (). After 7 d in culture, we found that treatment with IgG from non-atopic individuals induced elevated production of IFN-γ compared with non-treatment and to treatment with IgG from atopic individuals ( and S2). The evaluation of cytokine production revealed that treatment with IgG from non-atopic or atopic individuals induced similar increases in the production of IL-17A, tumor necrosis factor (TNF), transforming growth factor (TGF)-β and IL-10 compared with non-treatment with IgG ( and S2).
Figure 2. Effect of purified IgG on cytokine production by intra-thymic TDP cells. Thymocytes from children less than 7 d old (n = 14) were evaluated after 3 (A-E) or 7 d (F-J) in culture in RPMI medium supplemented with FBS in the absence (mock) or presence of 100 µg/mL IgG purified from atopic or non-atopic individuals. At each time point, the frequencies of cells displaying intracellular IFN-γ, IL-17A, TNF, TGF-β and IL-10 production were evaluated by flow cytometry. The results are illustrated by box and whiskers graphs with 25th percentiles, and the Tukey method was used to plot outliers; *p ≤ 0.05 between the indicated groups.
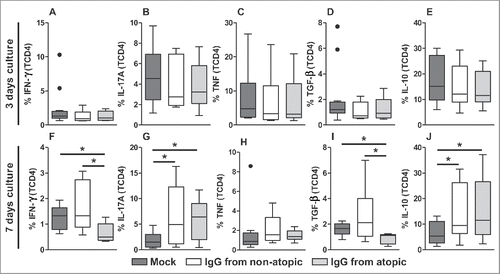
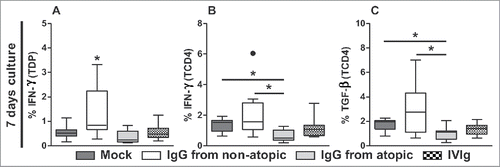
TCD4 cells, similar to TDP cells, did not appear to be influenced by IgG after 3 d in culture in terms of the production of any evaluated cytokine (). IFN-γ production in TCD4 cells appeared to be inhibited by treatment with atopic IgG compared with non-treatment or treatment with non-atopic IgG ( and S3). A similar inhibitory profile was detected with respect to TGF-β production ( and S3). IgG from both sources induced similar levels of IL-17A and IL-10 after 7 d in culture compared with the absence of IgG ( and S3). No effect of IgG on TNF production by TCD4 cells was observed.
Figure 3. Effect of purified IgG on cytokine production by intra-thymic TCD4 cells. Thymocytes from children less than 7 d old (n = 14) were evaluated after 3 (A-E) or 7 d (F-J) in culture in RPMI medium supplemented with FBS in the absence (mock) or presence of 100 µg/mL IgG purified from atopic or non-atopic individuals. At each time point, the frequencies of cells displaying intracellular IFN-γ, IL-17A, TNF, TGF-β and IL-10 production were evaluated by flow cytometry. The results are illustrated by box and whiskers graphs with 25th percentiles, and the Tukey method was used to plot outliers; *p ≤ 0.05 between the indicated groups.
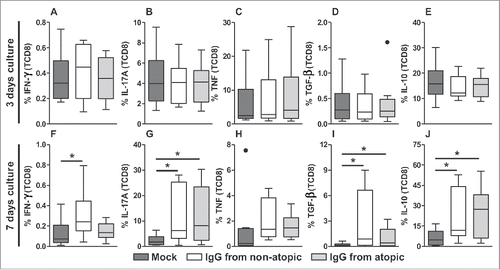
TCD8 cells also did not appear to be influenced by IgG after 3 d in culture in terms of the production of any evaluated cytokine (). Elevated IFN-γ levels were also observed in this cell population after 7 d in culture with IgG from non-atopic individuals, although only compared with the absence of IgG ( and S4). IgG from non-atopic or atopic individuals induced similar increases in the production of the cytokines IL-17A, TGF-β and IL-10 compared with the absence of IgG (, J and S4). However, IgG treatment did not influence TNF production.
Figure 4. Effect of purified IgG on cytokine production by intra-thymic TCD8 cells. Thymocytes from children less than 7 d old (n = 14) were evaluated after 3 (A-E) or 7 d (F-J) in culture in RPMI medium supplemented with FBS in the absence (mock) or presence of 100 µg/mL IgG purified from atopic or non-atopic individuals. At each time point, the frequencies of cells displaying intracellular IFN-γ, IL-17A, TNF, TGF-β and IL-10 production were evaluated by flow cytometry. The results are illustrated by box and whiskers graphs with 25th percentiles, and the Tukey method was used to plot outliers; *p ≤ 0.05 between the indicated groups.
Comparison of the effects of IVIg and atopic or non-atopic IgG
Next, we performed a comparative analysis of the effects of purified IgG that is used as an in vivo human therapy (IVIg) using a culture protocol that replicated our culture experiments. We analyzed the parameters and culture durations that showed a major influence of purified IgG from atopic or non-atopic individuals. Consistent with our above results, after 7 d in culture, TDP cells produced higher levels of IFN-γ in response to non-atopic IgG, and TCD4 cells produced lower levels of IFN-γ and TGF-β in response to atopic IgG (). Interestingly, in all comparative analyses, IVIg induced the production of intermediate levels of IFN-γ and TGF-β compared with purified IgG from atopic and non-atopic individuals ().
Figure 5. Comparison of the effects of IVIg and atopic or non-atopic IgG. Thymocytes from children less than 7 d old (n = 14) were evaluated after 7 d in culture in RPMI medium supplemented with FBS in the absence (mock) or presence of 100 µg/mL IgG purified from atopic or non-atopic individuals or IVIg. At this time point, the frequencies of TDP (A) and TCD4 cells displaying intracellular IFN-γ production (B) and of TCD4 cells displaying TGF-β production(C) were evaluated by flow cytometry. The results are illustrated by box and whiskers graphs with 25th percentiles, and the Tukey method was used to plot outliers; *p ≤ 0.05 between the indicated groups or compared with all other groups.
Evidence of a modulatory effect of IgG on human peripheral TCD4 cells
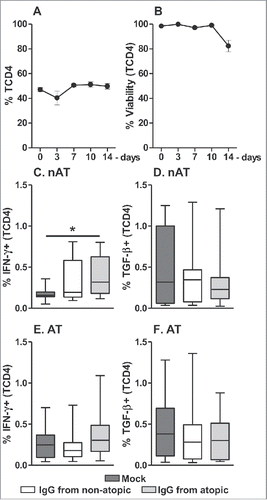
We also evaluated whether mature peripheral TCD4 cells show a similar profile of cytokine responses to that observed in intra-thymic TCD4 cells. For this purpose, we cultured PBMCs from atopic and non-atopic individuals in the presence of atopic or non-atopic IgG and evaluated INF-γ and TGF-β production by TCD4 cells. As the frequency and viability of these cells did not vary until 7 d in culture (), we evaluated this population at the same time points and under the same culture conditions as intra-thymic cells. Among non-atopic PBMCs, elevated production of IFN-γ by TCD4 cells was detected in response to IgG from atopic individuals () relative to the mock condition. However, this response was not observed for PBMCs from atopic individuals (). No influence of IgG on TGF-β production by TCD4 cells in non-atopic or atopic PBMC cultures was found.
Figure 6. Effect of purified IgG on the frequency and viability of human peripheral TCD4 cells. PBMCs from atopic or non-atopic individuals (n = 14) were evaluated at time 0 and after 3, 7, 10 and 14 d in culture in RPMI medium supplemented with FBS. At each time point, the frequency and viability of TCD4 cells were evaluated (A-B). Alternatively, these cells were cultured for 7 d in RPMI medium supplemented with FBS in the absence (mock) or presence of 100 µg/mL IgG purified from atopic or non-atopic individuals. At each time point, the frequency of TCD4 cells from non-atopic (C-D) or atopic (E-F) individuals displaying intracellular IFN-γ and TGF-β production were evaluated by flow cytometry. In the upper panels, the symbols represent the means with standard error. Other results are illustrated by box and whiskers graphs with 25th percentiles, and the Tukey method was used to plot outliers; *p ≤ 0.05 between the indicated groups.
Discussion
To evaluate the effect of IgG antibodies on cytokine production by αβT cells in the human thymus, we collected human thymus specimens from children less than 7 d old born from mothers without an allergic background and who did not exhibit allergic reactions until the surgery, thus avoiding any influence of ambient sensitization. We then performed in vitro experiments using human thymocytes, excluding thymic epithelial cells. We purified IgG using a method based on the principle of removing non-relevant proteins present in human sera without selective IgG binding to avoid interfering with its affinity and to exclude IgG purified from immune complexes.
We used this cell culture model to elucidate the direct effect of interactions between soluble IgG and cell populations in the human thymus. In this context, 4 main interactions can occur: I- IgG antibodies can idiotypically interact with membrane clonal receptors expressed by immature lymphocytes,Citation26-29 with the Fab regions of IgG being responsible for mediating this effect; II- IgG can interact via FcγR receptors expressed on B cells and possibly on thymic dendritic cells (tDCs) and macrophages,Citation30-32 with the Fc regions of IgG playing a pivotal role; III- IgG can permeate the membrane and mediate cytoplasmic interactions,Citation25 with the diversity of the Fab regions of IgG being responsible for mediating these interactions; and IV- IgG can be processed and presented by antigen-presenting cells (including tDCs and B cells) in the context of MHC molecules for recognition by clonal receptors on immature T cells,Citation33,34 with both Fc- and Fab-derived peptides of IgG being involved.
Regarding the interactions cited above, we can conclude that both the Fc and Fab regions of IgG can play a role in mediating their effects. Therefore, as the observed effects on our thymocyte culture are the results of direct interactions by whole IgG antibodies in contact with intra-thymic cells from individuals who were essentially unexposed to allergens and born from mothers without an allergic background, we cannot precisely conclude at this point whether a given IgG region is more important than another.
The induction of cytokine production in human thymocytes by purified IgG antibodies is a novel finding, even more so concerning the differential effects of IgG from atopic or non-atopic individuals. This previous lack of evidence could largely be due to the difficulty in obtaining human thymus samples, especially thymus samples from new-borns with a described allergic background.
Interestingly, our results also revealed that atopic and non-atopic IgG exhibit distinct cytokine-inducing potentials. TDP and TCD8 cells produced more IFN-γ only in response to non-atopic IgG, and TCD4 cells produced less IFN-γ in response to atopic IgG.
In the literature, as noted above, no study has examined the regulation of cytokine production in human thymocytes by purified IgG, but a similar profile was described in PBMC cultures. Using a pool of purified human IgG antibodies (IVIg), which is routinely used in the treatment of patients with primary immunodeficiencies,Citation35 IgG was found to decrease IFN-γ levels in PBMC culture supernatants.Citation21 Similar cell culture experiments that used immature cells from umbilical cord blood reported reduced IFN-γ secretion after stimulation with pooled IgG.Citation24 In this study, IgG treatment stimulated T cells, potentially suggesting that an idiotypic interaction can occur but not excluding the influence of other cells, mainly dendritic cells, which can process and present antibody-derived peptides. We did not evaluate whether our observations also reflect cytokine secretion modulations due to technical difficulties; all cytokines were produced at very low levels.
Due to the shortage of publications describing the use of purified IgG from atopic and non-atopic individuals, as a comparative analysis, the effect of IVIg on cytokine production by newborn thymocytes was examined. These experiments revealed the effect of non-atopic IgG on IFN-γ production by TDP cells and atopic IgG on IFN-γ production by TCD4 cells. Additionally, IVIg induced intermediate levels of IFN-γ production compared with the 2 tested sources of IgG and similar levels compared with the mock condition. The similarity between the IVIg and mock conditions is possibly due to the very low dose of IVIg used compared with other studies, which we discuss in further detail below.
IVIg is produced from the plasma of thousands of donors, and the exclusion criteria included only parameters regarding possible infection and prior transfusions. While the frequency of atopy in the population of developed countries is approximately 40%Citation36 and this parameter was not used as an exclusion criterion, the IVIg used in the treatment of patients possibly reflects a mixture of IgG from atopic and non-atopic individuals. This characteristic of IVIg may explain its intermediate effects in comparison to our pooled samples of atopic and non-atopic IgG.
Taken together, these results suggest that the repertoire of IgG antibodies from atopic and non-atopic individuals can differentially influence cytokine production by αβT cells during their maturation in humans. Considering that IgG from atopic and non-atopic individuals differs mainly in idiotypic diversity, we speculate that the IgG repertoire is responsible for the diverse effects of pooled IgG.
In the present work, we cannot conclude that the observed effect is mediated by direct interactions between IgG and αβT cells; for this, more experiments with purified αβT cells are required. Using purified populations, we could also overcome another limitation of this study by evaluating secreted proteins to certify that this modulation results in altered secretion by cells. Another limitation was the purity of the IgG used: we could not ensure the absence of other molecules from the IgG pools. However, any contaminants would have been present at very levels, and therefore we believe that the observed effects occurred as a consequence of the presence of IgG. Moreover, we used a commercial procedure to purify IgG that has been widely used to evaluate the effects of IgG in vitro and in vivo by our and other groups.Citation14,37-41
Furthermore, as it is possible that this phenomenon occurs in vivo, we can hypothesize that a child born of an atopic mother could be influenced during its gestational period to promote the maturation of T cells with impaired IFN-γ responses, which may favor their development into Th2 cells and allergy development. It is important to highlight that as our observations are unprecedented in the literature, the validation and relevance of our hypothesis must be corroborated with in vivo evidence from future studies.
To determine whether the most pronounced effects of atopic IgG can also be detected after the cells have matured, we also evaluated the effect of IgG antibodies from atopic or non-atopic individuals on mature PBMCs from atopic and non-atopic individuals.
Our results showed that among PBMCs from non-atopic individuals, TCD4 cells increased their production of IFN-γ after contact with IgG from atopic individuals, although this effect was not observed in PBMCs from atopic individuals.
Although the peripheral lymphocytes of atopic and non-atopic individuals were expected to exhibit differences in their potential to produce cytokines, this finding is novel. These results reveal that non-atopic individuals possess a TCD4 repertoire that can produce IFN-γ in response to the IgG pool from atopic individuals. As the IgG pool of atopic individuals contains a wide variety of anti-allergen idiotypes, it is possible that after reaching maturity, TCD4 cells from non-atopic individuals are able to facilitate the production of Th1 cytokines during humoral responses to new allergen idiotypes.
Furthermore, IgG from atopic subjects suppressed the production of TGF-β by intra-thymic TCD4 cells, indicative of a reduced Th3 response. Allergen-specific TCD4 cells that produce TGF-β can induce the suppression of immune responses and control allergic inflammation caused by exposure to an allergen.Citation42 These Th3 cells play an important role in the maintenance of immune tolerance to allergens in childrenCitation43; therefore, a reduced abundance of these cells could be associated with allergy development in children. We evaluated the frequency of Th3 cells among mature TCD4 cells in peripheral blood, but we did not detect any influence of IgG exposure on TGF-β production. This finding cannot rule out the possibility that modulation of Th3 cells can occur because this cell population preferentially migrates to mucosal sites, where these cells perform important functions in inducing oral tolerance.Citation44
These findings, particularly the observed effects of IgG on IFN-γ production by TCD4 cells, suggest that the hypothesis regarding a modulatory role of IgG in children from atopic mothers can become a reality.
Another notable parameter of this study is the selected dose of IgG. In general, studies that evaluated the effect of IVIg in vitro used 6.0 mg/mL IVIgCitation45 because this dose represents the expected elevated concentration in response to the in vivo administration of 500 mg/kg IVIg, which is the standard dose adopted as a therapy. In our study, we used a dose that was previously standardized in vitro according to the results of an assay of the inhibition of lymphocytes by purified IgG.Citation14 This dose is 10- to 50-fold lower than the dose typically used in the literature, and our results suggest that a modulatory effect can be observed at a lower dose.
In conclusion, we present evidence of a possible novel mechanism of immune modulation mediated by human IgG. The proposed mechanism consists of IgG influencing the intra-thymic maturation of αβT cells in a manner that can potentially modulate the peripheral immune status of children and may favor or inhibit the development of allergies depending on the maternal atopic state. Translating to in vivo conditions, our hypothesis may suggest that the classification of IVIg donors as atopic or non-atopic individuals might not only represent a novel alternative approach to elucidate the effects of IVIg from different sources and but also yield new alternatives to IVIg therapy.
Materials and methods
Patient samples
Thymic tissues were obtained from 14 patients who underwent corrective cardiac surgery at the Hospital do Coração (HCor), São Paulo, Brazil. The evaluated patients did not exhibit signs of immunodeficiency, genetic syndromes or allergic reactions, and a patient age of less than 7 d was used as an inclusion criterion (patient age, mean ± standard error (SE): 3.4 ± 0.54 days). Parental allergic background was evaluated, and only children from non-atopic mothers were included in this study.
Additionally, blood samples were collected from subjects who were previously clinical classified as atopic or non-atopic individuals and voluntarily submitted to a skin prick test (SPT) to confirm the atopic state. These individuals were classified into 2 groups: atopic individuals (clinically allergic and reactive to at least 2 allergens, n = 14, patient age, mean ± SE: 31.1 ± 2.86) and non-atopic individuals (without any clinical allergy symptoms and not reactive to any tested allergen, n = 14, patient age, mean ± SE: 28.2 ± 3.11).
Each sample of thymic tissue or PBMCs was provided from a different donor and was analyzed in 3 independent experiments. The ethics committees at the HCor and the School of Medicine at the University of São Paulo approved this study. Thymus removal is necessary for a successful surgery, and they are usually discarded. Informed consent was obtained from the parents of all children to use those organs for research, as well as from all volunteers who donated blood samples.
Thymic tissue dissociation, cell isolation, and storage
Thymocytes were released from the tissue samples using enzymatic dissociation, as described recently by our group.Citation46 The thymus was cut into small fragments and placed in 50-mL propylene conical centrifuge tubes. Next, an enzymatic solution (10 mL) consisting of RPMI medium pre-warmed to 37°C, 0.5 mg/mL collagenase A, 0.02 mg/mL DNase I (Roche Diagnostics, Mannheim, Germany), and 5% foetal bovine serum (FBS) was added, and the sample was incubated for 10 minutes at 37°C under continuous agitation. The digested fragments were homogenized gently and filtered through a plastic sieve with a 70-μM mesh screen (Cell Strainer, BD Falcon, CA, USA) to remove aggregates and stromal material. The resultant cell suspensions were washed twice with 50 mL of RPMI medium (Gibco - Life Technologies, Grand Island, NY, USA) pre-warmed to 37°C and containing 0.1 mg/mL collagenase A and 0.02 mg/mL DNase I (Roche Diagnostics, Mannheim, Germany), followed by centrifugation at 540 g for 5 minutes. Next, the pelleted cells were resuspended in 50 mL of cold PBS containing 5 mM EDTA (Sigma-Aldrich, Saint Louis, MO, USA), 0.02 mg/mL DNase I, and 5% FBS (Gibco, Life Technologies, USA), followed by centrifugation at 540 g for 5 minutes. The pelleted cells were immediately resuspended in RPMI medium, and the low-density fraction was collected following centrifugation through Ficoll-Paque (GE Healthcare Bio Science, Uppsala, Sweden) at 540 g for 20 minutes. The cells were washed twice with RPMI medium and centrifuged at 540 g for 10 minutes. The thymic cells were frozen and stored at −80°C until use.
SPT and blood sample collection
The SPTs were performed in accordance with European standardsCitation47 with an adapted panel of allergens that included the profile of Brazilian allergens (i.e., Blomia tropicalis, Canis familiaris, Periplaneta americana, Aspergillus fumigatus, Penicillium notatum, Alternaria alternata, Cladosporium herbarum, Dermatophagoides pteronyssinus, Dermatophagoides farinae, and Felis domesticus). Briefly, one drop of each allergen extract, histamine (positive control) or the allergen diluent (negative control) provided by IPI ASAC was applied to the volar forearm. A superficial skin puncture was made through each allergen or control drop using a hypodermic needle (Alko, Brazil) without inducing bleeding. After 15 minutes, the results were recorded by measuring the transverse diameter of each wheal reaction. Wheals with a diameter of 3 mm greater than that of the negative control were considered positive results. We excluded the results for patients who used antihistamines, glucocorticosteroids or certain other systemic drugs that can influence the SPT results within 15 d before the test. We also excluded volunteers with severe eczema or dermographism.
Thereafter, 2 blood samples were obtained from each individual via venipuncture and placed in tubes without anticoagulants. After the blood samples were centrifuged at 940 g for 10 minutes, the serum was fractionated, pooled and stored at -80°C.
Separation of PBMCs
PBMCs were obtained from atopic and non-atopic individuals via centrifugation in a Ficoll-Paque Plus density gradient. For this purpose, approximately 20-mL blood samples were collected via venipuncture into heparin sodium-containing tubes. The blood was diluted in physiological saline at a 1:1 ratio. Then, the blood sample was placed in conical tubes containing Ficoll-Paque Plus (GE Healthcare, Sweden) at a 1:3 ratio. This material was centrifuged at 540 g for 20 minutes. Subsequently, the cells were washed 2 times in saline and resuspended in 1 mL of RPMI 1640 medium containing 10% FBS. A sample of this suspension was diluted 1:2 in Trypan Blue to assess cell viability and perform cell counting using a Neubauer chamber under an optical microscope.
IgG purification
IgG was purified from pooled serum according to the specifications of the Melon Gel IgG Spin Purification Kit (Thermo, USA). Briefly, 2 mL of purification gel was placed in a column coupled to a sterile polypropylene conical tube and centrifuged for 1 minute at 2.0 x g. The supernatant was discarded, and the purification gel was resuspended in 1 mL of a mild purification buffer at physiological pH and centrifuged for one minute at 2.0 x g. The supernatant was discarded, and the purification gel was again suspended in 1 mL of the same purification buffer and centrifuged for one minute at 2.0 x g. A pooled serum sample was added to the gel at a 1:10 ratio, and the mixture was homogenized for 5 minutes. After centrifuging for one minute at 2.0 x g, the supernatant (purified IgG) was collected, sterilized and stored at -80°C for subsequent use in cell culture experiments. The IgG concentration was determined using Coomassie Protein Assay Reagent (Pierce, USA) according to the manufacturer's instructions. The purity of IgG, evaluated by SDS-PAGE, was above 90%. Thus, this method is more effective than using protein A, but we cannot exclude that other antibody isotypes were potentially present as contaminants at low or undetectable concentrations.
Cell culture and flow cytometry
Suspensions of thymocytes or freshly thawed, separated PBMCs were washed 2 times with 10 mL of RPMI medium at 37°C, followed by centrifugation at 400 x g for 10 minutes. After this procedure, the cells were resuspended in 1 mL of RPMI 1640 medium containing 10% FBS. A sample of this suspension was diluted 1:2 in Trypan Blue (Sigma, USA) to evaluate cell viability and perform cell counting using a Neubauer chamber under an optical microscope (Labor Optik, Germany). Then, 1×106 viable thymocytes or PBMCs were placed in each well of a 96-well culture plate (CoStar, USA) and cultured in 100 μg/mL IgG purified from pooled serum samples from atopic or non-atopic individuals or IVIg in RPMI 1640 medium containing 10% FBS in a total volume of 200 μL. The culture plate was incubated at 37°C in 5% CO2 for 2 or 6 days, as required for the kinetic assessment. Subsequently, 1 µg/mL Brefeldin A (Sigma, Israel) was added to each well of the culture plate, and after 12 hours, cell staining was performed to evaluate cell labeling via flow cytometry.
To evaluate cell viability and the kinetic rate of the investigated populations, thymocytes or PBMCs were cultured in RPMI 1640 culture medium containing 10% FBS, and the cell viability of each examined cell population was evaluated via flow cytometry at time zero (after thawing) and after 3, 7, 10 and 14 d of culture.
For the cell viability analysis, extracellular staining was performed as described above, and the cells were incubated with 0.04 μL of Live/Dead (PE-Texas red) fluorescent reagent (ThermoFisher, USA) diluted in 1 mL of 1X PBS. The cells were incubated at room temperature for 30 minutes while protected from light, washed and resuspended in 250 μL of 1X PBS. All extracellular and intracellular analyses were performed using viable cells.
To perform extracellular staining, thymocytes or PBMCs at a concentration of 0.5×106 cells/mL were transferred to test tubes, and 1 μg of each antibody was added to the cells (except to the unlabelled tubes). Then, the samples were incubated for 30 minutes at 4°C while protected from light. Thereafter, 500 μL of 1X PBS solution was added, and the tubes were centrifuged at 400 x g for 5 minutes. The supernatant was discarded by inverting each tube. Then, 300 μL of 1X PBS was added, followed by fixation in 200 μL of 1% formaldehyde for at least 10 minutes. Thymocytes were stained with mouse anti-human CD4-V605, CD8-APC-Cy7 or isotype control antibodies (BD PharMingen, NJ, USA) to identify populations of TDP cells (CD4+CD8+), TCD8 cells (CD4-CD8+) and TCD4 cells (CD4+CD8-), as illustrated (Fig. S1). Similarly, among PBMCs, we identified populations of TCD8 cells (CD4-CD8+) and TCD4 cells (CD4+CD8-).
To perform intracellular labeling, tubes were centrifuged at 400 x g for 5 minutes. The supernatant was discarded, and 1 μg of each antibody was added to the cells (except to the unlabelled tubes). Then, 100 µL of 1X PBS containing 0.05% saponin was added, and the tubes were stored at 4°C for 30 minutes while protected from light. After centrifugation at 400 x g for 5 minutes, the supernatant was discarded by inverting each tube, and the cells were resuspended in 300 μL of 1X PBS solution. Thymocytes and PBMCs were stained with mouse anti-human IFN-γ-V450, IL-17A-Alexa700, TNF-PE-Cy7, TGF-β-PE, IL-10-TexasRed or isotype control conjugated with the corresponding fluorochromes (BD PharMingen, New Jersey, USA).
Using an LSRII Fortessa flow cytometer (BD Biosciences, USA), 500,000 events per sample were acquired in the quadrant of lymphocytes (as determined by their relative size/granularity). Compensation was performed using adsorbed microspheres (CompBeads, BD Biosciences, USA) treated with the same antibodies used for extra- and intracellular staining. All antibodies were titrated to define 1 μg as an optimal concentration for specific staining. Cell gating was based on the specific isotype control values as well as the fluorochrome minus 1 setting, when needed. Data analysis was performed using FlowJo software (Tree Star, Ashland, OR, USA), and only the extra- and intracellular staining of viable cells was analyzed.
Statistical analysis
Comparisons among the evaluated groups were performed using the nonparametric Mann–Whitney test (to compare selected pairs of columns). Statistical significance was defined as a p value <0.05 determined using GraphPad Prism software (CA, USA).
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest related to this paper.
Supplementary_Materials.pdf
Download PDF (799.1 KB)Funding
This study was funded by grants from the Laboratory of Medical Investigation-56, Medical School, University of São Paulo, São Paulo, Brazil (LIM-56 HC-FMUSP); São Paulo Research Foundation (FAPESP - grant #2015/17256–3), the São Paulo Administrative Development Foundation (FUNDAP) and The National Council for Scientific and Technological Development (CNPq – grant #115603/2015–8).
References
- de Lima Lira AA, de Oliveira MG, de Oliveira LM, da Silva Duarte AJ, Sato MN, Victor JR. Maternal immunization with ovalbumin or Dermatophagoides pteronyssinus has opposing effects on FcγRIIb expression on offspring B cells. Allergy Asthma Clin Immunol 2014; 10(1):47; PMID:25221605; https://doi.org/10.1186/1710-1492-10-47
- Fusaro AE, Maciel M, Victor JR, Oliveira CR, Duarte AJS, Sato MN. Influence of maternal murine immunization with Dermatophagoides pteronyssinus extract on the type I hypersensitivity response in offspring. Int Arch Allergy Immunol 2002; 127(3):208-16; PMID:11979046; https://doi.org/10.1159/000053865
- Fusaro AE, Brito CA, Victor JR, Rigato PO, Goldoni AL, Duarte AJS, Sato MN. Maternal-fetal interaction: preconception immunization in mice prevents neonatal sensitization induced by allergen exposure during pregnancy and breastfeeding. Immunology 2007; 122(1):107-15; PMID:17608811; https://doi.org/10.1111/j.1365-2567.2007.02618.x
- Victor JR, Fusaro AE, Duarte AJD, Sato MN. Preconception maternal immunization to dust mite inhibits the type I hypersensitivity response of offspring. J Allergy Clin Immunol 2003; 111(2):269-77; PMID:12589344; https://doi.org/10.1067/mai.2003.39
- Fusaro AE, de Brito CA, Taniguchi EF, Muniz BP, Victor JR, Orii NM, da Silva Duarte AJ, Sato MN. Balance between early life tolerance and sensitization in allergy: dependence on the timing and intensity of prenatal and postnatal allergen exposure of the mother. Immunology 2009; 128(1):e541-50; PMID:19740315; https://doi.org/10.1111/j.1365-2567.2008.03028.x
- Rigato PO, Fusaro AE, Victor JR, Sato MN. Maternal immunization to modulate the development of allergic response and pathogen infections. Immunotherapy 2009; 1(1):141-56; PMID:20635979; https://doi.org/10.2217/1750743X.1.1.141
- Muniz BP, Victor JR, Oliveira LDM, de Lima Lira AA, Perini A, Olivo CR, Arantes-Costa FM, Martins MA, da Silva Duarte AJ, Sato MN. Tolerogenic microenvironment in neonatal period induced by maternal immunization with ovalbumin. Immunobiology 2014; 219(5):377-84; PMID:24582301; https://doi.org/10.1016/j.imbio.2014.01.002
- Oliveira CR, Taniguchi EF, Fusaro AE, Victor JR, Brito CA, Duarte A, Sato MN. Bystander effect in synergy to anergy in oral tolerance of Blomia tropicalis/ovalbumin murine co-immunization model. J Clin Immunol 2005; 25(2):153-61; PMID:15821892; https://doi.org/10.1007/s10875-005-2821-3
- Brito CA, Fusaro AE, Victor JR, Goldoni AL, Rigato PO, Duarte AJS, Sato MN. CpG induces Th1 response in neonatal mice but does not suppress anaphylactic IgG1 response: possible role of low TLR-9 expression. Clin Immunol 2007; 123:S78-9; https://doi.org/10.1016/j.clim.2007.03.403
- Futata E, de Brito CA, Victor JR, Fusaro AE, Oliveira CR, Maciel Y, Duarte AJD, Sato MN. Long-term anergy in orally tolerized mice is linked to decreased B7.2 expression on B cells. Immunobiology 2006; 211(3):157-66; PMID:16530083; https://doi.org/10.1016/j.imbio.2005.08.006
- Jarrett EE, Hall E. IgE suppression by maternal IgG. Immunology 1983; 48(1):49-58; PMID:6848454
- Jenmalm MC, Björkstén B. Cord blood levels of immunoglobulin G subclass antibodies to food and inhalant allergens in relation to maternal atopy and the development of atopic disease during the first 8 years of life. Clin Exp Allergy 2000; 30(1):34-40; PMID:10606928; https://doi.org/10.1046/j.1365-2222.2000.00771.x
- Vance GH, Grimshaw KE, Briggs R, Lewis SA, Mullee MA, Thornton CA, Warner JO. Serum ovalbumin-specific immunoglobulin G responses during pregnancy reflect maternal intake of dietary egg and relate to the development of allergy in early infancy. Clin Exp Allergy 2004; 34(12):1855-61; PMID:15663559; https://doi.org/10.1111/j.1365-2222.2004.02111.x
- Victor JR, Muniz BP, Fusaro AE, de Brito CA, Taniguchi EF, Duarte AJS, Sato MN. Maternal immunization with ovalbumin prevents neonatal allergy development and up-regulates inhibitory receptor Fc gamma RIIB expression on B cells. BMC Immunol 2010; 11(1):11; PMID:20222978; https://doi.org/10.1186/1471-2172-11-11
- Macchiaverni P, Arslanian C, Frazão JB, Palmeira P, Russo M, Verhasselt V, Condino-Neto A. Mother to child transfer of IgG and IgA antibodies against Dermatophagoides pteronyssinus. Scand J Immunol 2011; 74(6):619-27; PMID:21883350; https://doi.org/10.1111/j.1365-3083.2011.02615.x
- Chan YC, Ramadani F, Santos AF, Pillai P, Ohm-Laursen L, Harper CE, Fang C, Dodev TS, Wu SY, Ying S, et al. “Auto-anti-IgE:” naturally occurring IgG anti-IgE antibodies may inhibit allergen-induced basophil activation. J Allergy Clin Immunol 2014; 134(6):1394-401; PMID:25112697; https://doi.org/10.1016/j.jaci.2014.06.029
- Kukkonen AK, Savilahti EM, Haahtela T, Savilahti E, Kuitunen M. Ovalbumin-specific immunoglobulins A and G levels at age 2 years are associated with the occurrence of atopic disorders. Clin Exp Allergy 2011; 41(10):1414-21; PMID:21771118; https://doi.org/10.1111/j.1365-2222.2011.03821.x
- Victor JR. Influence of maternal immunization with allergens on the thymic maturation of lymphocytes with regulatory potential in children: a broad field for further exploration. J Immunol Res. 2014; 2014:780386; PMID:25009823; https://doi.org/10.1155/2014/780386
- Victor JR. Allergen-specific IgG as a mediator of allergy inhibition: Lessons from mother to child. Hum Vaccin Immunother 2017; 13(3):507–513; PMID:27808600; https://doi.org/10.1080/21645515.2016.1244592
- Chen ST, Tobe T, Iosobe Y, Chiu AF. Cellular sites of immunoglobulins. VI. Localization of immunoglobulins in the human thymus. Acta Pathol Jpn 1975; 25(1):69-73; PMID:1094798
- Sticherling M, Trawinski H. Effects of intravenous immunoglobulins on peripheral blood mononuclear cell activation in vitro. Ann N Y Acad Sci 2007; 1110(1):694-708; PMID:17911484; https://doi.org/10.1196/annals.1423.072
- Siedlar M, Strach M, Bukowska-Strakova K, Lenart M, Szaflarska A, Węglarczyk K, Rutkowska M, Baj-Krzyworzeka M, Pituch-Noworolska A, Kowalczyk D, et al. Preparations of intravenous immunoglobulins diminish the number and proinflammatory response of CD14+CD16++ monocytes in common variable immunodeficiency (CVID) patients. Clin Immunol 2011; 139(2):122-32; PMID:21300572; https://doi.org/10.1016/j.clim.2011.01.002
- Gille C, Dreschers S, Spring B, Tárnok A, Bocsi J, Poets CF, Orlikowsky TW. Differential modulation of cord blood and peripheral blood monocytes by intravenous immunoglobulin. Cytometry B Clin Cytom 2012; 82(1):26-34; PMID:21812105; https://doi.org/10.1002/cyto.b.20609
- Tawfik DS, Cowan KR, Walsh AM, Hamilton WS, Goldman FD. Exogenous immunoglobulin downregulates T-cell receptor signaling and cytokine production. Pediatr Allergy Immunol 2012; 23(1):88-95; PMID:21265884; https://doi.org/10.1111/j.1399-3038.2010.01129.x
- Sali AD, Karakasiliotis I, Evangelidou M, Avrameas S, Lymberi P. Immunological evidence and regulatory potential for cell-penetrating antibodies in intravenous immunoglobulin. Clin Transl Immunol 2015; 4(10):e42; https://doi.org/10.1038/cti.2015.18
- Borghesi C, Nicoletti C. Autologous anti-idiotypic antibody response is regulated by the level of circulating complementary idiotype. Immunology 1996; 89(2):172-7; PMID:8943710; https://doi.org/10.1046/j.1365-2567.1996.d01-724.x
- Vakil M, Sauter H, Paige C, Kearney JF. In vivo suppression of perinatal multispecific B cells results in a distortion of the adult B cell repertoire. Eur J Immunol 1986; 16(9):1159-65; PMID:2428628; https://doi.org/10.1002/eji.1830160921
- Bogen B, Dembic Z, Weiss S. Clonal deletion of specific thymocytes by an immunoglobulin idiotype. EMBO J. 1993; 12(1):357-63; PMID:8428591
- Ghosh SK, Chakrabarti D. Immunoregulation by processed immunoglobulin on B-cells. Indian J Biochem Biophys 1993; 30(6):414-21; PMID:7911781
- Rabinovitch N, Gelfand EW. Expression of functional activating and inhibitory Fcgamma receptors on human B cells. Int Arch Allergy Immunol 2004; 133(3):285-94; PMID:14976398; https://doi.org/10.1159/000076836
- Evans VA, Cameron PU, Lewin SR. Human thymic dendritic cells: regulators of T cell development in health and HIV-1 infection. Clin Immunol 2008; 126(1):1-12; PMID:17916443; https://doi.org/10.1016/j.clim.2007.08.016
- Soga H, Nakamura M, Yagi H, Kayaba S, Ishii T, Gotoh T, Itoh T. Heterogeneity of mouse thymic macrophages: I. Immunohistochemical analysis. Arch Histol Cytol 1997; 60(1):53-63; PMID:9161689; https://doi.org/10.1679/aohc.60.53
- Weist BM, Kurd N, Boussier J, Chan SW, Robey EA. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat Immunol 2015; 16(6):635-41; PMID:25939026; https://doi.org/10.1038/ni.3171
- Perera J, Meng L, Meng F, Huang H. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proc Natl Acad Sci U S A 2013; 110(42):17011-6; PMID:24082098; https://doi.org/10.1073/pnas.1313001110
- Lemieux R, Bazin R, Néron S. Therapeutic intravenous immunoglobulins. Mol Immunol 2005; 42(7):839-48; PMID:15829272; https://doi.org/10.1016/j.molimm.2004.07.046
- Weinberger MM. What is the problem with asthma care for children? Arch Pediatr Adolesc Med 2011; 165(5):473-5; PMID:21536964; https://doi.org/10.1001/archpediatrics.2011.46
- Mara-Koosham G, Hutt JA, Lyons CR, Wu TH. Antibodies contribute to effective vaccination against respiratory infection by type A Francisella tularensis strains. Infect Immun 2011; 79(4):1770-8; PMID:21282410; https://doi.org/10.1128/IAI.00605-10
- Mahan AE, Tedesco J, Dionne K, Baruah K, Cheng HD, De Jager PL, Barouch DH, Suscovich T, Ackerman M, Crispin M, et al. A method for high-throughput, sensitive analysis of IgG Fc and Fab glycosylation by capillary electrophoresis. J Immunol Methods 2015; 417:34-44; PMID:25523925; https://doi.org/10.1016/j.jim.2014.12.004
- Dugast AS, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, Streeck H, Suscovich TJ, Ghebremichael M, Ackerman ME, et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS One 2014; 9(5):e97229; PMID:24820481; https://doi.org/10.1371/journal.pone.0097229
- Ankeny DP, Guan Z, Popovich PG. B cells produce pathogenic antibodies and impair recovery after spinal cord injury in mice. J Clin Invest 2009; 119(10):2990-9; PMID:19770513; https://doi.org/10.1172/JCI39780
- Rigato PO, Maciel M, Goldoni AL, Piubelli OG, Orii NM, Marques ET, August JT, Duarte AJ, Sato MN. Maternal LAMP/p55gagHIV-1 DNA immunization induces in utero priming and a long-lasting immune response in vaccinated neonates. PLoS One 2012; 7(2):e31608; PMID:22355381; https://doi.org/10.1371/journal.pone.0031608
- Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol 2003; 33(5):1205-14; PMID:12731045; https://doi.org/10.1002/eji.200322919
- Pérez-Machado MA, Ashwood P, Thomson MA, Latcham F, Sim R, Walker-Smith JA, Murch SH. Reduced transforming growth factor-beta1-producing T cells in the duodenal mucosa of children with food allergy. Eur J Immunol 2003; 33(8):2307-15; PMID:12884306; https://doi.org/10.1002/eji.200323308
- MacDonald TT. T cell immunity to oral allergens. Curr Opin Immunol 1998; 10(6):620-7; PMID:9914215; https://doi.org/10.1016/S0952-7915(98)80079-4
- Wu KH, Wu WM, Lu MY, Chiang BL. Inhibitory effect of pooled human immunoglobulin on cytokine production in peripheral blood mononuclear cells. Pediatr Allergy Immunol 2006; 17(1):60-8; PMID:16426257; https://doi.org/10.1111/j.1399-3038.2005.00344.x
- Bento-de-Souza L, Victor JR, Bento-de-Souza LC, Arrais-Santos M, Rangel-Santos AC, Pereira-Costa É, Raniero-Fernandes E, Seixas-Duarte MI, Oliveira-Filho JB, Silva Duarte AJ. Constitutive expression of genes encoding notch receptors and ligands in developing lymphocytes, nTreg cells and dendritic cells in the human thymus. Results Immunol 2016; 6:15-20; PMID:27504259; https://doi.org/10.1016/j.rinim.2016.04.001
- Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, Durham S, Fokkens W, Gjomarkaj M, Haahtela T, et al. The skin prick test-European standards. Clin Transl Allergy 2013; 3(1):3; PMID:23369181; https://doi.org/10.1186/2045-7022-3-3
