?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Herpes zoster (HZ) or “shingles” results from a reactivation of the varicella zoster virus (VZV) acquired during primary infection (chickenpox) and surviving in the dorsal root ganglia. In about 20% of cases, a complication occurs, known as post-herpetic neuralgia (PHN). A live attenuated vaccine against VZV is available for the prevention of HZ and subsequent PHN. The present study aims to update an earlier evaluation estimating the cost-effectiveness of the HZ vaccine from a Swiss third party payer perspective. It takes into account updated vaccine prices, a different age cohort, latest clinical data and burden of illness data. A Markov model was developed to simulate the lifetime consequences of vaccinating 15% of the Swiss population aged 65–79 y. Information from sentinel data, official statistics and published literature were used. Endpoints assessed were number of HZ and PHN cases, quality-adjusted life years (QALYs), costs of hospitalizations, consultations and prescriptions. Based on a vaccine price of CHF 162, the vaccination strategy accrued additional costs of CHF 17,720,087 and gained 594 QALYs. The incremental cost-effectiveness ratio (ICER) was CHF 29,814 per QALY gained. Sensitivity analyses showed that the results were most sensitive to epidemiological inputs, utility values, discount rates, duration of vaccine efficacy, and vaccine price. Probabilistic sensitivity analyses indicated a more than 99% chance that the ICER was below 40,000 CHF per QALY. Findings were in line with existing cost-effectiveness analyses of HZ vaccination. This updated study supports the value of an HZ vaccination strategy targeting the Swiss population aged 65–79 y.
Introduction
Infection with the varicella zoster virus (VZV) primarily causes chickenpox. It can cause herpes zoster (HZ) or “shingles” in the later life of persons with a primary infection. HZ causes rash and (severe) pain. Pain persists for an extended time period after the cutaneous eruption has healed, in about 25% of cases with post-herpetic neuralgia (PHN).Citation1 PHN is defined as pain persisting after the typical cutaneous rash has healed. This is in contrast to pain due to swelling and inflammation when the rash is still persisting and not due to nerve damage. The time during which pain must persist after healing for the rash to be classified as PHN varies between guidelines (1 week, 28 days, 3 months). While there is no international consensus on the definition of PHN, the most commonly accepted one is based on a time of 3 months, and this definition was also used in this article.Citation2-4 Patients with PHN can develop severe physical, occupational and social disabilities as a consequence of the enduring pain,Citation5-7 with a profound impact on daily live.Citation8
Because of age-related decrease in cell-mediated immunity, the individual lifetime risk of developing HZ increases with higher age.Citation1,9 The annual incidence of HZ is as high as 10 per 1,000 patient-years in persons over 75 y of age.Citation10 According to incidence estimates for Switzerland, about 11,000 people older than 50 y old are affected each year by HZ.Citation11
Due to the fact that almost everyone has had chickenpox as a child, almost everyone is at risk. However, it is currently not possible to predict who will develop HZ, ophthalmic zoster and PHN. There is evidence that the proportion of patients experiencing PHN increases with age to about 34% in HZ patients aged 80 years.Citation12
A live attenuated vaccine against varicella zoster virus infection is available (Zostavax®, MERCK & CO., INC) for the prevention of HZ and subsequent PHN. The efficacy of this vaccine has been tested in 2 randomized studies.Citation4,13,14 Furthermore, retrospective cohort studies have confirmed the effectiveness under real-life conditions.Citation15,16 The vaccine received a marketing authorization for immunocompetent individuals aged 50 y or older by the European Agency for the Evaluation of Medicinal Products (EMA), and several European countries recommend and/or fund HZ vaccination, including Austria, Greece, the UK and France, as well as some regions in Germany, Italy and Spain.Citation17 However, the vaccine has not been introduced in the Swiss vaccination plan.Citation18,19
An economic evaluation of HZ vaccination in elderly people aged 70–79 y in the Swiss population was conducted in 2011 by one of the authors of the present study (TD Szucs) and colleagues. Since this economic evaluation was conducted, new data on the duration of the vaccine efficacy based on long-term efficacy data have become available and input parameter values (demographics, hospitalization costs, coverage and vaccine price assumptions) have changed.Citation4,20
The present study aims to update the earlier economic evaluation of the cost-effectiveness of HZ vaccination from a third party payer perspective, focusing on the Swiss population aged 65–79 y. We incorporated the latest data or estimates of vaccine efficacy, demographics, hospitalization costs, coverage rate and vaccine price.
Results
Base-case analysis on public health and economic impacts
The target population included individuals aged 65 to 79 y in Switzerland. The vaccination strategy assumed a vaccination coverage of 15%. Hence, 1,034,026 individuals were included, whereof 155,104 (15% of the total target population) persons were assumed to be vaccinated in the vaccination strategy. The base case results () indicated that the vaccination of people aged 65–79 years, in comparison with no vaccination and over a lifetime horizon, would yield public health benefits in the form of additional QALYs and HZ and PHN cases avoided, and economic benefits through a reduced use of health care resources required to treat HZ and PHN. In the base case, the HZ vaccination strategy avoided 3,489 HZ cases and 1,244 PHN cases. QALYs accrued were 12,192,998 and 12,192,394 for the vaccination and no vaccination strategies, respectively, implying a difference of 594 QALYs in favor of the vaccination strategy. The total mean cost was CHF 156,136,484 and CHF 138,416,397 for the vaccination and the no vaccination strategies, respectively, resulting in additional costs of CHF 17,720,087 for the vaccination strategy. The incremental cost-effectiveness ratio (ICER) for vaccination of those aged 65–79 was CHF 29,814 per QALY gained.
Table 1. Strategy-specific results and base case ICER.
The number needed to vaccinate (NNV) quantifies the number of people that needs to be vaccinated to prevent one case of the disease, and is an indicator of vaccination effectiveness. In the population aged 65–79 years, 44 people would need to be vaccinated to avoid one case of HZ and 125 people would need to be vaccinated to avoid one case of PHN. The cost per HZ case avoided was estimated at CHF 5,078. On the other hand, the average cost of avoiding one case of PHN was higher, partially because the number of incident PHN cases was much lower than for HZ: in fact, patient had to experience HZ before PHN.
Sensitivity analysis
Deterministic sensitivity analysis
illustrates the impact on the ICER resulting from a series of sensitivity analyses performed for the included population. The results were sensitive to several parameters.
Table 2. Deterministic sensitivity analysis (lifetime results).
Assuming discount rates of 0% or 5% for both costs and outcomes had a strong impact on the ICER (range: CHF 24,254/QALY to CHF 33,293/QALY). Using alternative utility inputs extracted from other publications had a detrimental effect on the ICER (), since these values did not penalize severe pain states as much as the base case inputs (range: CHF 36,279/QALY to CHF 42,886/QALY).Citation21-23 On the contrary, the use of alternative inputs for both HZ and PHN incidence had a very favorable effect, with resulting ICERs ranging from CHF 16,997/QALY to CHF 23,189/QALY.Citation24,25 Assuming a vaccine efficacy duration of 10 y for both HZ and PHN with no waning had a small impact on the ICER (CHF 26,978/QALY).
Increasing or decreasing the vaccine price by 10% (CHF 178.20 or CHF 145.80) had a substantial impact on the ICER (CHF 30,621/QALY or CHF 23,454/QALY, respectively). Increasing healthcare resource use for HZ and PHN care by 20% resulted in an increased ICER (CHF 29,913/QALY) whereas decreasing healthcare resource use by 20% resulted in a decreased ICER (CHF 24,162/QALY). Assuming no hospitalization or using alternative values for hospitalization costs ( ± 30%) had a limited effect on the cost-effectiveness results.Citation26 The variation of the vaccine efficacy ( ± 10%; consistent with the 95% confidence interval seen in the clinical trialCitation4) at vaccine uptake led to a variation of 10% of the ICER in both directions.
Probabilistic sensitivity analysis
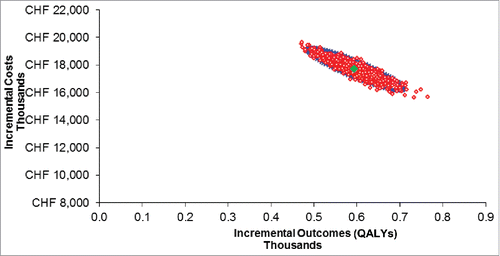
shows the cost-effectiveness scatter plot resulting from the PSA, for the outcome of cost per QALY gained. There was an evident negative correlation between costs and outcomes, meaning that high effectiveness and lower costs were associated, and vice versa.
The probability of not surpassing the cost-effectiveness threshold of CHF 40,000 from a third party payer perspective was 99% for the vaccination strategy. The corresponding cost-effectiveness acceptability curve (CEAC) over a series of thresholds is shown in Appendix 1. The CEAC represents the probability of the vaccination strategy being cost-effective, over a range of cost-effectiveness thresholds.
Discussion
The present cost-effectiveness analysis provides an update of an earlier economic evaluation of HZ vaccination in Switzerland in comparison with no vaccination. It is based on new information regarding the age group to be targeted and new data on the duration of vaccine efficacy based on long-term efficacy data, coverage rate and price. The main results of the present analysis show that vaccinating persons in Switzerland aged 65–79 y is likely to lead to both substantial clinical and economic benefits. These benefits include a reduced number of HZ and PHN cases, an increase in health-related quality of life (measured as QALYs), in addition to a reduction in hospitalizations, consultations and prescription costs for the vaccinated population. These results are explained by the association of both good vaccine efficacy and a substantial burden of disease in this age group.
Nevertheless, the costs of the vaccination strategy could not be fully compensated by the saved costs of treating HZ and PHN cases. The analysis indicates an ICER of CHF 29,814 per QALY gained, over a lifetime horizon. In Switzerland, there is no formally accepted cost-effectiveness threshold. The most frequently cited cost-effectiveness thresholds are from the British National Institute for Health and Clinical Excellence (NICE) (GBP 20,000 to 30,000 per QALY gained).Citation27 To transfer these values to Switzerland, they could be put in relation to the per capita gross domestic product (GDP) per year. For example, in 2013 the ratio of the ICER threshold to per capita GDP was 0.71 in the UK. Applying this value to the Swiss per capita GDP in 2013 would lead to an ICER threshold in the range of CHF 40,117 to CHF 60,171 per QALY. The World Health Organization (WHO) suggests cost-effectiveness thresholds of 2–3 times the GDP per person.Citation28 Considering the 2013 Swiss GDP per person of CHF 84,748, the WHO recommendation would yield Swiss thresholds in a range of CHF 169,496 to CHF 254,244. In the present study, the base-case ICER is well below the range of discussed thresholds.
Results of this study are very similar to those obtained in the earlier Swiss analysis by Szucs et al., which assessed the cost-effectiveness of the HZ vaccine in the Swiss population aged 70–79 y and estimated the ICER at CHF 25,538 per QALY gained from the third party payer perspective (versus CHF 29,814 per QALY gained in the current study for the 65–79 y old population).Citation11,29 The present, updated analysis includes the most recent available data and estimates of vaccine efficacy, demographics, hospitalization costs, coverage rate and vaccine price. The most important change related to the assumptions on vaccine efficacy duration. In the base case analysis by Szcus et al, the vaccine was assumed to offer lifetime protection against HZ and PHN.Citation11 Even in the sensitivity analysis, only annual waning of the achieved protection against HZ of 8.3% was applied. In contrast, we applied estimates from a recent durability model for the input of vaccine efficacy, and also used only 10 y of protection against PHN in the base case analysis.Citation21,30
In a recent systematic review, the cost-effectiveness of routine varicella and HZ vaccination was assessed in high-income countries.Citation31 In total, 15 studies were included which considered the health economic impact of HZ vaccination exclusively. From a payer perspective, ICERs ranged from EUR 5,412 to 140,125 per QALY gained. The majority of the studies reported ICERs between EUR 10,000 to 40,000/QALY, which is in line with our results. Interestingly, studies which included waning of vaccine-induced immunity showed a u-shape age-dependence of the cost-effectiveness results (i.e. ICERs decreased with increasing age up to 60–70 y and then increased further with increasing age). Furthermore, many studies indicated that cost-effectiveness results were dependent on the duration of vaccine-induced protection, the vaccine price and the applied cost per QALY threshold. Other recent health economic evaluations from Portugal, France and Italy reported favorable cost-effectiveness results for HZ vaccination among elderly.Citation32-34
Strengths of the present study are the robustness of the model that was validated through an internal validation with results exactly matching the trail results, and investigated by several health authorities (e.g. in the NetherlandsCitation35 and FranceCitation36). Furthermore, the use of local data sources allowed producing country-specific results. This advantageous property of the model has been attested by the external validation and led to analyses for several countries (e.g., UK,Citation37 Belgium,Citation38 France,Citation39 Germany,Citation40 SpainCitation41 or SwitzerlandCitation11).
The results are indeed consistent with the conclusions drawn by 2 recent literature reviews of existing health economic evaluations of HZ vaccination among adults over 50 years.Citation42,43 Another strength is the incorporation of the vaccine waning effect, which dramatically reduces the uncertainty around vaccine efficacy duration. Indeed, consideration of vaccine efficacy waning since the time of vaccination along with the age at vaccination is crucial in estimating the cost-effectiveness of HZ vaccination.Citation44 Whereas the previous version of the model was using efficacy waning assumptions that were not age specific given an absence of follow-up data, in the present analysis, real life waning in efficacy was taken into account.
This study has several limitations, most of which are related to the model input parameters. First, the sources used for some parameters (PHN incidence, utility values, disease severity split) were not specific to Switzerland because no Swiss data were available. However, Swiss specific references were used as much as possible and the most uncertain parameters were extensively assessed in sensitivity analyses. The latter demonstrated that the conclusions of the study were robust to input parameters changes since ICERs remained below or close to CHF 40,000 per QALY gained. Second, resource use and corresponding cost data were taken from a Swiss burden of illness study based on Swiss expert opinions conducted in 2007 In the absence of better data, these were inflated to represent 2014 costs.Citation26 Sensitivity analysis indicated an only mild impact of these parameters on the ICER result. Third, hospitalization costs were assessed using hospitalization records extracted from only one hospital in Switzerland (CHUV). Thus, hospitalization costs may not be representative for the entire Swiss population. Moreover, the same ICD codes were used to assess HZ-related and PHN-related hospitalizations, and may have led to a flawed estimation of PHN hospitalization costs (no differentiation was possible). It is also possible that following a diagnosis of HZ or PHN, hospitalizations were not coded as being specifically for HZ or PHN, and could therefore not be considered. This implies a possibility that the estimated costs were under or over-estimated. However, since an underestimation of costs favors the vaccine arm this limitation makes the results more conservative.
The percentage of elderly people in Switzerland increases. The Swiss Federal Office of Statistics estimated that by 2045, the percentage of residents over the age of 65 may reach 27.4% of total population.Citation45 People are living longer but are experiencing more chronic and infectious diseases as they age.Citation46 Investing in the health of the senior population, including the prevention of disease, is important in light of this demographic change and ultimately to preserve healthcare system sustainability.Citation47,48
In summary, health economic evidence provided by this study supports the value of an HZ vaccination strategy targeting the Swiss population aged from 65 to 79 y. Comprehensive sensitivity analyses demonstrated that results are robust and aligned with existing cost-effectiveness analyses of HZ vaccination.Citation42,43 With the elderly population in Switzerland steadily growing in size, the number of patients presenting with HZ is also likely to increase and this will place a greater burden on the healthcare systems. No preventative or curative treatment of HZ exists. A cost-effective prevention strategy by vaccination which was demonstrated to be may offer a good option for the future.
Materials and methods
Overview of the model
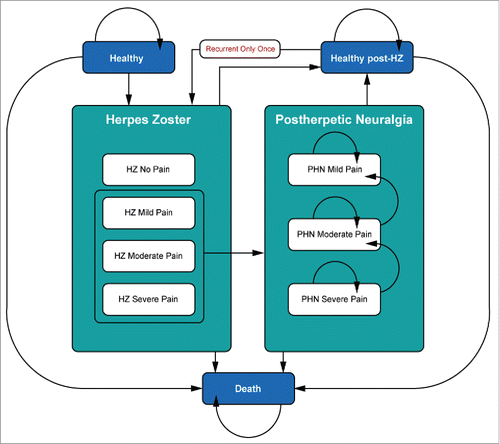
The model simulates the lifetime incidence and consequences of HZ in the Swiss population aged 65–79 y at the time of vaccination based on earlier publications.Citation11,37 Two strategies are compared: the adoption of a vaccination strategy in Switzerland, for which a 15% vaccine coverage rate is assumed to be achieved in the relevant age group, and the current Swiss policy of no vaccination (not taking into account vaccination paid by customers). It comprises 4 different health states including healthy (no HZ symptoms); HZ; PHN; and death. The HZ and PHN health states are further divided into different pain severity levels. Recurrent HZ and subsequent neuropathic pain are also allowed states, but are constrained to a one-time-only recurrent episode (). It was assumed that there was no additional recurrent HZ episode.
The model uses a Markov cycle length of one month. Within each cycle, members of the cohort can remain in their current health state or make a transition into one of the allowable states. Transitions are governed by a matrix of probability values. The transition probabilities determine the proportion of the cohort moving through the HZ and PHN states, and eventually to death, in each monthly cycle. The model runs until the entire cohort has died.
For most epidemiological and cost parameters, age-specific Swiss data were retrieved. HZ-related transition probabilities were derived from published literature. Costs were assessed from a third party payer perspective. Discount rates applied in the base case were 3.5% for costs and for 1.5% for outcomes,Citation49,50 to maintain comparability with the previous analysis.Citation11 The model was developed in Microsoft Excel®, with additional programming in Visual Basic for Applications (VBA). We updated the earlier analysis by Szucs et al.Citation11 by incorporating the latest data or estimates of vaccine efficacy duration, demographics, hospitalization costs, coverage rate and vaccine price.
Model input parameters
Demographic data
The population was analyzed as separate 5-year age cohorts (65–69 years, 70–74 years, and 75–79 years). Results were subsequently combined. The Swiss population size and monthly mortality rates () were obtained from the Swiss Federal Statistical Office.Citation51,52 In total, 1,034,026 individuals were included in the model, representing the population aged 65–79 y.
Table 3. Input parameters related to HZ/PHN vaccine and epidemiology.
Epidemiology of herpes zoster
Herpes zoster
Data from the Swiss Sentinel Surveillance Network were used as a source of data for HZ incidence rates ().Citation53 For HZ duration, information from a clinical trial was used.Citation4 Most patients experienced a duration of 30 days, as also reported elsewhere in the literature.Citation4,54,55
Evidence from the UK indicates no mortality directly linked to HZ.Citation25 As no information on HZ mortality was available for Switzerland, the UK data was applied in the model and HZ mortality was set at 0% for all age groups.
Post-herpetic neuralgia
The proportion of PHN has been reported as a proportion of HZ patients who develop PHN, and has been applied only to those HZ cases presenting with pain ().Citation25 The literature provides evidence that patients have a higher risk of developing PHN if they experience severe pain during HZ. To reflect the increased (decreased) odds of developing PHN by patients having a severe (mild) HZ pain episode relative to moderate HZ pain, odds ratios of 2.39 for severe pain and 0.88 for mild pain, obtained from the literature, were applied.Citation55 Hence, patients with severe pain were estimated to be 2.8 times more likely to develop PHN than patients with lower levels of pain or no pain.
Neuropathic pain following recurrent HZ was assumed to occur as frequently as the same proportions as first time PHN. No mortality was deemed to be associated with PHN. In the absence of literature on the subject, this was confirmed by expert opinion.
Furthermore, the duration of PHN is reduced through vaccination, and this ultimately affects the pain severity experienced by the patients, as they spend a shorter period of time in each painful PHN health state (). Reported pain splits by age group are shown in
HZ and PHN pain split
It was important to model the split between the different pain states for HZ and PHN as these were linked to PHN probability and treatment costs. The pain split results from Oxman et al. were used in the base case analysis.Citation4 In this trial, pain duration and severity were measured using the HZ severity-of-illness score (HZSOI) and Zoster Brief Pain Inventory (ZBPI). Pain split data were reported as proportions of patients with no, mild, moderate or severe pain, and were investigated at study recruitment ().
Data for gender split in HZ and PHN cases were available from Gauthier et al. reporting a proportion of women of 61.10% in all HZ cases and of 65.4% in all PHN cases.Citation25 This information was incorporated in the model where gender specific values were relevant (e.g., for utility decrements).
Vaccine efficacy
Reduction of HZ incidence and PHN proportion
Vaccine efficacy was derived from clinical trials.Citation4,20 The reduction in the number of PHN cases is due to the indirect reduction in the HZ cases. The vaccine efficacy data on HZ and PHN cases are expressed as relative risks and differ by age group ().Citation4,20
In scenario analyses, alternative inputs for both HZ and PHN incidence were tested.Citation24,25
PHN duration
In addition, vaccination has a positive effect on the duration of PHN. In the model this indirectly impacted the pain severity experienced by the patient since it implied a shorter period of time spent in each painful PHN health state. Within the vaccination group, the PHN duration was assumed to be 8.07 months and 9.58 months for the 60–69 y and the 70+ years old individuals, respectively. Unvaccinated individuals experienced a longer PHN duration of 10.34 months and 12.90 months for the 60–69 y and the 70+ years old individuals, respectively ()Citation4,56.
Vaccine efficacy duration and waning rate for HZ
The HZ vaccine efficacy was assumed to decrease over time. Available published data on efficacy decline following vaccination vary between 0% and 8.3% per year.Citation21 Given that the waning rate is not constant over time and unequal among different age groups, data from a durability model were incorporated. The durability model was developed to assess the efficacy of HZ vaccine based on long-term efficacy data, the age at time of vaccination and time since vaccination.Citation21,30
The assumption was made that at 13 y, 18 y and 23 y after vaccination the vaccine efficacy would rebound to zero for the 75–79 y, 70–74 y and 65–69 y old individuals, respectively (Appendix 2). Appendix 2 shows the assumed decline in efficacy over time for various age groups.Citation21,30
Vaccine efficacy duration on PHN
Due to the lack of statistically significant data for PHN, the durability model could only be estimated for efficacy regarding HZ incidence. As a consequence, the duration of protection from PHN was set to 10 y in the base case, meaning that after 10 y with full efficacy it would rebound to zero.
Transition probabilities
HZ and PHN
The direct effects on occurrence of HZ were applied to the transition probabilities (TP) “Healthy to HZ” and “HZ to PHN“ as follows:
The indirect effect on PHN occurrence was included implicitly as this was an additional effect of the direct HZ effect.
PHN duration
The values for PHN durations, differing by age (≤ 69 and ≥ 70) and vaccination status, were included in the model by adjusting the transition probabilities associated with each of the 4 distinct 5-year age groups. Modeling PHN duration required transition probabilities resulting in PHN patients remaining in PHN states for several cycles equal to the average PHN duration (in months). The main assumption in calculating PHN pain state transition probabilities was that pain would decrease over time and eventually cease.
PHN pain split
Calculating the transition probabilities required a calibration exercise using a basic Markov chain. An initial cohort with the Oxman et al. pain split was considered during the first cycle.Citation4 A constant probability was assumed for all PHN patients transitioning to the next lower pain state (severe to moderate; moderate to mild; mild to full health). A sufficient number of cycles were calculated until nearly all patients (> 99%) were no longer in pain. Finally, average duration of pain was calculated for this population. The initial, constant probability was then adjusted until the average duration of pain was equal to the PHN duration provided by Oxman et al. The resulting constant probability was the PHN pain state transition probability. This exercise was conducted 4 times, to reflect the available information on PHN duration, which differed by age and vaccination status. The transition probabilities included were 23.0% and 22.0% for the vaccination policy and 18.2% and 16.2% for the no-vaccination policy for the 60–69 y old and ≥ 70 y old individuals, respectively.
Utilities
The model contains different pain states and specific utilities associated with these states. The pain states related to HZ cover no pain; mild pain; moderate pain; and severe pain. The pain states related to PHN exclude the no-pain state, as pain is a prerequisite for a PHN diagnosis.
The utilities of the various pain states were used to obtain decrements which were subsequently applied to age-specific utilities.Citation7 Decrements were defined as the part of the baseline utility (i.e., HZ with no pain) that needed to be subtracted to obtain the utility value for a given health state (). These were combined with age-specific utilities for the general population. UK data from the Health Survey for England were used for this purpose, since no age-specific utility values by gender are available for the Swiss population (65–74 years: 0.92 male, 0.85 female; 75–79 years: 0.85 male, 0.80 female).Citation57 Since non-gender-separated 5-year age groups in the Swiss population were evaluated at one time, overall utility values were calculated from this data by weighting the male and female utility values by the gender distribution in the relevant age groups in Switzerland.
Table 4. Monthly costs and utilities of HZ and PHN management.
The final utility values were calculated as the age-specific utilities minus the applicable decrement of the specific health state. Additionally, alternative utility inputs were extracted from other publications and assessed in sensitivity analysesCitation21-23 ().
Costs
Vaccine cost
Unit cost of vaccination to the third party payer was estimated at CHF 162.00 (by subtracting a 10% co-payment of CHF 16.20). We assumed that 10% of administrations occurred at the same time as the administration of a flu vaccine; thus administration costs were estimated at CHF 22.68 including an inflation rate as used in the earlier publication.Citation11
Management of HZ and PHN
Data on healthcare utilization and costs in the model were based on a Swiss burden of illness study conducted in 200726. The study investigated the consumption of health care-related resources associated with HZ and PHN and the cost of current HZ/PHN management in Swiss patients aged 50 y and older. The information was derived from an interview with 2 Swiss experts with a recognized expertise in HZ and PHN diseases. All costs parameters provided by this study were inflated to 2014 costs using the Swiss consumer price index ().Citation58
Hospitalization
The mean length of hospital stay and the mean hospitalization cost during the acute and chronic phases of severe HZ cases and moderate to severe PHN cases were estimated using hospitalizations records from the University of Vaud Hospital Centre (CHUV). Hospitalization records were extracted when a HZ related ICD-10 (International classification of diseases, Version 10) was registered as main diagnosis code between 2010 and 2013. In total, 78 hospitalizations were extracted. From these hospitalizations a mean cost per day of CHF 1,472 and a mean length of stay of 9.80 d were estimated (CHF 14,421).Citation26 The hospitalization costs were calculated as monthly costs to match the Markov cycle length of one month.
For PHN, the hospitalization cost for patients with moderate pain was CHF 72.11 and CHF 144.21 for patients with severe pain (average hospitalization costs). For HZ, no hospitalization was estimated for the patients with mild or moderate pain. For patient with severe pain, the hospitalization was CHF 648.95 ().
Sensitivity-analysis
Deterministic sensitivity analyses
Deterministic sensitivity analyses (DSAs) were performed to assess the impact of parameter uncertainty and alternative assumptions on the model results. By changing one parameter at a time and leaving all other parameters constant, possible boundaries of the cost-effectiveness results were defined and parameters with a strong impact on the ICER were identified. Variables subjected to variation included epidemiological inputs (HZ and PHN incidence, HP pain split and HZ mortality), discount rates, descriptors of management of HZ care, vaccine price, vaccine efficacy, vaccine efficacy duration and utility values.
Probabilistic sensitivity analyses
Probabilistic sensitivity analyses (PSA) were conducted to describe and account for the uncertainty surrounding input parameters, jointly. The principle is to run the model a large number of times (i.e., 1,000 runs), with different sets of inputs drawn randomly from pre-specified distributions.
Parameters most influential in the DAS were included in the PSA (health care resource costs, HZ/PHN utility values, HZ incidence and PHN proportion). shows all variables included with their distributions and respective values.
Table 5. Parameters and distribution type selected for PSA.
Disclosure of potential conflicts of interest
PB, ZA, TS and MS received funding from Sanofi Pasteur MSD; XL was an employee of Sanofi Pasteur MSD during the period of the present study.
KHVI_A_1308987_SUPPLEMENTS.zip
Download Zip (231.2 KB)Funding
This research received funding from Sanofi Pasteur MSD, Lyon France.
References
- Stankus SJ, Dlugopolski M, Packer D. Management of herpes zoster (Shingles) and postherpetic neuralgia. Am Fam Physician 2000; 61:2437-48; PMID:10794584
- Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract 2002; 19(5):471-5; PMID:12356697; https://doi.org/10.1093/fampra/19.5.471
- Scott FT, Leedham-Green ME, Barrett-Muir WY, Hawrami K, Gallagher WJ, Johnson R, Breuer J. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol 2003; 70(Suppl 1):S24-30; PMID:12627483; https://doi.org/10.1002/jmv.10316
- Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, Arbeit RD, Simberkoff MS, Gershon AA, Davis LE, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352(22):2271-84; PMID:15930418; https://doi.org/10.1056/NEJMoa051016
- Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain 2002; 18(6):350-4; PMID:12441828; https://doi.org/10.1097/00002508-200211000-00002
- Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain 1996; 67(2–3):241-51; PMID:8951917; https://doi.org/10.1016/0304-3959(96)03122-3
- Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use, and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain 2005; 6(6):356-63; PMID:15943957; https://doi.org/10.1016/j.jpain.2005.01.359
- Katz J, Cooper EM, Walther RR, Sweeney EW, Dworkin RH. Acute pain in herpes zoster and its impact on health-related quality of life. Clin Infect Dis 2004; 39(3):342-8; PMID:15307000; https://doi.org/10.1086/421942
- Pinchinat S, Cebrián-Cuenca AM, Bricout H, Johnson RW. Similar herpes zoster incidence across Europe: results from a systematic literature review. BMC Infect Dis 2013; 13:170; PMID:23574765; https://doi.org/10.1186/1471-2334-13-170
- Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med 1995; 155(15):1605-9; PMID:7618983; https://doi.org/10.1001/archinte.1995.00430150071008
- Szucs TD, Kressig RW, Papageorgiou M, Kempf W, Michel JP, Fendl A, Bresse X. Economic evaluation of a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in older adults in Switzerland. Hum Vaccin 2011; 7(7):749-56; PMID:21606685; https://doi.org/10.4161/hv.7.7.15573
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract 1975; 25(157):571-5; PMID:1195231
- Schmader KE, Oxman MN, Levin MJ, Johnson G, Zhang JH, Betts R, Morrison VA, Gelb L, Guatelli JC, Harbecke R, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis 2012; 55(10):1320-8; PMID:22828595; https://doi.org/10.1093/cid/cis638
- Morrison VA, Johnson GR, Schmader KE, Levin MJ, Zhang JH, Looney DJ, Betts R, Gelb L, Guatelli JC, Harbecke R, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis 2015; 60(6):900-9; PMID:25416754; https://doi.org/10.1093/cid/ciu918
- Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, Saag KG, Baddley JW, Curtis JR. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA 2012; 308(1):43-9; PMID:22760290; https://doi.org/10.1001/jama.2012.7304
- Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med 2013; 10(4):e1001420; PMID:23585738; https://doi.org/10.1371/journal.pmed.1001420
- EMA. European Medicines Agency: Zostavax; zoster vaccine (live)
- Vionnet J, Hart K, Spertini F. Vaccin contre le zona : quelles recommandations en 2014? Rev Med Suisse 2014; 426:869-75
- BAG. Bundesamt für Gesundheit: Vaccination contre le zona: pas d'introduction dans le plan suisse de vaccination. Bulletin OFSP. 2010:97-101
- Schmader KE, Levin MJ, Gnann JW Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis 2012; 54(7):922-8; PMID:22291101; https://doi.org/10.1093/cid/cir970
- Pellissier JM. Evaluation of the cost-effectiveness in the United States of a vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. Vaccine 2007; 25:8326-37; PMID:17980938; https://doi.org/10.1016/j.vaccine.2007.09.066
- Bala MV, Wood LL, Zarkin GA, Norton EC, Gafni A, O'Brien B. Valuing outcomes in health care: a comparison of willingness to pay and quality-adjusted life-years. J Clin Epidemiol 1998; 51(8):667-76; PMID:9743315; https://doi.org/10.1016/S0895-4356(98)00036-5
- Van Hoek AJ, Gay N, Melegaro A, Opstelten W, Edmunds WJ. Estimating the cost-effectiveness of vaccination against herpes zoster in England and Wales. Vaccine 2009; 27(9):1454-67; PMID:19135492; https://doi.org/10.1016/j.vaccine.2008.12.024
- Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine 2001; 19(23–24):3076-90; PMID:11312002; https://doi.org/10.1016/S0264-410X(01)00044-5
- Gauthier A, Breuer J, Carrington D, Martin M, Rémy V. Epidemiology and cost of herpes zoster and post-herpetic neuralgia in the United Kingdom. Epidemiol Infect 2008, 137(1):38-47; PMID:18466661; https://doi.org/10.1017/S0950268808000678
- Michel JP, Kempf W. Data collection related to the management of herpes zoster (HZ) and post-herpetic neuralgia (PHN) and demographic parameters in Switzerland. 2007
- National Institute for Health and Clinical Excellence. www.nice.org.uk 2009
- Organization., W.H. Cost-effectiveness thresholds. 2015
- Szucs TD, Largeron N, Dedes KJ, Rafia R, Bénard S. Cost-effectiveness analysis of adding a quadrivalent HPV vaccine to the cervical cancer screening programme in Switzerland. Curr Med Res Opin 2008; 24(5):1473-83; PMID:18413014; https://doi.org/10.1185/030079908X297826
- Li X, Zhang JH, Betts RF, Morrison VA, Xu R, Itzler RF, Acosta CJ, Dasbach EJ, Pellissier JM, Johnson GR, et al. Modeling the durability of ZOSTAVAX(R) vaccine efficacy in people >/=60 years of age. Vaccine 2015; 33(12):1499-505; PMID:25444784; https://doi.org/10.1016/j.vaccine.2014.10.039
- Damm O, Ultsch B, Horn J, Mikolajczyk RT, Greiner W, Wichmann O. Systematic review of models assessing the economic value of routine varicella and herpes zoster vaccination in high-income countries. BMC Public Health 2015; 15:533; PMID:26041469; https://doi.org/10.1186/s12889-015-1861-8
- Uhart M, Préaud E, Brandao A, Silva AP, Vandewalle B, Félix J. Herpes Zoster and Post-Herpetic Neuralgia Vaccination- Cost-Effectiveness Analysis In Portugal. Value Health 2015; 18(7):A584; PMID:26533283; https://doi.org/10.1016/j.jval.2015.09.1960
- Belchior E, Lévy-Bruhl D, Le Strat Y, Herida M. Cost-effectiveness of a herpes zoster vaccination program among the French elderly people. Hum Vaccin Immunother 2016; 12(9):2378-82; PMID:27484158; https://doi.org/10.1080/21645515.2016.1184801
- Coretti S CP, Romano F, Ruggeri M, Cicchetti A. COST-EFFECTIVENESS ANALYSIS OF HERPES ZOSTER VACCINATION IN ITALIAN ELDERLY PERSONS. Int J Technol Assess Health Care 2016; 32(4):233-40; PMID:27624398; https://doi.org/10.1017/S0266462316000337
- College voor Zorgverzekeringen (CVZ). GVS-rapport 14/06: herpes zoster vaccine (Zostavax®). 2014
- HAUTE AUTORITE DE SANTE: Transprency Committee opinion on Zostavax
- Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK. Cost Eff Resour Alloc 2010; 8:7; PMID:20433704; https://doi.org/10.1186/1478-7547-8-7
- Annemans L, Bresse X, Gobbo C, Papageorgiou M. Health economic evaluation of a vaccine for the prevention of herpes zoster (shingles) and post-herpetic neuralgia in adults in Belgium. J Med Econ 2010; 13(3):537-51; PMID:20707768; https://doi.org/10.3111/13696998.2010.502854
- Bresse X, Annemans L, Préaud E, Bloch K, Duru G, Gauthier A. Vaccination against herpes zoster and postherpetic neuralgia in France: a cost-effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 2013; 13(3):393-406; PMID:23537397; https://doi.org/10.1586/erp.13.19
- Preaud E, Uhart M, Böhm K, Aidelsburger P, Anger D, Bianic F, Largeron N. Cost-effectiveness analysis of a vaccination program for the prevention of herpes zoster and post-herpetic neuralgia in adults aged 50 and over in Germany. Hum Vaccin Immunother 2015; 11(4):884-96; PMID:25933182; https://doi.org/10.1080/21645515.2015.1011561
- Lopez-Belmonte JL, Cisterna R, Gil de Miguel A, Guilmet C, Bianic F, Uhart M. The use of Zostavax in Spain: the economic case for vaccination of individuals aged 50 years and older. J Med Econ 2016; 19(6):576-86; PMID:26808422; https://doi.org/10.3111/13696998.2016.1146726
- Szucs TD, Pfeil AM. A systematic review of the cost effectiveness of herpes zoster vaccination. Pharmacoeconomics 2013; 31(2):125-36; PMID:23335045; https://doi.org/10.1007/s40273-012-0020-7
- Kawai K, Preaud E, Baron-Papillon F, Largeron N, Acosta CJ. Cost-effectiveness of vaccination against herpes zoster and postherpetic neuralgia: a critical review. Vaccine 2014; 32(15):1645-53; PMID:24534737; https://doi.org/10.1016/j.vaccine.2014.01.058
- Bilcke J, Ogunjimi B, Hulstaert F, Van Damme P, Hens N, Beutels P. Estimating the age-specific duration of herpes zoster vaccine protection: a matter of model choice? Vaccine 2012; 30(17):2795-800; PMID:21964056; https://doi.org/10.1016/j.vaccine.2011.09.079
- Kohli R. Swiss Federal Statistical Office: Szenarien zur Bevölkerungsentwicklung der Schweiz 2015–2045. 2015
- Salisbury C, Johnson L, Purdy S, Valderas JM, Montgomery AA. Epidemiology and impact of multimorbidity in primary care: a retrospective cohort study. Br J Gen Pract 2011; 61(582):e12-21; PMID:21401985; https://doi.org/10.3399/bjgp11X548929
- Council of the European Union - Note from Presidency to Permanent Representatives Committee/Council. Investing in Health: The ‘Missing Dimension’ of the Europe 2020. 2016
- Lang PO, Aspinall R. Vaccination in the elderly: what can be recommended? Drugs Aging 2014; 31(8):581-99; PMID:24928553; https://doi.org/10.1007/s40266-014-0193-1
- Bos JM, Postma MJ, Annemans L. Discounting health effects in pharmacoeconomic evaluations: current controversies. Pharmacoeconomics 2005; 23(7):639-49; PMID:16173156; https://doi.org/10.2165/00019053-200523070-00001
- Bonneux L, Birnie E. The discount rate in the economic evaluation of prevention: a thought experiment. J Epidemiol Community Health 2001; 55(2):123-5; PMID:11154251; https://doi.org/10.1136/jech.55.2.123
- BFS Swiss Federal Statistical Office: Permanent resident population by age, sex and category of citizenship, on 31.12.2013. 2014
- BFS. Swiss Federal Statistical Office: Mortality, all causes of death. 2014
- BAG Federal Office of Public Health: The Swiss Sentinel Surveillance Network. 2015
- Johnson RW, Dworkin RH. Treatment of herpes zoster and postherpetic neuralgia. BMJ 2003; 326(7392):748-50; PMID:12676845; https://doi.org/10.1136/bmj.326.7392.748
- Nagasako EM, Johnson RW, Griffin DR, Dworkin RH. Rash severity in herpes zoster: correlates and relationship to postherpetic neuralgia. J Am. Acad Dermatol 2002; 46(6):834-9; PMID:12063479; https://doi.org/10.1067/mjd.2002.120924
- Merck Data on File. 2006
- Health Survery for England 2012. 2012
- BFS. Swiss Federal office of statistics: Kaufkraftparitäten – Daten, Indikatoren Preisniveauindizes. 2014; Available from: http://www.bfs.admin.ch/bfs/portal/de/index/themen/05/07/blank/key/01.html
- BFS. Swiss Federal Statistical Office: Medical Statistics of hospitals 2013. 2013