ABSTRACT
Objective To describe the epidemiological characteristics of contraindications in children routine vaccination, to evaluate vaccination doctors’ ability to determine contraindications. Method Using cross-section study, 34 urban and 15 suburb units were selected from 206 Community Health Center (CHC) in Hangzhou, China. Subjects were all children coming to CHCs for routine vaccination. All situations considered to be unsuitable for vaccination were recorded as contraindications. 3 experts were used to classify these abnormal records as true or false contraindications. Then, the multi-analysis was used to find factors related with the rate of false contraindications. Results There were 2801 children with 2969 contraindications in the present study. The prevalence of contraindications was 3.03‰ by dose of vaccines. Cough (24.78%), fever (21.86%) and medication (19.54%) were the most common contraindications in children routine vaccination. Measles-rubella vaccine (MR) (6.78‰), measles-mumps-rubella vaccine (MMR) (5.87‰) and hepatitis B vaccine (Hep B) (5.25‰) had higher prevalence of contraindications than other vaccines. According to the evaluation of 3 experts, about 13.53% of contraindications were misdiagnosed by vaccination doctor. The rate of misdiagnosed contraindications was correlated with the sex, age and educational background of vaccination doctor, total dose of vaccination of CHC. Conclusion A portion of children might miss the routine vaccination because of misdiagnosed contraindications. More investigations are needed to report the epidemiological distribution of contraindication in routine vaccination of children.
Introduction
Contraindications of vaccination were referring to conditions caused by vaccines unsuitable to inject, which might increase the chance or severity of adverse reactions or interfere the immunity of vaccines.Citation1 The consensus among most experts is that there are very few conditions where vaccines are contraindicated. The risks associated with vaccine administration need to be weighed against the benefits. The judgment of contraindications are mainly based on Specification of Product Characteristics (SPC), also rely on physician's clinical experience in evidence of excess risk. Therefore, rates of contraindications in vaccination are often varied in countries and over time. An early research showed that the percent correct responses on the contraindications of vaccination questions was 55∼73% for public physicians, private physicians and public health nurses.Citation2 This rate for vaccination doctor found in China reported as 56%.Citation3 Even toward same vaccine, there was no consensus in contraindications among pediatricians, general practitioners and family practitioners in a catch-up campaign.Citation4 Additional, the SPC of vaccine often has non-specific description about contraindications, such as serious disease history, suffering moderate or severe chronic disease and ingredient allergy. In China, vaccination personnel have medical education background of preventive medicine, nursing or general practice was responsible for vaccination activities. Before children accepting vaccination, the vaccination doctor need collect health condition. Most of them did not specialize on clinical diagnosis and virology. The criterion of contraindications to immunization usually became an obstacle in their medical activities.
Vaccines were divided into 2 types according to whether need to pay in China. Type one vaccines was under the Chinese National Immunization Scheme and supplied free of charge by the government, which strongly required targeted population to vaccinate unless had contraindications. In contrast, type 2 vaccines need to pay and vaccinate by voluntary choice. Some parents usually choice type 2 vaccines as replacement of type one vaccines in routine vaccination. For example, absorbed diphtheria-tetanus- acellular pertusis-inactivated poliovirus-haemophilus influenzae type b combined vaccine (DTaP-IPV/Hib, type two vaccines) could replace DTaP (type one vaccines) and oral poliomyelitis vaccine (OPV, type one vaccines) in the recommended schedule.
In the present study, all condition considered to be unsuitable for vaccination by vaccination doctors was contraindication (genuine, suspected or perceived). Investigations were performed to describe the epidemiological characteristics about contraindications of types one vaccines and alternative vaccines in children's routine vaccination, as also as to evaluate the ability of vaccination doctors to determine contraindications.
Results
General description of subjects
A total of 2801 children with 2969 contraindications were collected from 49 CHCs. Male 1584(56.55%), female 1217(43.45%).The age ranged 0 month to 12 y old with an 8 month of median age. The characteristics of vaccination doctors were showed in . There were 280,865 children (≤ 6 years) registered in the electronic registration system from the 49 CHCs and had injected 977,202 dose listed vaccines in 2014. The prevalence of contraindications was 3.03‰ calculated by dose of vaccines, and 1.06% by registered children.
Table 3. Recommended schedule of vaccines.
Table 1. Characteristics of vaccination doctors.
Distribution and prevalence of contraindications
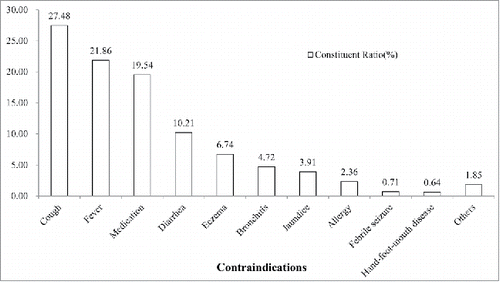
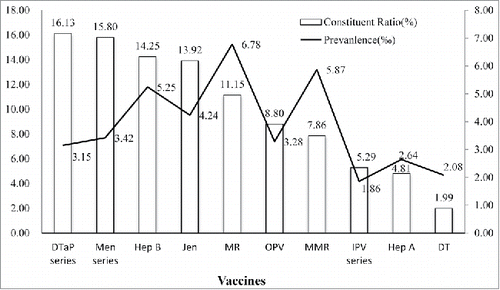
showed the top 10 proportions of contraindications. Cough (24.78%), fever (21.86%) and medication (19.54%) were the most common abnormal examination. According to variety of vaccines, the proportions of DTaP and Men series were 16.13% and 15.80%. MR (11.15%) and MMR (7.86%) had a moderated proportion with the highest prevalence of contraindications by injecting dose among vaccines. The prevalence of Hep B was 5.25‰. DT had the least proportion and IPV series had the lowest prevalence among all vaccines ().
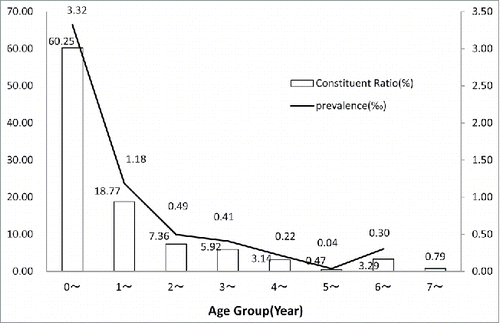
Children aged more than 7 y almost completed the routine vaccination. A small part of children in this age group need catch-up vaccination for missing before. The present study did not calculated the prevalence of ≥ 7 y group because the vaccinated age would be sporadic in catch-up vaccination. Below the 6 y group, most of contraindications were happened in less than one year old group (60.25%). The highest prevalence was also found in the same age group. A low curve was presented from 2 to 6 y age groups ().
Ability of vaccination doctors to identify contraindications
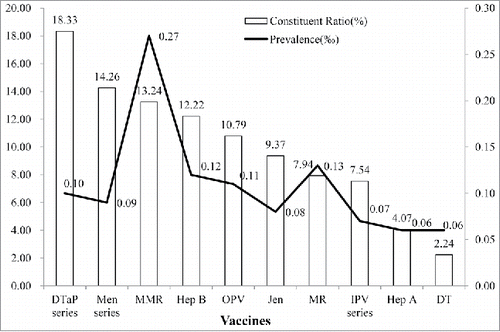
Three experts were used to independently assess the ability to of vaccination doctors identify contraindications. 41.52% abnormal examination reached the consensus by 3 experts. 44.95% of records considered as contraindications by 2 experts. At last, 8.07% (considered as true contraindications by 1 expert) and 5.46% (considered as false contraindications by 3 experts) of records were judged as false contraindications. A higher rate of false contraindications was found in MMR (0.27‰) and MR (0.13‰) vaccination ().
Factors related with contraindications identification of vaccination doctors
Compared with male group, female vaccination doctor had a lower prevalence of contraindications false identified. The ORs of factors age and education were 0.95(0.92∼0.97) and 0.68(0.50∼0.94), which indicated that higher degree of age and education background could promote vaccination doctor to make reasonable decision. There was no difference in prevalence of false contraindication between urban and suburban CHCs. However, the results showed that the CHC performed more doses of vaccination, there were more false contraindications identified by vaccination doctors ().
Table 2. Factors affected the prevalence of false contraindications.
Discussion
Rare publication had described the epidemiological characteristics of contraindication in routine vaccination among children. In the present study, the contraindications diagnosed by vaccination doctors were based on SPC of vaccines or relied on physician's clinical experience. As investigation, approximately 3 contraindications been examined by 1000 dose of vaccination. Measles contained vaccine (MR and MMR) caused a higher prevalence of contraindications than other type one vaccines and alternative vaccines. According to the assessment by experts, 13.53% of contraindications were improperly decision by vaccination doctors. The rate of false contraindication correlated with sexual, age and education background of vaccination doctor, and the total doses of vaccination.
Early in 1980s, World Health Organization (WHO) noticed that contraindications to immunization had substantially affected the vaccination coverage, and recommended that situations as slight fever, mild respiratory infections or diarrhea, and other minor illnesses should not be considered as contraindications.Citation5 In the present study, the most contraindications were identified as cough, fever and medication. In fact, except fever, cough and most of medication were not a reasonable abnormal situation to withhold vaccination according to the specification. A Czech study indicated that the proportion of children with contraindications was increasing over time and would cause incompletely vaccination to children.Citation6 Also, a rapidly increased rate of contraindication was found in a single survey about smallpox vaccine.Citation7 The Czech investigation reported DTaP-HiB had highest rate of contraindications by number of children.Citation6 These results did not consider the factors of dose and consumption of vaccines. In the present study, the proportion of DTaP, Men and Jen series ranked in the former because the consumption ranked at the top of listed vaccines. It seemed to be more reasonable that using the prevalence to illustrate the frequency of contraindication. Compared to other vaccines, more abnormal diagnosis was found before vaccinating MR and MMR. According to specification, the contradictions of MR and MMR contained fever, immunodeficiency, history of convulsion, epilepsy, and ambiguous description like infectious disease, severe chronic disease, ingredient allergy, which was more than other vaccines. Especially, it was early proved that children with egg allergy could be administered measles contained vaccines.Citation8,9 But egg allergy was considered as contraindication in the specification until 2012 in China. With the specification updated, vaccination doctors’ confusion substantial induced. For example, the Advisory Committee on Immunization Practices (ACIP) would update advice and guidance regarding use of influenza vaccines in each epidemic season.Citation10 Egg allergy, as a special topic, it would be described with situations in detail about suitable and unsuitable to accept influenza vaccine. In contrast, measles contained vaccine was lack of a necessary explanation about the change. Furthermore, measles contained vaccines easily caused adverse events following immunization (AEFI). The AEFI rate of MV, MR and MMR once reported as 140.96/100,000 38.94/100,000 and 17.00/100,000, were multiple of other vaccines.Citation11 The confusion in contraindication and frequent AEFI might result vaccination doctor making a conservative decision. They would like to postpone or refuse to vaccinate MR and MMR for children. The decreasing trend could found in age distribution. Apparently, the decline was related with frequency of vaccination and feature of physical development of children. Most of vaccinations happened within one year of age. And, subjects in this age group were unbalanced in physical development and were susceptible to a variety of infection.
A review indicated that false contraindication happened to health workers including belief as sick child should not be vaccinated, disallow multiple vaccinations on the same visit, underweight infant should not be vaccinated.Citation12 A survey showed only 64% of providers on average could correctly answer questions about the schedule and contraindication of immunization.Citation13 Undoubtedly, heath worker's knowledge influenced a reasonable vaccination.Citation14,15 According to assessment by experts, about 13% of contraindication resulted in a miss opportunity to vaccination in the present study. Some presence of common minor illnesses, such as cough, cold without fever, supportive medication, light diarrhea were considered as contraindication, similar results had been reported in former literatures.Citation16-18 The prevalence of false contraindication had similar curve like the whole records. Children had more chances of missing MMR and MR vaccination than other vaccines. In most cases, localized eczema was improperly considered as contraindication by vaccination doctor in measles contained vaccination. A cohort study showed that the prevalence of eczema was 6.7% and deceased along with age among children aged from 0 to 17 month.Citation19
Besides following the specification, identified contraindications also relied on experience of vaccination doctors in vaccination. Higher education level and older age could guarantee vaccination doctors had a scientific experience in vaccination. In the present study, the worker year did not distinguished time in vaccination and other medical position. Subjects might be a nurse just changed work position not a long time ago, which might induce the factor of longer work year was not significant related to the outcome. Numerous dose of vaccination means heavy working pressure, seemed as a risk factor could bring about more false contraindication.
There were limitations in the present study. Those parents know contraindication well might not come to vaccination clinic when their children had suspected situation. Therefore, the prevalence of contraindication might be underestimated. More detailed data would help diagnosed specific contraindication. Information like temperature of fever, frequency of diarrhea, kinds of medicine, degree of allergy was not collected in the present study, resulted in a possible bias of false contraindication. Subjective variable of vaccination doctor as opinion on doctor-patient relationship, knowledge of infectious disease, confidence on vaccination should included in the analysis, to found the factors could affect the prevalence of false contraindication. However, literature on the frequency of contraindications in children vaccination was surprisingly sparse around countries. The present study first added the epidemiological distribution of contraindications in Chinese children in the routine immunization.
Methods
Subjects
There were 204 Community Health Centers (CHCs) located at 5 urban districts(42) and 8 suburb counties (162) in Hangzhou, China. Vaccination clinic was setup in CHC at each administrative street or town. The present study included 34 urban CHCs and 15 suburb CHCs from 4 districts and 7 counties. Vaccination clinic had to meet the official admission criteria formulated by administrative department of government. Therefore, there was no difference between selected and unselected CHCs in the baseline. In 2014, all the children come to selected CHCs for vaccinating types one vaccines and the replaced vaccines were the subjects. Every vaccination clinic must assign the specialized doctors for health examination before vaccination. The characteristics of these doctors were also investigated in study. All participants had read and sign the informed consent.
The judgment of contraindications
Doctors in vaccinated clinic must carry out health examination for all subjects before vaccination, and record the situations which were not suitable to vaccination. Then, 3 experts who have chief academic title and more than 15 y work experience in immunization was used to classify these records as true or false contraindications. Each true contraindication needed consent by 2 experts at least. In contrast, those records admitted by single or no experts considered as false one.
Questionnaire
The contraindications record consists of 3 parts. Part one listed as name, gender, birth date. Part 2 registered the planed vaccine, included hepatitis A vaccine (Hep A), hepatitis B vaccine (Hep B), oral poliomyelitis vaccine (OPV), Japanese encephalitis vaccine (Jen) (live and inactivated), measles-rubella vaccine (MR), measles-mumps-rubella vaccine (MMR), diphtheria- tetanus vaccine (DT), the series of DTaP (DTaP and DTaP-IPV /Hib), the series of inactivated poliomyelitis vaccine (IPV series) (IPV and DTaP-IPV /Hib), the series of meningococcal vaccine (Men series) (Group A polysaccharide Men, Group A and C protein conjugate Men, Group A and C polysaccharide Men, Group A, C, Y and W135 polysaccharide Men). The IPV, DTaP-IPV/Hib, inactivated Jen and Group A and C protein conjugate Men, Group A, C, Y and W135 polysaccharide Men were type 2 vaccines, which cloud replace the type one vaccines. Part 3 was the contraindications, which recorded the clinic diagnosis, symptom and medication in detail. With the completely information collected, the vaccination doctor recorded the dominated reason as contraindications.
Vaccination schedule
listed the schedule of type one vaccines. Most of bacillus of calmette-guerin vaccine (BCG) and the first dose of Hep B injected in 24 hrs after birth in hospital. Thus, we did not calculate the data of BCG and the first dose of Hep B in the present study. IPV used as replacement of OPV. According to recommended schedule, DTaP-IPV/Hib was provided as an alternative choice of OPV and DTap for containing same preventive function MMR must be vaccinated at the age of more than 18 month. Group A polysaccharide Men and Group A and C protein conjugate Men were used from 6 to 18 month. Group A and C polysaccharide Men, Group A, C, Y and W135 polysaccharide Men) were planned to be vaccinated at the age of 3 and 6 y old. MMR was the booster of MV at more than 18 months. DT was recommended as a booster of DTaP at 6 y. As for Hep A and Jen, 2 inactivate dose could substitute one live dose with an interval time in accordance with the SPC. All vaccines did not allow to be vaccinated before recommended age.
Statistical analysis
Data entry was performed in duplicate using Epidata version 3.1 (www.epidata.dk). And statistical analysis was performed with SPSS version 15.0 (SPSS Inc., Chicago, IL).Variables mean (standard deviation) and median (quartile) were used to describe the data distribution. The significant difference was analyzed by t-test for quantitative data and χ2 test for categorical variables. Take Hep A as an example, rate of contraindications = count of contraindications for planning of Hep A divided by total of vaccinating Hep A in 2014 years×1000‰. Constituent ratio = count of contraindications for planning of Hep A divided by total of contraindications in 2014 years×100%. Multi-logistic regression was used to calculate correlation between the discriminated ability and characteristics of vaccination doctor and CHCs. All of analysis was 2-tailed, when p value was less than 0.05, the difference was significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Atkinson W, Wolfe S, Hamborsky J. CDC. Epidemiology and Prevention of Vaccine Preventable Disease. 12th ed. Washington D.C.: Public Health Foundation. 2011:6-15.
- Wood D, Halfon N, Pereyra M, Hamlin JS, Grabowsky M. Knowledge of the childhood immunization schedule and of contraindications to vaccinate by private and public providers in Los Angeles. Pediatr Infect Dis J. 1996; 15:140-45; PMID:8822287; https://doi.org/10.1097/00006454-199602000-00010
- Li Feng, Hang Tongwu, Liu Dawei, Feng Zijian. Survey on the knowledge of vaccination contraindication among expanded program of immunization. Zhongguo Yi Miao He Mian Yi 2009; (6):498-500. (Chinese).
- Cohen NJ, Lauderdale DS, Shete PB, Seal JB, Daum RS. Physician knowledge of catch-up regimens and contraindications for childhood immunizations. Pediatrics. 2003; 111(5 Pt 1):925-32; PMID:12728067; https://doi.org/10.1542/peds.111.5.925
- Galazka AM, Lauer BA, Henderson RH, Keja J. Indications and contraindications for vaccines used in the Expanded Programme on Immunization. Bull World Health Organ 1984; 62(3):357-66.
- Danova J, Gopfertova D, Bobak M. Rates of contraindications and use of alternative vaccines in routine immunisation of children: a population based study in the Czech Republic[J]. Vaccine 2007; 25(19):3890-5; PMID:17316928; https://doi.org/10.1016/j.vaccine.2007.01.099
- Fogel I, Brosh-Nissimov T, Vager G, Aviv Y, Kassirer M. Estimated prevalence of smallpox vaccine contraindications in Israeli adolescents. Vaccine 2016; 34(29):3331-4; PMID:27206387; https://doi.org/10.1016/j.vaccine.2016.05.025
- James JM, Burks AW, Roberson PK, Sampson HA. Safe administration of the measles vaccine to children allergic to eggs. N Engl J Med. 1995; 332: 1262-6; PMID:7708070; https://doi.org/10.1056/NEJM199505113321904
- Baxter DN. Measles immunization in children with a history of egg allergy. Vaccine 1996; 14: 131-4; PMID:8852409; https://doi.org/10.1016/0264-410X(95)00154-S
- Grohskopf Lisa A, Sokolow Leslie Z, Broder Karen R, Olsen Sonja J, Karron Ruth A, Jernigan Daniel B, Bresee Joseph S. Prevention and Control of Seasonal Influenza with Vaccines Recommendations of the Advisory Committee on Immunization Practices - United States, 2016-2017 Influenza Season. MMWR 2016; 65(5):1-54.
- wendi Wu, Dawei Liu, Keli Li, Jingshan Zheng, Disha Xu, Yaming Wang, Lei Cao, Lingsheng Cao, Ping Yuan, Huaqing Wang, Li Li. Analysis on Surveillance Data of Adverse Events Following Immunization in China, 2012. Zhongguo Yi Miao He Mian Yi 2014; 20(1):1-12.
- Favin M, Steinglass R, Fields R, Banerjee K, Sawhney M. Why children are not vaccinated: a review of the grey literature. Int Health 2012; 4(4):229-38; PMID:24029668; https://doi.org/10.1016/j.inhe.2012.07.004
- Wood D, Halfon N, Pereyra M, Hamlin JS, Grabowsky M. Knowledge of the childhood immunization schedule and of contraindications to vaccinate by private and public providers in Los Angeles. Pediatr Infect Dis J 1996; 15(2):140-5; PMID:8822287; https://doi.org/10.1097/00006454-199602000-00010
- Stevens D, Baker R, Hands S. Failure to vaccinate against whooping cough. Arch Dis Child 1986; 61: 382-7; PMID:3707190; https://doi.org/10.1136/adc.61.4.382
- Brink SG. Provider reminders: changing information format to increase infant. Med Care 1989; 27: 648-53; PMID:2725091; https://doi.org/10.1097/00005650-198906000-00007
- Wood DL, Pereyra M, Halfon N, Hamlin J, Grabowsky M. Increasing immunizations in the public sector: missed opportunities and other contributing factors. Am J Public Health. 1995; 85: 850-3; PMID:7762724; https://doi.org/10.2105/AJPH.85.6.850
- McConnochie KM, Roghmann KJ. Immunization opportunities missed among urban poor children. Pediatrics 1992; 89: 1019-26; PMID:1594341
- Hutchins SS, Jansen HAFM, Robertson SE. Missed opportunities for immunization: the magnitude, reasons, strategies and recommendations. Geneva: Expanded Programme on Immunization (WHO), 1991.
- Hill DA, Grundmeier RW, Ram G, Spergel JM. The epidemiologic characteristics of healthcare provider-diagnosed eczema, asthma, allergic rhinitis, and food allergy in children: a retrospective cohort study. BMC Pediatr 2016; 16: 133; PMID:27542726; https://doi.org/10.1186/s12887-016-0673-z