ABSTRACT
Staphylococcus aureus is the leading cause of nosocomial and community-acquired infections, including soft tissue and skin infections and bacteremia. However, efforts to develop an effective vaccine against S. aureus infections have not been successful. We evaluated serotypes 5 and 8 capsule polysaccharides (CP) CRM197 conjugates as vaccine candidates in murine models of bacteremia, lethal sepsis, and skin infection. The conjugate vaccines elicited a good antibody response, and active immunization of CP5-CRM or CP8-CRM conjugates protected against staphylococcal bacteremia. In the skin infection model, CP8-CRM but not CP5-CRM protected against dermonecrosis, and CP8-CRM immunization significantly decreased the bacterial burden in the lesion. However, neither CP5-CRM nor CP8-CRM protected against mortality in the lethal sepsis model. The results indicate the capsular vaccines elicit protection against some, but not all, aspects of staphylococcal infection.
Introduction
Community-associated methicillin-resistant Staphylococcus aureus (MRSA) is the most common causative bacterial pathogen of skin and soft tissue infections (SSTI) in the United States.Citation1,2 The increasing prevalence of MRSA infections in previously healthy individuals without identified risk factors,Citation3,4 and the identification of S. aureus strains resistant to many licensed antibioticsCitation5 have marked an urgent need to develop an effective vaccine to prevent S. aureus infections.
The approach of targeting the capsular polysaccharides (CP) as vaccine candidates has been employed successfully against many bacterial pathogens, including S. pneumoniae, H. influenzae, and N. meningitidis.Citation6 In S. aureus, CP serotype 5 and 8 CPs comprise the majority of clinical isolates.Citation7-9 A polysaccharide capsule envelopes the surface of many bacterial pathogens and can confer resistance to phagocytic clearance by the host innate immune response, thereby prolonging persistence of pathogen in the bloodstream of the host (reviewed in ref. Citation10). However, the resistance can be overcome by the opsonophagocytic antibodies targeting the capsule.Citation11-15 In addition to the critical role of the CPs in bacteremia,Citation11,12 the S. aureus CPs have also been shown to enhance virulence in rodent models of surgical wound infection,Citation16 septic arthritis,Citation17 subcutaneous abscess formation,Citation16 and renal abscess formation.Citation18 Due to differences in immunological defense against S. aureus infections, we have speculated that CP may be a less desirable target for S. aureus than typically encapsulated bacterial pathogens.Citation19-21 Furthermore, the advent of the epidemic CP− USA300 strain in the US suggests that unencapsulated strains can be fully virulent.Citation22,23
Fattom et al. first demonstrated that CP5-EPA and CP8-EPA conjugate vaccines were immunogenic in mice and humans, and that these vaccines induced opsonic antibodies that showed efficacy in protecting rodents from lethal peritonitis and reducing the bacterial burden in nonlethal staphylococcal infection.Citation24-26 More recently, active immunization with CP-conjugate vaccines has been shown to reduce bacteremia,Citation14,15,27,28 and prevent osteomyelitis in rodents.Citation28 Likewise, capsular antibodies have shown protective efficacy in rodent models of mastitis, endocarditis, and skin abscesses.Citation27,29-31 However, the S. aureus CP vaccines have not been examined for protective efficacy against dermonecrotic skin lesions or lethal sepsis. They also have not been tested against unencapsulated USA300 strains. In this study, we prepared CP5 and CP8 conjugate vaccines, evaluated their optimal dosage, and determined whether active immunization protected against S. aureus bacteremia, lethality and skin infection (SSTI). The assessment of CP5 and CP8 conjugate vaccines in relevant animal infection models is important in guiding future trials of multivalent vaccine that include CP5 and CP8 as components.
Results and discussion
CP5-CRM and CP8-CRM were protective against S. aureus bacteremia
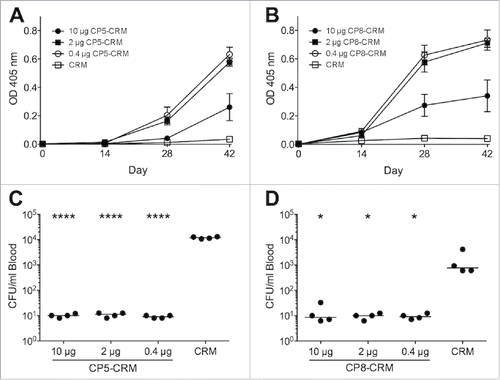
Purified CP has poor immunogenicity in animals. To mitigate this, we conjugated CP to a nontoxic mutant of diphtheria toxin, cross reacting mutant (CRM) 197, to increase the immunogenicity of CP. To determine the optimal immunogenic dose of our CP5-CRM and CP8-CRM vaccines, we actively immunized mice with CP doses ranging from 0.4 to 10 µg per mouse. Optimal immunogenicity was achieved at doses of 0.4 and 2 µg (). To evaluate whether the vaccines would protect against bacteremia in an established bacteremia model,Citation12,15,32,33 we challenged the animals IP with ∼107 CFU Reynolds (CP5) or Reynolds (CP8), both encapsulated strains. Quantitative blood cultures performed on mouse blood collected 2 h after challenge showed that CP5-CRM and CP8-CRM decreased the bacterial load in mice challenged with either strain (). Subsequent immunizations were performed with a polysaccharide dose of 1 µg CP5-CRM or CP8-CRM.
Figure 1. Immunogenicity and protective efficacy of CP5 and CP8 conjugate vaccines in BALB/c mice. Groups of four BALB/c mice were immunized on days 0, 14, and 28 with 10, 2, or 0.4 µg of CP5-CRM, CP8-CRM, or CRM alone adsorbed to 100 µg aluminum phosphate adjuvant. Blood was collected from each mouse before each immunization and prior to challenge. Serum was diluted 1:100 for evaluation of antibodies to CP5 or CP8 by ELISA (A). On Day 42, the mice were challenged intraperitoneally with ∼107 CFU of (C) Reynolds (CP5) or (D) Reynolds (CP8). Quantitative blood cultures were performed on heparinized blood collected by tail vein puncture 2 h after challenge. Data are presented as means ± standard errors and were analyzed by one-way ANOVA with multiple comparisons made to the group receiving CRM alone. ****, P < 0.0001; *, P < 0.05. For each experiment, the results of one representative experiment are presented; each was repeated at least twice.
CP5-CRM and CP8-CRM did not protect against mortality in a lethal sepsis model
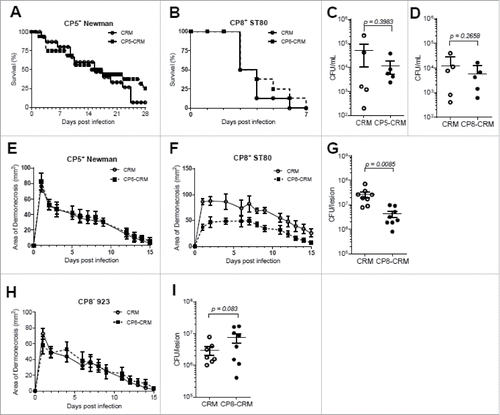
To determine whether active immunization with CP5-CRM or CP8-CRM protected mice from S. aureus lethal sepsis, immunized BALB/c mice were infected with CP5+ Newman or CP8+ ST80 strains, respectively. We observed no significant differences in survival in mice vaccinated with either CP5-CRM () or CP8-CRM () compared with control animals given CRM only. Bacterial burdens at 24h post-infection in both vaccinated groups were also statistically not different ().
Figure 2. Evaluation of CP5-CRM and CP8-CRM as vaccines against S. aureus strains Newman and ST80 in the murine models of lethal sepsis and skin infection. BALB/c mice were immunized with 1 µg of CP5-CRM or CP8-CRM adsorbed to 100 µg aluminum phosphate adjuvant on days 0, 14, and 28. On day 42, mice were challenged in the bacteremia model with (A) Newman or (B) ST80 and in the dermonecrosis model with (E) Newman or (F) ST80 (n = 8 to 10 mice/group). In the lethal sepsis model, blood was collected at 24 hours post-infection and plated for CFU analysis (C-D) and mice were monitored for survival for 28 days. In the skin infection model, the area of dermonecrosis was photographed and measured for 15 days. (G) Bacterial burden on 7 days post-infection in the lesions of CP8-CRM or CRM immunized BALB/c mice challenged with ST80 in the skin infection model. (H) Immunized mice were challenged in the skin infection model with USA300 strain 923 (n = 8/group). (I) Bacterial burden on 7 days post-infection in the skin lesions of CP8-CRM or CRM immunized BALB/c mice challenged with USA300 strain 923. Data are presented as means ± standard errors and considered significant at a confidence level of 95%. For each experiment, the results of one representative experiment are presented; each was repeated at least twice.
CP8-CRM but not CP5-CRM protected against dermonecrotic skin lesions
Mice immunized with CP5-CRM or CRM only showed no significant differences in lesion size during the course of skin infection with CP5+ S. aureus Newman (36 mm2 vs. 33 mm2 mean area of dermonecrosis on day 7 post-infection (PI), ). In contrast, CP8-CRM immunized mice infected with CP8+ ST80, a community-associated MRSA strain from Europe, had ameliorated skin lesions compared with the control group in the skin infection model (82 mm2 vs. 49 mm2 mean area of dermonecrosis on day 7 PI, , p < 0.01). The smaller lesions in CP8-CRM vaccinated ST80 infected mice also showed a significantly (P = 0.0085) decreased tissue bacterial burden, compared with the control group 7 days after inoculation ().
Because USA300, the predominant community-associated MRSA genetic background in the United States is CP serotype-negative, we hypothesized that a CP-based vaccine would not protect against this strain. As expected, immunization with CP8-CRM did not result in a decrease in the severity of dermonecrosis following infection with the USA300 isolate 923 (37 mm2 vs. 36 mm2 mean area of dermonecrosis on day 7, ). There were also no significant differences in the number of bacteria recovered from the skin lesions (). This result underscores the specificity of the protective effect of immunization with CP8-CRM, since the vaccine protected against CP8+ ST80, but not against CP− USA300.
We found that CP5-CRM and CP8-CRM vaccines were also immunogenic, and that active immunization protected mice against S. aureus bacteremia, consistent with previous studies.Citation15,32 Neither vaccine, however, was protective in an IV challenge model of lethal sepsis. In contrast with a previous reportCitation29 wherein CP5 antibodies significantly reduced the bacterial burden in a subcutaneous abscess model, CP5-CRM did not reduce lesion size in the dermonecrosis model. In contrast, CP8-CRM did ameliorate the severity of dermonecrosis caused by a CP8-expressing strain. However, no benefit was seen during skin infection caused by a USA300 strain, which is not surprising given that such strains do not express capsule due to three conserved mutations in the cap5 operon.Citation34
As we have previously summarized,Citation19-21 encapsulated bacterial pathogens infection are more common and often severe in mice and humans with B lymphocyte and antibody deficiencies. In contrast, the same phenomenon is not observed with S. aureus infections, which has led us to question the utility of targeting CP with antibody as a protective mechanism of vaccination targeting S. aureus. In preclinical models of infection, CP-based vaccines have shown protection against bacteremia, osteomyelitis, mastitis, endocarditis, and SSTI, but not in a lethal sepsis model. Clearly, a CP vaccine alone is insufficient to prevent infection, as documented by clinical trials of CP5(8)-Epa vaccines that were immunogenic but not protective in patients undergoing hemodialysis.Citation35,36
The present study has limitations. We have not tested the CP conjugates in a variety of murine backgrounds, S. aureus strains or carrier proteins. We found that antigenic protection against S. aureus infection is dependent on the route of infection and the disease model. Another limitation of our study was that mouse models are imperfect in translating into success in human clinical trials. Compared with humans, a mouse is different in terms of immune response and resistance to S. aureus superantigens and cytotoxins. The present study also has strengths. Sometimes immunization with the capsule antigens successfully decreased the bacterial burden of the infection, while sometimes it did not. USA300, the epidemic strain in the US, does not elaborate a capsule. Perhaps other strains do not as well. A capsule containing vaccine would be unlikely to prevent an infection caused by a USA300 strain. Thus, a vaccine containing capsular polysaccharide antigens would require testing into whether the targeted S. aureus strains elaborated a capsule and whether the vaccine prevented an infection caused by this strain.
Materials and methods
Mouse models of skin infection and bacteremia
Strains Reynolds (CP5) and Newman are prototype CP5+ strains, and Reynolds (CP8) and ST80 are prototype CP8+ strains, as described previously.Citation32,37,38 ST80-16 is a serotype 8 community acquired methicillin-resistant S. aureus strain that is prevalent in Europe.Citation21,39 The USA300 clinical isolate 923 was included since this genetic background is the most common cause of SSTI in the United States.Citation1,2 The virulence of S. aureus USA300 clinical isolate 923 has been described.Citation40,41
For the bacteremia experiments, the bacteria were cultivated for 24 h on Columbia agar plates supplemented with 2% NaCl to enhance CP production.Citation10,32 For the lethal sepsis and dermonecrotic lesion models, the bacterial isolates were grown overnight in tryptic soy broth (TSB) with 5% NaCl in a 37°C shaking incubator set to 250 rpm. The overnight culture was diluted 1:100 in fresh TSB with 5% NaCl and grown to exponential phase (OD600: 1.8), a growth phase in which little CP is produced.Citation10 The bacteria were then centrifuged and washed in sterile phosphate-buffered saline (PBS) before suspension in PBS to a concentration of 3 × 108 CFU/mL. The inocula were confirmed by plating serial dilutions on tryptic soy agar.
Female BALB/c mice, 6–7 weeks old, were purchased from Taconic Biosciences Inc., Hudson NY, and allowed to acclimate for 1 week before vaccination, with unlimited access to food and water. All procedures involving mice were approved by the Institutional Biosafety Committee (IBC) and Institutional Animal Care and Use Committee (IACUC) at the University of Chicago, the University of Southern California, or Harvard Medical School.
CP5-CRM and CP8-CRM
CP5 and CP8 were purified as described.Citation42 The CPs were conjugated to cross-reacting mutant (CRM) 197 (Reagent Proteins), a nontoxic recombinant mutant of diphtheria toxin, in a conjugation process using 1,1-carboyldiimidazole/1,1-carboyl-di-1,2,4-triazole (described in US patent US 8568735 B2). A single CP5-CRM vaccine was used for all the mouse experiments; two different CP8-CRM conjugates were used. The conjugate vaccines were analyzed for protein content with the Pierce BCA protein assay kit (ThermoFisher Scientific), and the CP content was determined by ELISA inhibition assays that included a purified CP standard curve, as described.Citation43 The CP5-CRM vaccine had a CP:protein ratio of 1:1.78, whereas the CP8-CRM vaccines had CP:protein ratios of 1:1.12 and 1:0.93. Effective conjugation was evaluated by subjecting the vaccines to SDS-PAGE and staining with Coomassie blue. After transfer to nitrocellulose, the samples were detected by their reactivity with rabbit antibodies to CRM (Abcam). A capture ELISA was performed by coating 96-well plates with anti-CRM antibodies. After blocking and washing the plate, serial dilutions of CRM alone, CP alone, or conjugate vaccine were added. Following incubation and washes, the samples were incubated with a mouse anti-CP5 or anti-CP8 antibody. Following washes, the conjugate vaccine (but not free CP or CRM) was detected by a goat anti-mouse IgG Fc alkaline phosphatase conjugate and appropriate substrate (p-nitrophenyl phosphate).
Animal experiments
To determine the optimal dose of the conjugate vaccines, we immunized mice with polysaccharide doses of 0.4, 2, or 10 µg per mouse. The animals were immunized subcutaneously on days 0, 14, and 28 with CP5-CRM or CP8-CRM and 100-µg aluminum phosphate adjuvant (Adju-Phos, Accurate Chemical & Scientific Corp.) in sterile 0.15 M saline (Teknova). Mice immunized with CRM and aluminum phosphate adjuvant served as controls. The mice were bled before each immunization for antibody determination. A pre-challenge bleed was performed on day 41, followed by infection 1–2 days later.
For the bacteremia model, the mice were challenged intraperitoneally with a 0.5 ml inoculum containing 107 CFU S. aureus. After two hours the mice were bled by tail vein nicking, and the heparinized blood was diluted and plated quantitatively on blood agar plates. The lower limit of sensitivity (∼10 CFU/mL) was determined by plating ∼50 µl aliquots of undiluted blood in duplicate for each sample.
For the skin infection model,Citation40 the mice were sedated, and their flanks were shaved and disinfected. Mice were injected subcutaneously with 50 µl of the S. aureus suspension at a concentration of 1.5 × 107 CFU/50 µl. Skin lesions were photographed each day for 15 days using a 100-mmCitation2 square as a standard. The size of the lesion was measured using Adobe Photoshop software. To determine the bacterial burden, skin lesions were excised on day 7 post-infection. Serial dilutions of the tissue homogenates were plated on mannitol salt agar to quantify the bacterial load. For the lethal sepsis model, mice were challenged by tail vein injection of a 250-µl suspension of S. aureus of 2.5 × 108 CFU/mL and monitored for survival up to 28 days. At 24 hours post-infection, blood collected from tail vein is diluted and plated on mannitol salt agar to quantify bacterial burden.
Antibody quantification by ELISA
Blood was collected from mice prior to infection and prepared using serum separator tubes (BD Biosciences). The serum was diluted 1:100 and tested by ELISA on microtiter plates coated with 4 µg/mL of purified CP5 or CP8 coupled to poly-L-lysine, as described.Citation15,44
Statistical analyses
Quantitative blood culture data were analyzed by the Mann-Whitney U test. Lesion sizes in the skin infection model and ELISA data were compared between groups using the unpaired two-tailed Student's t test. Survival in the bacteremia model was compared by the non-parametric Log Rank test. Analyses were considered significant when p < 0.05. All statistical analyses were performed with GraphPad Prism or KyPlot software.
Disclosure of potential conflicts of interest
In the last 12 months, BS has received consulting fees from Paratek, OvaGene, Roche, Pfizer, Cempra, Forge, The Medicines Company, MedImmune/AstraZeneca, Entasis, Tetraphase, DSMB fees from Dipexium, and owned equity in Motif, BioAIM, Novadigm, and Synthetic Biologics.
Funding
This work was supported by a grant from the National Institute of Health (R01 AI103342).
References
- Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, EMERGEncy ID Net Study Group. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 2006; 355:666-74; PMID:16914702; https://doi.org/10.1056/NEJMoa055356
- David MZ, Medvedev S, Hohmann SF, Ewigman B, Daum RS. Increasing burden of methicillin-resistant Staphylococcus aureus hospitalizations at US academic medical centers, 2003-2008. Infect Control Hosp Epidemiol 2012; 33:782-9; PMID:22759545; https://doi.org/10.1086/666640
- Herold BC, Immergluck LC, Maranan MC, Lauderdale DS, Gaskin RE, Boyle-Vavra S, Leitch CD, Daum RS. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 1998; 279:593-8; PMID:9486753; https://doi.org/10.1001/jama.279.8.593
- Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ, Etienne J, Johnson SK, Vandenesch F, Fridkin S, O'Boyle C, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 2003; 290:2976-84; PMID:14665659; https://doi.org/10.1001/jama.290.22.2976
- Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol 2009; 7:629-41; PMID:19680247; https://doi.org/10.1038/nrmicro2200
- Trotter CL, McVernon J, Ramsay ME, Whitney CG, Mulholland EK, Goldblatt D, Hombach J, Kieny MP, SAGE subgroup. Optimising the use of conjugate vaccines to prevent disease caused by Haemophilus influenzae type b, Neisseria meningitidis and Streptococcus pneumoniae. Vaccine 2008; 26:4434-45; PMID:18617296; https://doi.org/10.1016/j.vaccine.2008.05.073
- Cocchiaro JL, Gomez MI, Risley A, Solinga R, Sordelli DO, Lee JC. Molecular characterization of the capsule locus from non-typeable Staphylococcus aureus. Mol Microbiol 2006; 59:948-60; PMID:16420363; https://doi.org/10.1111/j.1365-2958.2005.04978.x
- Arbeit RD, Karakawa WW, Vann WF, Robbins JB. Predominance of two newly described capsular polysaccharide types among clinical isolates of Staphylococcus aureus. Diagn Microbiol Infect Dis 1984; 2:85-91; PMID:6232086; https://doi.org/10.1016/0732-8893(84)90002-6
- Hochkeppel HK, Braun DG, Vischer W, Imm A, Sutter S, Staeubli U, Guggenheim R, Kaplan EL, Boutonnier A, Fournier JM. Serotyping and electron microscopy studies of Staphylococcus aureus clinical isolates with monoclonal antibodies to capsular polysaccharide types 5 and 8. J Clin Microbiol 1987; 25:526-30; PMID:2437148
- O'Riordan K, Lee JC. Staphylococcus aureus capsular polysaccharides. Clin Microbiol Rev 2004; 17:218-34; PMID:14726462; https://doi.org/10.1128/CMR.17.1.218-234.2004
- Thakker M, Park JS, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 1998; 66:5183-9; PMID:9784520
- Watts A, Ke D, Wang Q, Pillay A, Nicholson-Weller A, Lee JC. Staphylococcus aureus strains that express serotype 5 or serotype 8 capsular polysaccharides differ in virulence. Infect Immun 2005; 73:3502-11; PMID:15908379; https://doi.org/10.1128/IAI.73.6.3502-3511.2005
- Nanra JS, Buitrago SM, Crawford S, Ng J, Fink PS, Hawkins J, Scully IL, McNeil LK, Aste-Amézaga JM, Cooper D. Capsular polysaccharides are an important immune evasion mechanism for Staphylococcus aureus. Hum Vaccin Immunother 2013; 9:480-7; PMID:23249887; https://doi.org/10.4161/hv.23223
- Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, Shields K, Park S, Alaimo C, Kelley KA, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2014; 209:1551-61; PMID:24308931; https://doi.org/10.1093/infdis/jit800
- Park S, Gerber S, Lee JC. Antibodies to Staphylococcus aureus serotype 8 capsular polysaccharide react with and protect against serotype 5 and 8 isolates. Infect Immun 2014; 82:5049-55; PMID:25245803; https://doi.org/10.1128/IAI.02373-14
- McLoughlin RM, Solinga RM, Rich J, Zaleski KJ, Cocchiaro JL, Risley A, Tzianabos AO, Lee JC. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc Natl Acad Sci U S A 2006; 103:10408-13; PMID:16801559; https://doi.org/10.1073/pnas.0508961103
- Nilsson IM, Lee JC, Bremell T, Ryden C, Tarkowski A. The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis. Infect Immun 1997; 65:4216-21; PMID:9317029
- Portoles M, Kiser KB, Bhasin N, Chan KHN, Lee JC. Staphylococcus aureus Cap5O has UDP-ManNAc dehydrogenase activity and is essential for capsule expression. Infect Immun 2001; 69:917-23; PMID:11159986; https://doi.org/10.1128/IAI.69.2.917-923.2001
- Spellberg B, Daum R. A new view on development of a Staphylococcus aureus vaccine: Insights from mice and men. Hum Vaccin 2010; 6:857-9; PMID:20930569; https://doi.org/10.4161/hv.6.10.12469
- Daum RS, Spellberg B. Progress toward a Staphylococcus aureus vaccine. Clin Infect Dis 2012; 54:560-7; PMID:22186773; https://doi.org/10.1093/cid/cir828
- Spellberg B, Daum R. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol 2012; 34:335-48; PMID:22080194; https://doi.org/10.1007/s00281-011-0293-5
- Sutter DE, Summers AM, Keys CE, Taylor KL, Frasch CE, Braun LE, Fattom AI, Bash MC. Capsular serotype of Staphylococcus aureus in the era of community-acquired MRSA. FEMS Immunol Med Microbiol 2011; 63:16-24; PMID:21631600; https://doi.org/10.1111/j.1574-695X.2011.00822.x
- Montgomery CP, Boyle-Vavra S, Adem PV, Lee JC, Husain AN, Clasen J, Daum RS. Comparison of virulence in community-associated methicillin-resistant Staphylococcus aureus pulsotypes USA300 and USA400 in a rat model of pneumonia. J Infect Dis 2008; 198:561-70; PMID:18598194; https://doi.org/10.1086/590157
- Fattom A, Schneerson R, Watson DC, Karakawa WW, Fitzgerald D, Pastan I, Li X, Shiloach J, Bryla DA, Robbins JB. Laboratory and clinical evaluation of conjugate vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides bound to Pseudomonas aeruginosa recombinant exoprotein A. Infect Immun 1993; 61:1023-32; PMID:8432585
- Fattom A, Schneerson R, Szu SC, Vann WF, Shiloach J, Karakawa WW, Robbins JB. Synthesis and immunologic properties in mice of vaccines composed of Staphylococcus aureus type 5 and type 8 capsular polysaccharides conjugated to Pseudomonas aeruginosa exotoxin A. Infect Immun 1990; 58:2367-74; PMID:2114365
- Fattom AI, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun 1996; 64:1659-65; PMID:8613375
- Lee JC, Park JS, Shepherd SE, Carey V, Fattom A. Protective efficacy of antibodies to the Staphylococcus aureus type 5 capsular polysaccharide in a modified model of endocarditis in rats. Infect Immun 1997; 65:4146-51; PMID:9317020
- Lattar SM, Noto Llana M, Denoel P, Germain S, Buzzola FR, Lee JC, Sordelli DO. Protein antigens increase the protective efficacy of a capsule-based vaccine against Staphylococcus aureus in a rat model of osteomyelitis. Infect Immun 2014; 82:83-91; PMID:24126523; https://doi.org/10.1128/IAI.01050-13
- Skurnik D, Merighi M, Grout M, Gadjeva M, Maira-Litran T, Ericsson M, Goldmann DA, Huang SS, Datta R, Lee JC, et al. Animal and human antibodies to distinct Staphylococcus aureus antigens mutually neutralize opsonic killing and protection in mice. J Clin Invest 2010; 120:3220-33; PMID:20739753; https://doi.org/10.1172/JCI42748
- Tuchscherr LP, Buzzola FR, Alvarez LP, Lee JC, Sordelli DO. Antibodies to capsular polysaccharide and clumping factor A prevent mastitis and the emergence of unencapsulated and small-colony variants of Staphylococcus aureus in mice. Infect Immun 2008; 76:5738-44; PMID:18809660; https://doi.org/10.1128/IAI.00874-08
- Pozzi C, Wilk K, Lee JC, Gening M, Nifantiev N, Pier GB. Opsonic and protective properties of antibodies raised to conjugate vaccines targeting six Staphylococcus aureus antigens. PLoS One 2012; 7:e46648; PMID:23077517; https://doi.org/10.1371/journal.pone.0046648
- Wacker M, Wang L, Kowarik M, Dowd M, Lipowsky G, Faridmoayer A, Shields K, Park S, Alaimo C, Kelley KA, et al. Prevention of Staphylococcus aureus infections by glycoprotein vaccines synthesized in Escherichia coli. J Infect Dis 2014; 209:1551-61; PMID:24308931; https://doi.org/10.1093/infdis/jit800
- Thakker M, Park JS, Carey V, Lee JC. Staphylococcus aureus serotype 5 capsular polysaccharide is antiphagocytic and enhances bacterial virulence in a murine bacteremia model. Infect Immun 1998; 66:5183-9; PMID:9784520
- Boyle-Vavra S, Li X, Alam MT, Read TD, Sieth J, Cywes-Bentley C, Dobbins G, David MZ, Kumar N, Eells SJ, et al. USA300 and USA500 clonal lineages of Staphylococcus aureus do not produce a capsular polysaccharide due to conserved mutations in the cap5 locus. MBio 2015; 6:02585-14; PMID:25852165; https://doi.org/10.1128/mBio.02585-14
- Shinefield H, Black S, Fattom A, Horwith G, Rasgon S, Ordonez J, Yeoh H, Law D, Robbins JB, Schneerson R, et al. Use of a Staphylococcus aureus conjugate vaccine in patients receiving hemodialysis. N Engl J Med 2002; 346:491-6; PMID:11844850; https://doi.org/10.1056/NEJMoa011297
- Fattom A, Matalon A, Buerkert J, Taylor K, Damaso S, Boutriau D. Efficacy profile of a bivalent Staphylococcus aureus glycoconjugated vaccine in adults on hemodialysis: Phase III randomized study. Hum Vaccin Immunother 2015; 11:632-41; PMID:25483694; https://doi.org/10.4161/hv.34414
- Duthie ES, Lorenz LL. Staphylococcal coagulase; mode of action and antigenicity. J Gen Microbiol 1952; 6:95-107; PMID:14927856; https://doi.org/10.1099/00221287-6-1-2-95
- Linde H, Wagenlehner F, Strommenger B, Drubel I, Tanzer J, Reischl U, Raab U, Holler C, Naber KG, Witte W, et al. Healthcare-associated outbreaks and community-acquired infections due to MRSA carrying the Panton-Valentine leucocidin gene in southeastern Germany. Eur J Clin Microbiol Infect Dis 2005; 24:419-22; PMID:15937659; https://doi.org/10.1007/s10096-005-1341-7
- Stegger M, Price LB, Larsen AR, Gillece JD, Waters AE, Skov R, Andersen PS. Genome sequence of Staphylococcus aureus strain 11819-97, an ST80-IV European community-acquired methicillin-resistant isolate. J Bacteriol 2012; 194:1625-6; PMID:22374956; https://doi.org/10.1128/JB.06653-11
- Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS, Daum RS. Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. Infect Immun 2014; 82:2125-34; PMID:24614654; https://doi.org/10.1128/IAI.01491-14
- Spellberg B, Ibrahim AS, Yeaman MR, Lin L, Fu Y, Avanesian V, Bayer AS, Filler SG, Lipke P, Otoo H, et al. The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 2008; 76:4574-80; PMID:18644876; https://doi.org/10.1128/IAI.00700-08
- Tzianabos AO, Wang JY, Lee JC. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc Natl Acad Sci USA 2001; 98:9365-70; PMID:11470905; https://doi.org/10.1073/pnas.161175598
- Lee JC, Takeda S, Livolsi PJ, Paoletti LC. Effects of in vitro and in vivo growth conditions on expression of type 8 capsular polysaccharide by Staphylococcus aureus. Infect Immun 1993; 61:1853-8; PMID:8478074
- Gray BM. ELISA methodology for polysaccharide antigens: Protein coupling of polysaccharides for adsorption to plastic tubes. J Immunol Methods 1979; 28:187-92; PMID:38284; https://doi.org/10.1016/0022-1759(79)90340-5
