ABSTRACT
Autologous dentritic cell immunotherapy has been proven effective in treating tumors outside the central nervous system. Current evidence from phase I and II trials suggest a similar efficacy for central nervous system tumors as well and that an active immune response against these tumors can be generated. We aim to review the literature to identify the types of immune responses against gliomas found to be generated by dendritic cell vaccinations and the types of immune cells subsequently infiltrating the glioma microenvironment. A systematic review of the literature was performed by searching the online databases PubMEd, Google Scholar, and EMBASE with use of the keywords intratumoral, infiltration, lymphocytic, vaccination and gliomas. Seven studies reporting lymphocytic infiltration of gliomas microenvironment were identified. Three studies (42.8%) reported presence of tumor infiltrating lymphocytes in 50%, 50% and 28.6% of included patients respectively in the post–vaccination specimens that were not present in the pre–vaccination samples. The remaining 4 (57.2%) reported an up to 6-fold increase in the number of pre-existing lymphocytes following vaccination. Present data indicate that tumor infiltration by lymphocytes can be induced by dentritic cell immunotherapy and that this may positively affect clinical outcome. It still remains unclear which factors influence the above reaction and therefore prediction of response to treatment is still not possible.
Introduction
Background
Glioblastoma multiforme (GBM) remains one of the most lethal tumors despite recent treatment advances in neuro-oncology. Treatment with combination of surgical resection, radiotherapy and chemotherapy is standard of care and provides a statistically significant survival benefit with minimal additional toxicity when compared with treatment with resection and radiotherapy alone.Citation1 Despite the latter though, overall survival remains very poor with median overall survival of 14.6 months, 2 y survival of 26.5% and 5 y survival of 9.7%.Citation1 Different approaches are currently being investigated with immunotherapy being one of the most appealing and currently under intense investigation. Immunotherapy aims at boosting the patients' immune system to recognize and attack tumor cells.
The infiltration of both animal and human tumors by lymphocytes is known for more than a century now and in the 1960s a possible relation between infiltration and prognosis was considered.Citation2,3 Hamlin was the first to show the relation between lymphocytic infiltration and prognosis in patients with breast cancer.Citation4 Other studies showed that the majority of cells infiltrating tumors were T cells (80%) and that there was positive correlation between the degree of lymphocytic infiltration of primary tumors and the absence of metastases.Citation5 Bertrand and Mannen, in 1960, were the first to report infiltration of glioma microenvironment by lymphocytes,Citation6 followed by Lucio Palma who showed that glioblastoma patients with definite lymphocytic infiltration had significantly longer postoperative survival.Citation7
The presence of the blood-brain barrier, the unique lymphatic drainage of the central nervous system (CNS),Citation8 as well as the distinct and localized actions of the microglia create an immunologically challenging environment within which brain tumors grow, escaping regular immune surveillance observed in peripheral tissues.Citation9 Additionally, it has been shown that in patients with GBMs, there is under-expression of immunostimulatory MHC class I molecules and overexpression of suppressive surface proteins (PD-L1, FasL) and cytokines (IL-10, TGF-b, CCL) which stimulate the accumulation of T regulatory cells and myeloid derived suppressor cells. This leads to impaired proliferation and activation of cytotoxic lymphocytes. Thus accumulation of natural killer and regulatory T cells causes leukopenia and immunological compromiseCitation10-12 ().
Figure 1. Schematic illustration showing the production, administration and in vivo activation of DC vaccines for the treatment of high grade gliomas.
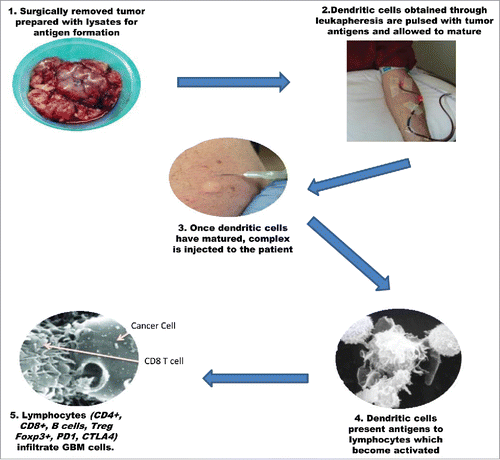
Evidence that the “immunologically privileged” environment of the central nervous system can be overcome and that an immune response against brain tumors can be generated by vaccination with cytokine–producing tumor cells was first described almost 20 y ago. In this study it was shown that tumor–bearing mice had increased overall survival (OS) following subcutaneous vaccination with genetically engineered tumor cells. Interestingly, in the same study, they concluded that, unlike tumors outside the CNS, where the presence of both CD4 and CD8 cells is necessary for an effective response, relative depletion of CD4 cells with increased CD8 cells is associated with better outcome with CNS tumors.Citation13 A significant number of other preclinical studies on various grades of tumors showed similar results with reports of an increase in the OS of animals injected with tumor cells previously cultured with antigen presenting cells.Citation14,15 Notably, even complete resolution was reported in some cases.Citation16
The above observations, combined with the need for a treatment that is both patient and tumor specific as well as safe, triggered research efforts on passive and active immunotherapy for brain tumors. The latter is almost exclusively based on dendritic cell (DC). Of significance, peripheral vaccination with DCs pulsed with tumor antigens has already shown positive results in other types of tumors, including prostate, melanoma, lymphoma and renal cell carcinoma, with minimal side effects and no observed autoimmunity, rendering the technique safe.Citation17-20
Dendritic cells are the most powerful of antigen presenting cells and are able to activate both naïve and memory immune responses. In dendritic cell based immunotherapy for tumors, immature cells are isolated from the patients via leukapheresis. Addition of proinflammatory cytokines induces their maturation which is followed by loading with tumor antigens.Citation21 Once the complex is mature, it is injected back to the patient where dendritic cells act to present the antigens to CD8 and CD4 T cells via MHC class I and II, inducing tumor specific responseCitation22 ().
Objectives
In this study we aim to review the literature and identify all studies reporting lymphocytic infiltration of the tumor microenvironment following peripheral (subcutaneous or intradermal) vaccination with antigen pulsed DCs for the treatment of intracranial high-grade gliomas (HGGs). Additionally we aim to present the evidence relating to the importance of lymphocytic infiltration in human gliomas.
Methods
Inclusion criteria
phase I/II or prospective studies evaluating the efficacy of adjuvant vaccination using DCs previously pulsed with tumor antigens in the treatment of HGGs managed with standard of care protocols.
Human patients
Studies reporting intracranial/intratumoral lymphocytic infiltration
Published in English
Literature review
Using the keywords intratumoral (intratum$), infiltration (infiltr$), lymphocytic (lymphocyte$), vaccination (vaccin$) and gliomas (gliom$) a systematic review of the literature was performed by searching the online databases PubMEd, Google Scholar, and EMBASE. To ensure no studies were missed, the references of included studies were also reviewed. Last search of the literature was performed on 08 November 2015.
Data collection
• General data
○ Author, country, number of patients, trial phase, mean age, I.D or S.C vaccination
• Treatment protocol
• Intratumoral infiltration
○ Number of patients, means of identification
• Outcome
Results
Studies
Original search revealed 29 studies. Removing duplicates left 20 studies for full review. Following further removal of studies not commenting on inctracranial or intratumoal infiltration, animal and non – English studies left 7 that fulfilled all inclusion criteria. Main characteristics of the included studies are presented in .
Table 1. Main characteristics of included studies.
General characteristics of included studies
Three of the above studies took place in the USA (42.8%), one in Taiwan (14.2%), one in Germany (14.2%), one in Australia (14.2%) and one in Japan (14.2%). Four studies were phase I (57.1%), 2 were phase I/II (28.5%) and one prospective study (14.2%). The number of patients included varied from 1222 to 2423 with the mean age varying from 44.724,25 to 51.Citation26,27 The vaccination was administered intradermally (ID) in 5 out of 7 studies (71.4%), subcutaneously (SC) in 1 (14.2%) and either intradermally (ID) or intrathecally (IT) in one (14.2%).
In 6 out of 7 studies the vaccine was produced with tumor antigens pulsed with dendritic cells (DC) that were harvested from the patients' peripheral circulation (85.7%), while in 1 the vaccine was produced from tumor cell cultures infected with Newcastle Disease VirusCitation28 (14.2%).
Treatment protocols
The reviewed studies used different inclusion and exclusion criteria, treatment protocols and tumor infiltration identification techniques. Walker included patients with surgically accessible malignant glioma (WHO grade III or IV), with ECOG (Eastern Cooperative Oncology Group) performance status of 0, 1 or 2 and absence of other significant disease or pregnancy. There was no specific treatment protocol followed before vaccination and patients could receive the vaccine with or without chemotherapy or radiotherapy following surgery.Citation27 Yamanaka, included patients with HGGs and Karnofsky scores varying from 30 to 80 who received vaccination following radiological confirmation of disease progression.Citation23 Steiner, studied patients with Karnofsky scores greater than 60 all of whom had undergone maximum tumor resection and radiotherapy before receiving the vaccine.Citation28 A similar protocol was used in the study of Liau which included GBM patients receiving surgical resection and external beam radiotherapy before vaccination.Citation22 In the study of Prins, the “Stupp” protocol was applied, comprising of surgical resection and concomitant temozolimide chemo radiation, before the vaccination.Citation26 Lastly, Chang, recruited patients with newly diagnosed or recurrent HGGs, newly diagnosed patients received surgical resection with radiation therapy whereas patients with recurrent gliomas were treated solely with surgical resection.Citation24
Immunohistochemical analysis
Similar immunohistochemical techniques were used in all studies to identify tumor microenvironment infiltrating lymphocytes. Serial paraffin sections were cut and stained with antihuman antibodies against CD3, CD8, CD4, CD45, CD45RO and transforming growth factor-b2 in the study of LiauCitation22; versus p53, glial fibrillary acidic protein (GFAP), nestin, CD3, CD4 and CD8 in the study of ChangCitation24; vs. CD8, CD45RO and mouse anti-human in the study of WalkerCitation27; vs. CD3, CD4, CD8, CD20 and CD56 in the study of YamanakaCitation23; vs. CD3 and CD8 for the study of PrinsCitation26; vs. CD8, EGFR (epidermal growth factor) type III deletion, neurofilament protein, GFAP, platelet endothelial cell adhesive molecule in the study of SteinerCitation28; and CD8, CD45RO, CD20 and CD56 for the study of Yu.Citation25 ().
Table 2. Markers used for immunochemistry, reported treatments and performance score before initial vaccination, use of corticosteroids during vaccination treatment and histological diagnosis.
Lymphocytes infiltrating tumor microenvironment
The presence of tumor infiltrating lymphocytes (TILs) and the significance of it was analyzed to various degrees in the included studies. Tumor samples were collected following radiologically proven progression/recurrence and analyzed with immunohistochemistry as mentioned above. Three studies (42.8%) reported presence of TILs in the post–vaccination specimens that were not present in the pre–vaccination samples examined,Citation25,29,30 whereas the remaining 4 (57.2%) reported increase (to various extents) in the numbers of pre-existing lymphocytes following vaccination.
To summarize the specifics, Yu, reported the presence of cytotoxic CD8 lymphocytes as well as the memory CD45RO cells that were not present before vaccination in 3 out of 6 patients (50%) who underwent re-operation for tumor progression.Citation25 Liau reported a robust T-cell infiltration in 4 out of 8 patients (50%) who underwent re-operation, mainly consisting of CD8 and CD45RO and to a lesser degree CD4 helper cells.Citation29 Similar results were published by Yamanaka with a significant accumulation of CD8 and CD4 tumor infiltrating cells in 2 out of 7 (28.6%) patients following vaccination, while no such increase was observed in 5 non-vaccinated patients who underwent re-operation.Citation30 An increase in the number of already pre-existing infiltrating CD8 and CD45RO cells, in all 4 patients undergoing re-operation, was detected by Walker.Citation27 Steiner also reported an up to 6-fold increase in CD8 cytotoxic cells in vaccinated patients compared with very low numbers in the non-vaccinated group.Citation28
Interestingly, Chang, observed 2 major changes in tumor samples received pre- and post-vaccination. Firstly, a shift from perivascular to relatively diffuse TIL infiltration following vaccination and in addition a reversal in the CD4 and CD8 ratio with an increase in the number of CD8 TILs.Citation24 Lastly, Prins, described increased infiltration with CD3 and CD8 lymphocytes following dendritic cell vaccination in patients that had undergone re-operation for progression.Citation26
Clinical outcome and correlation with tumor microenvironment infiltration
Most of the studies included here were originally designed to assess the feasibility and safety of the treatment. Most authors agreed that there was a benefit in terms of OS and progression free survival following peripheral DC vaccination with Liau et al. going into further detail describing their findings.
They reported that out of the 8 patients who underwent re-operation for progression, 4 survived for more than 30 months while 3 survived for less than a year and the remaining one had intermediate survival. The patients that survived more than 30 months had shown a robust CD3 and CD8 lymphocytic tumor infiltration that was not present in the pre-operative tissue samples. This infiltration was not observed in the tumor samples of the 3 patients who survived less than a year.Citation29
Despite not directly correlating their outcomes with immune infiltration, all 6 remaining studies reported improved overall survival in the vaccination treatment group. Overall survival varied from 348 to 1077 d for the vaccination group. In the studies of Yu, Liau, Steiner and Yamanaka, comparison with control groups was performed which showed statistically significant differences between the 2 groups. Most interestingly, Prins et al. showed statistically significant improvement in overall survival, not only when comparing the vaccination group with the controls but also when comparing patients who received treatment at first diagnosis to those receiving treatment following recurrence (p = 0.03).Citation26 ().
Table 3. Survival rates and subgroup analysis.
The most relevant prognostic factors described in these studies, in keeping with the existing literature on high grade gliomas, were patients' age, performance score and treatment protocols used. No information, however, was provided on other potentially relevant parameters such as tumor size, location or its molecular and genetic makeup.
Discussion
Immunotherapy for HGGs is a relatively new concept. Unlike HGGs, in other common types of cancer, such as ovarian, colorectal and melanoma, the presence of TILs is consistently related to better clinical outcome.Citation31-36 The relevant literature on DC-immunotherapy for HGGs is still limited and therefore the number of studies included in our review is small, potentially limiting any didactic conclusions. Nonetheless, data provide indication of the types of lymphocytes that infiltrate malignant gliomas following vaccination, suggest that infiltration can be boosted and also imply a positive correlation between induced infiltration and survival.
There appears to be unanimous agreement on the type of tumor infiltrating cells that appear or increase in numbers following peripheral DC vaccination. These mainly include CD8 cytotoxic, CD45RO memory and to a lesser degree CD4 helper cells. The presence of CD8 and the absence of CD20 lymphocytes in peripherally vaccinated patients suggest a Th1 immune response initiation rather than Th2, without excluding preferential CD8 immigration though.Citation37
Most studies suggested improved clinical outcomes following immunotherapy, potentially indicating that the observed recruitment of immune cells in the tumor's microenvironment could be the determining factor. Such increased survival rates due to increased lymphocytic infiltration would be in agreement with the study of Yang et al. who investigated the correlation between the presence of CD8 cells in the initial tumor specimen and overall survival. They found that 65 out of 108 patients (60.1%) who had extended survival (> 403 days) had an intermediate to extensive T-cell infiltration (p<o.oo6).Citation38 Several other studies, including the study of Brooks et al. showed positive correlation between lymphocytic infiltration and better clinical outcomes.Citation7,39,40 In contrast though, a study by Safdari et al. who assessed the prognostic implications of lymphocytic infiltration in 342 patients with WHO grade III and IV gliomas, showed negative correlation between the presence of TILs and survival.Citation41 Furthermore, the teams of Schiffer and Rossi reported no correlation between infiltration and survival in their studies.Citation42,43 Such observation is hard to explain but glioblastoma molecular heterogeneity, varying treatment and sampling protocols, as well as the lack of lymphocytic sub-type classification (CD4, CD8, T-reg) across studies could have potentially led to these contradicting results.
In our view, the small number of studies together with the relative lack of detailed description of prognostic factors limits any firm conclusions on the relevance of lymphocytic tumor infiltration to survival. The quality of the data and particularly its consistency across a range of studies reviewed here, does however raise the possibility that DC immunotherapy may improve patient outcomes, warranting further studies. It is important to highlight at this point the heterogeneity of the patients included in the mentioned studies. With the exception of 2, the remaining included both patients with newly diagnosed and recurrent malignant gliomas with overall survival data referring to the combination of the groups. Subgroup analysis comparing recurrent to newly diagnosed disease was performed by 2 studies with contradicting results (). Additionally, comparison with control groups is not the result of randomized trials but a comparison to matched or historical controls.
Furthermore, data regarding the safety of the technique is limited. Authors report that the technique was well tolerated with the most commonly reported adverse events being headaches, fever, myalgia, fatigue, lymphopenia and seizures, in a small number of patients and no author has reported any grade 3 or 4 National Cancer Institute Common Toxicity Criteria adverse events. The above indicate that conclusions regarding survival rates, safety and recommendation or not of the technique cannot be safely drawn and that the results of ongoing phase III trials should be awaited.
Although it is well established that glioblastoma induces immunosuppression, the exact mechanisms and patient specific factors underlying those processes remain unclear. Great emphasis is now given on the presence of T regulatory cells that have been shown to downregulate CD4 and CD8 cells and also produce IL-10 and TGB-b which block effector T cell response.Citation44,45 Treatment related immunosuppression investigated by Authier et al indicated that treated glioma cells were more immunosuppressive and formed tumors at a faster rate in vitro and in an animal models, compared with untreated ones.Citation44 Interestingly, increased apoptotic rates of lymphocytes were first described by Walker et al. who demonstrated that T cells expressing Fas ligand (Fas-L, CD95L) were 8 times more susceptible to apoptosis compared with those not expressing Fas ligand.Citation37
Liau proposed that active tumor recurrence and/or bulky residual tumor further negatively influence the ability of T lymphocytes to accumulate within the local tumor microenvironment affecting infiltration following DC vaccination.Citation22 Notably, Yu implied that T cell infiltration is a characteristic of a subset of patients that undergo re-operationCitation25,45 whereas Prins found a positive correlation between increased T lymphocytic infiltration and the mesenchymal gene expression.Citation26 Controversially, a recent study by Yang et al. showed that dendritic cells loaded with autologous tumor lysate increased tumor angiogenesis and indicated for the first time that the latter process could potentially promote tumor progression.Citation46 Of note though, the study was based on an animal model and there is no evidence to date indicating that similar findings would be expected in human cancer and more specifically glioblastoma. Nonetheless, the findings create an important question for future research.
The most important finding of this review is the wide variation in the degree of response to DC-immunotherapy measured by the increase in the numbers of infiltrating lymphocytes. The variations could arguably be attributed to the different protocols followed by each study group but there were significant differences even within individual studies with some patients exhibiting significant, some moderate and some no infiltration at all. The above observations could indicate that there are intrinsic factors, specific to each patient, or group of patients, that determine their response to treatment. Genetic, immunological and molecular profiling of patients, ideally from peripheral blood, tumor tissue or CSF could potentially help identify the characteristics of those patients who are more likely to respond to the treatment as well as help develop patient specific treatment protocols.
In the face of these challenges, dendritic cell immunotherapy for high grade gliomas, has attracted significant interest from the scientific community. There are currently 2 ongoing phase III trials, namely DCVax which uses activated monocytes loaded with antigens from the patient's own tumor tissue, and ICT-107 which uses dendritic cells, pulsed with 6 synthetic peptides derived from tumor associated antigens from glioblastoma cells. At least 5 more phase one and 2 trials, due for completion between 2017 and 2020 are also currently running (from http://clinicaltrials.gov).
Future studies are likely to aim to maximize success of immunotherapy by specifically catering for the peculiarities of the central nervous system and the immunosuppressive properties of glioblastoma. Thus various techniques that can amplify dendritic cell based, tumor specific, immune response, are currently under investigation. Targeted patient selection (pSTAT signaling, mesenchyman gene expression signature, TCR),Citation47,48 administration of adjuvant treatments (tetanus, polyICLC, imiquimod),Citation26,49 as well as novel tumor antigen generation and delivery strategies (CMV RNA, cocktail of peptides)Citation50,51 are being trialed aiming to improve the specificity and the efficiency of the technique.
Conclusion
Within the limitations imposed by small numbers and potential biases, we reviewed the literature in an attempt to evaluate the degree of immune cell infiltration of malignant glioma microenvironment induced by peripheral DC immunotherapy. Present data indicate that tumor infiltration by lymphocytes is feasible, that it can be induced/boosted by DC immunotherapy and that this may positively affect clinical outcome. It still remains unclear which factors enhance or restrict the phenomenon and therefore determine patients' response to treatment. However, current evidence create numerous opportunities for further research, not only to characterize the types of immune changes following immunotherapy but also to identify the patients that will benefit from it the most.
Abbreviations
| CNS | = | central nervous system |
| CD | = | cluster of differentiation |
| DC | = | dendritic cell |
| EGFR | = | epidermal growth factor |
| GBM | = | glioblastoma multiforme |
| GFAP | = | glial fibrillary acidic protein |
| HGGs | = | high grade gliomas |
| ID | = | intradermally |
| IT | = | intrathecally |
| MHC | = | major histocompatibility complex |
| OS | = | overall survival |
| SC | = | subcutaneously |
| TILs | = | tumor infiltrating lymphocytes |
| WHO | = | world health organization |
Disclosure of potential conflict of interests
No potential conflicts of interest or financial benefits to disclose.
References
- Stupp R, Mason WP, Van Den Bent Martin J, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi:10.1056/NEJMoa043330. PMID:15758009
- MacCarty WC. Factors which influence longevity in cancer. Ann Surg. 1922;76(1):9–12; PMID:17864672
- Wade H. Of its relationship to cancer. J Pathol Bacteriol. 1908;12:384. doi:10.1002/path.1700120221
- Hamlin IM. Possible host resistance in carcinoma of the breast: A histological study. Br J Cancer. 1968;22(3):383–401. doi:10.1038/bjc.1968.47. PMID:5681002
- Husby G, Hoagland PM, Strickland RG, Williams RC, Jr. Tissue T and B cell infiltration of primary and metastatic cancer. J Clin Invest. 1976;57(6):1471–1482. doi:10.1172/JCI108417. PMID:180052
- Bertrand I, Mannen H. Etude des reactions vasculaires dans les astrocytomes. Rev Neurol. 1960;102:3–19
- Palma L, Di Lorenzo N, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas: Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg. 1978;49(6):854–61. doi:10.3171/jns.1978.49.6.0854. PMID:731302
- Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80(4):797–801. doi:10.1189/jlb.0306176. PMID:16885505
- Polyzoidis S, Tuazon J, Brazil L, Beaney R, Al-Sarraj ST, Doey L, Logan J, Hurwitz V, Jarosz J, Bhangoo R, et al. Active dendritic cell immunotherapy for glioblastoma: Current status and challenges. Br J Neurosurg. 2015;29(2):197–205. doi:10.3109/02688697.2014.994473. PMID:25541743
- Ochs K, Sahm F, Opitz CA, Lanz TV, Oezen I, Couraud PO, von Deimling A, Wick W, Platten M. Immature mesenchymal stem cell-like pericytes as mediators of immunosuppression in human malignant glioma. J Neuroimmunol. 2013;265(1):106–16. doi:10.1016/j.jneuroim.2013.09.011. PMID:24090655
- Sims JS, Ung TH, Neira JA, Canoll P, Bruce JN. Biomarkers for glioma immunotherapy: The next generation. J Neurooncol. 2015;123(3):359–72
- Smyth MJ, Godfrey DI, Trapani JA. A fresh look at tumor immunosurveillance and immunotherapy. Nat Immunol. 2001;2(4):293–99. doi:10.1038/86297. PMID:11276199
- Sampson JH, Archer GE, Ashley DM, Fuchs HE, Hale LP, Dranoff G, Bigner DD. Subcutaneous vaccination with irradiated, cytokine-producing tumor cells stimulates CD8+ cell-mediated immunity against tumors located in the “immunologically privileged” central nervous system. Proc Natl Acad Sci U S A. 1996;93(19):10399–404. doi:10.1073/pnas.93.19.10399. PMID:8816812
- Liau LM, Black KL, Prins RM, Sykes SN, DiPatre PL, Cloughesy TF, Becker DP, Bronstein JM. Treatment of intracranial gliomas with bone marrow-derived dendritic cells pulsed with tumor antigens. J Neurosurg. 1999;90(6):1115–24. doi:10.3171/jns.1999.90.6.1115. PMID:10350260
- Broder H, Anderson A, Kremen TJ, Odesa SK, Liau LM. MART-1 adenovirus-transduced dendritic cell immunization in a murine model of metastatic central nervous system tumor. J Neurooncol. 2003;64(1-2):21–30. doi:10.1007/BF02700017. PMID:12952283
- Siesjö P, Visse E, Sjögren HO. Cure of established, intracerebral rat gliomas induced by therapeutic immunizations with tumor cells and purified APC or adjuvant IFN-U03B3 treatment. Journal of Immunotherapy. 1996;19(5):334–45. doi:10.1097/00002371-199609000-00003. PMID:8941873
- Hsu FJ, Benike C, Fagnoni F, Liles TM, Czerwinski D, Taidi B, Engleman EG, Levy R. Vaccination of patients with b–cell lymphoma using autologous antigen–pulsed dendritic cells. Nat Med. 1996;2(1):52–8. doi:10.1038/nm0196-52. PMID:8564842
- Nestle FO, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Vaccination of melanoma patients with peptide-or tumorlysate-pulsed dendritic cells. Nat Med. 1998;4(3):328–32. doi:10.1038/nm0398-328. PMID:9500607
- Tjoa B, Simmons S, Bowes V, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, et al. Evaluation of phase I/II clinical trials in prostate cancer with dendritic cells and PSMA peptides. Prostate. 1998;36(1):39–44. doi:10.1002/(SICI)1097-0045(19980615)36:1%3c39::AID-PROS6%3e3.0.CO;2-6. PMID:9650914
- Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P, Trefzer U, Ullrich S, Müller CA, Becker V, Gross AJ, Hemmerlein B, Kanz L, Muller GA, Ringert R-H. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell–dendritic cell hybrids. Nat Med. 2000;6(3):332–36. doi:10.1038/73193. PMID:10700237
- Sabado RL, Bhardwaj N. Dendritic cell immunotherapy. Ann N Y Acad Sci. 2013;1284(1):31–45. doi:10.1111/nyas.12125. PMID:23651191
- Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, Roth MD. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–25. doi:10.1158/1078-0432.CCR-05-0464. PMID:16061868
- Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, Yoshida S, Abe T, Narita M, Takahashi M, Tanaka R. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: Results of a clinical phase I/II trial. Clin Cancer Res. 2005;11(11):4160–67. doi:10.1158/1078-0432.CCR-05-0120. PMID:15930352
- Chang C, Huang Y, Yang D, Kikuta K, Wei KJ, Kubota T, Yang WK. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18(8):1048–54. doi:10.1016/j.jocn.2010.11.034. PMID:21715171
- Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64(14):4973–79. doi:10.1158/0008-5472.CAN-03-3505. PMID:15256471
- Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, Nelson SF, Liau LM. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–15. doi:10.1158/1078-0432.CCR-10-2563. PMID:21135147
- Walker DG, Laherty R, Tomlinson FH, Chuah T, Schmidt C. Results of a phase I dendritic cell vaccine trial for malignant astrocytoma: Potential interaction with adjuvant chemotherapy. J Clin Neurosci. 2008;15(2):114–21. doi:10.1016/j.jocn.2007.08.007. PMID:18083572
- Steiner HH, Bonsanto MM, Beckhove P, Brysch M, Geletneky K, Ahmadi R, Schuele-Freyer R, Kremer P, Ranaie G, Matejic D, et al. Antitumor vaccination of patients with glioblastoma multiforme: A pilot study to assess feasibility, safety, and clinical benefit. J Clin Oncol. 2004;22(21):4272–81. doi:10.1200/JCO.2004.09.038. PMID:15452186
- Liau LM, Jensen ER, Kremen TJ, Odesa SK, Sykes SN, Soung MC, Miller JF, Bronstein JM. Tumor immunity within the central nervous system stimulated by recombinant listeria monocytogenes vaccination. Cancer Res. 2002;62(8):2287–93; PMID:11956085
- Yamanaka R, Homma J, Yajima N, Tsutiya N, Takahashi M, Tanaka R. Vaccination of recurrent glioma patients with tumour lysate-pulsed dendritic cells elicits immune responses: Results of a clinical phase I/II trial. Cancer Res. 2004;64(7 Supplement):283–283
- Clark WH, Jr, Elder DE, Guerry D, 4th, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81(24):1893–904. doi:10.1093/jnci/81.24.1893. PMID:2593166
- Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77(7):1303–10. doi:10.1002/(SICI)1097-0142(19960401)77:7%3c1303::AID-CNCR12%3e3.0.CO;2-5. PMID:8608507
- Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: A histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74(1):43–7; PMID:8569196
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–949. doi:10.1038/nm1093. PMID:15322536
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, Rubin SC, Coukos G. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13. doi:10.1056/NEJMoa020177. PMID:12529460
- Naito Y, Saito K, Shiiba K, Ohuchi A, Saigenji K, Nagura H, Ohtani H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998;58(16):3491–94; PMID:9721846
- Walker DG, Chuah T, Rist MJ, Pender MP. T-cell apoptosis in human glioblastoma multiforme: Implications for immunotherapy. J Neuroimmunol. 2006;175(1):59–68. doi:10.1016/j.jneuroim.2006.03.006. PMID:16631933
- Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, Parsa AT. CD8 T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci. 2010;17(11):1381–5. doi:10.1016/j.jocn.2010.03.031. PMID:20727764
- Boker DK, Kalff R, Gullotta F, Weekes-Seifert S, Mohrer U. Mononuclear infiltrates in human intracranial tumors as a prognostic factor. influence of preoperative steroid treatment. I. glioblastoma. Clin Neuropathol. 1984;3(4):143–7; PMID:6478676
- Brooks W, Markesbery W, Gupta G, Roszman T. Relationship of lymphocyte invasion and survival of brain tumor patients. Ann Neurol. 1978;4(3):219–24. doi:10.1002/ana.410040305. PMID:718133
- Safdari H, Hochberg FH, Richardson EP. Prognostic value of round cell (lymphocyte) infiltration in malignant gliomas. Surg Neurol. 1985;23(3):221–26. doi:10.1016/0090-3019(85)90086-2. PMID:2983448
- Rossi M, Jones N, Candy E, Nicoll JA, Compton JS, Hughes JT, Esiri MM, Moss TH, Cruz-Sanchez FF, Coakham HB. The mononuclear cell infiltrate compared with survival in high-grade astrocytomas. Acta Neuropathol. 1989;78(2):189–93. doi:10.1007/BF00688208. PMID:2750489
- Schiffer D, Cavicchioli D, Giordana MT, Palmucci L, Piazza A. Analysis of some factors effecting survival in malignant gliomas. Tumori. 1979;65(1):119–25; PMID:220763
- Authier A, Farrand KJ, Broadley KW, Ancelet LR, Hunn MK, Stone S, McConnell MJ, Hermans IF. Enhanced immunosuppression by therapy‐exposed glioblastoma multiforme tumor cells. Int J Cancer. 2015;136(11):2566–78. doi:10.1002/ijc.29309. PMID:25363661
- Yu JS, Wheeler CJ, Zeltzer PM, Ying H, Finger DN, Lee PK, Yong WH, Incardona F, Thompson RC, Riedinger MS, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61(3):842–7; PMID:11221866
- Yang Y, Lu J, Liu H, Jin G, Bai R, Li X, Wang D, Zhao J, Huang Y, Liu K, et al. Dendritic cells loading autologous tumor lysate promote tumor angiogenesis. Tumor Biol. 2016;37(12):15687–95
- Hsu M, Sedighim S, Wang T, Antonios JP, Everson RG, Tucker AM, Du L, Emerson R, Yusko E, Sanders C, et al. TCR sequencing can identify and track glioma-infiltrating T cells after DC vaccination. Cancer Immunol Res. 2016;4(5):412–8.
- Everson RG, Jin RM, Wang X, Safaee M, Scharnweber R, Lisiero DN, Soto H, Liau LM, Prins RM. Cytokine responsiveness of CD8 T cells is a reproducible biomarker for the clinical efficacy of dendritic cell vaccination in glioblastoma patients. J Immunother Cancer. 2014;2(1):1. doi:10.1186/2051-1426-2-10. PMID:24829758
- Kamran N, Calinescu A, Candolfi M, Chandran M, Mineharu Y, Asad AS, Koschmann C, Nunez FJ, Lowenstein PR, Castro MG. Recent advances and future of immunotherapy for glioblastoma. Expert Opin Biol Ther. 2016;16(10):1245–64. doi:10.1080/14712598.2016.1212012. PMID:27411023
- Libard S, Popova SN, Amini R, Kärjä V, Pietiläinen T, Hämäläinen KM, Sundström C, Hesselager G, Bergqvist M, Ekman S, et al. Human cytomegalovirus tegument protein pp65 is detected in all intra-and extra-axial brain tumours independent of the tumour type or grade. PloS one. 2014;9(9):e108861. doi:10.1371/journal.pone.0108861. PMID:25268364
- Shamran HA, Kadhim HS, Hussain AR, Kareem A, Taub DD, Price RL, Nagarkatti M, Nagarkatti PS, Singh UP. Detection of human cytomegalovirus in different histopathological types of glioma in iraqi patients. BioMed Res Int. 2015;2015:642652
