ABSTRACT
Macrophages are located in essentially all tissues due to their “janitor” function. Macrophages can exert either anti- or pro-tumor activities depending upon the specific tumor microenvironment they inhabit. Substantial evidence indicates that macrophages, owing to their plasticity, can be reeducated to adopt a protumoral phenotype within a tumor microenvironment through the help of growth factors in the microenvironment and intercellular interactions. As the lethality of malignant melanoma is due to its aggressive capacity for metastasis and resistance to therapy, considerable effort has gone toward treatment of metastatic melanoma. In the present review, we focus on the pro-tumor activities of macrophages in melanoma. Based upon the information presented in this review it is anticipated that new therapies will soon be developed that target pro-tumor activities of macrophages for use in the treatment of melanoma.
Introduction
Malignant melanoma is considered as one of the most lethal cancers due to its aggressive metastasis and resistance to therapy. Worldwide it has been reported to affect 232,000 people, leading to 55,000 deaths in 2012.Citation1 In western countries, people, especially those with diminished skin pigment, are at increased risk as a result of sunbathing.Citation1 In mainland China, it is anticipated that melanoma will result in 20,000 new cases annually,Citation2 of which primary skin melanoma will account for 50–70% and mucous membrane 22.6% of the cases.Citation3
Macrophages, originally identified by Elie Metchnikoff,Citation4 can engulf and digest malignant cells within the body.Citation5 Initially, macrophages were considered to be the guardians of our body against microbes as well as tumors,Citation6 including melanoma.Citation7 Macrophages are classified into 2 different extremes of a continuum ranging from M1 to M2 macrophages in terms of the Th1/Th2 paradigm. M1 is a pro-inflammatory macrophage exhibiting defense reactions against tumors while M2 is an anti-inflammatory macrophage which is beneficial to tumors.Citation8,9 Recently, evidence has been presented which suggests that macrophages within the context of a tumor microenvironment show a preference to adopt to a protumoral phenotype (M2)in primary and/or metastatic sites in vivo with the help of growth factors in the microenvironment and through intercellular interactions.Citation10 We have described targeting macrophage anti-tumor activity to suppress melanoma progression in another review.Citation11 In the current review, we focus on discussing the protumor activities of macrophages in melanoma and how such activities can be used for therapeutic purposes in the treatment of melanoma.
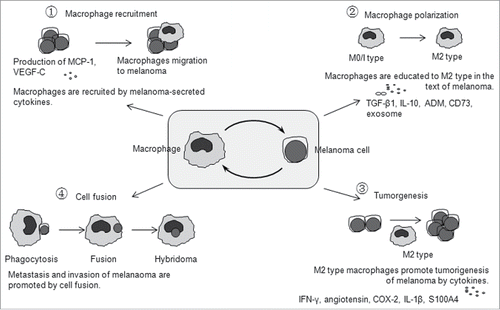
Macrophage recruitment to melanoma
Melanomas release molecules that can recruit macrophages to melanoma sites. Alterations in macrophage population patterns are observed during the progression of a malignant melanoma.
Monocyte chemoattractant protein-1 (MCP-1)
MCP-1, acting as a potent macrophage-recruiting molecule,Citation12 is expressed in human malignant melanoma.Citation13 A mutant of MCP-1 that lacks the amino acids 2–8 at the N-terminal was reported to be overexpressed when transfected in thigh muscle and secreted into the systemic blood circulation.Citation13 Such an effect in turn leads to a reduction in MCP-1 expression by melanoma cells.Citation13 Blocking of MCP-1 function inhibits macrophage recruitment and partially reduces the angiogenesis and growth of malignant melanomas.Citation13 The capacity of MCP-1to enhance tumor angiogenesis is related with inducing the secretion of TNF-α, IL-1α and vascular endothelial growth factor (VEGF) through macrophage recruitment as well as exerting potential direct autocrine/paracrine effects upon the melanoma cells.Citation13 In a melanoma xenograft study, the tissue growth was substantially reduced, which is due to no production of MCP-1 of human melanoma cell line IIB-MEL-J.Citation14 When transfected with an MCP-1-expression vector, MCP-1 was produced and in vivo tissue growth increased.Citation14 The application of MCP-1 inhibitor, as well as macrophage depletion with clodronate-laden liposomes, have been shown to reduce tumor growth and macrophage recruitment, which then induces necrotic tumor masses.Citation14 Anti-tumor effects with restraint stress could reduce macrophage trafficking by suppressing MCP-1 production.Citation15
However, MCP-1 may exert a biphasic effect in melanoma, with high levels promoting tumor rejection, whereas low or intermediate levels of MCP-1 support tumor growth.Citation16
VEGF-C
Vascular endothelial growth factor C (VEGF-C) is a protein that is a member of the platelet-derived growth factor / vascular endothelial growth factor (PDGF/VEGF) family.Citation17 A substantial number of human tumors express VEGF-C, including malignant melanomas.Citation17 One of the most critical steps in tumor progression is completed through the interactions of tumor cells with lymphatic vessels. VEGF-C-overexpressing human melanomas result in enhanced macrophage recruitment as well as melanoma progression.Citation18 Furthermore, in skin areas surrounding VEGF-C-transfected melanomas, increased levels of peritumoral macrophages have been observed. VEGF-C does not appear to exert any direct effects on tumor cells, as VEGF-C-overexpressing cells do not change the proliferation of control cells, and addition of recombinant VEGF-C to control cells did not affect their growth rate in vitro.Citation18 Like MCP-1, VEGF-C may exert biphasic effects in melanoma. An increase in the recruitment of macrophages, which can enhance host-tumor defense capabilities, may be related to the reduction in growth observed in VEGF-C-overexpressing melanomas.Citation18 This conclusion follows from results showing that increased densities of peritumoral macrophages were correlated with tumor growth suppression.Citation18
Polarization to pro-tumor M2 type
Polarization of macrophages to M2 plays a vital role in the outcome of melanoma patients. It results from the presence of growth factors and exsomes that can be released by both melanoma cells and macrophages, or from Treg cells, as well as by intercellular interactions.
Transforming growth factor-β1
All 3 isoforms of TGF-β has been found to be expressed in cultured malignant cellsCitation19-21 and in situ.Citation22-24 TGF-β1, that can be produced by M2 type macrophages, plays a pivotal role in macrophage polarization to the pro-tumor M2 type.Citation9 It has been reported that tumor cells which produce high levels of TGF-β1 can stimulate monocytes/macrophages, and, in this way, support tumor growth and immune escape.Citation25,26 Alternative activation (M2) was shown to be mostly associated with responses of macrophages to anti-inflammatory mediators, such as glucocorticoids.Citation27 This mechanism appears to represent a major component involved with increasing surface contact sites of the TGF-β1 receptor.Citation28 In the presence of glucocorticoids, TGF-β1 stimulates mature macrophages to polarize to the M2 type.Citation28 TGF-β1 can also suppress nitric oxide release resulting from M1 polarized macrophagesCitation29 and suppress M1 polarized macrophages to increase melanoma survival through stroma remodeling.Citation30 However, it has also been reported that tumor cells exposed to TGF-β1 experience enhanced susceptibility to NK-mediated extermination.Citation31
IL-10
Interleukins are a group of cytokines (secreted proteins and signal molecules) that were first seen to be expressed by white blood cells (leukocytes).Citation32 Alternative activation (M2) can be a response of macrophages to Th2 cytokines, such as IL-10. IL-10 acts on macrophages by down-regulating class-II MHC antigensCitation32 and expression of the co-stimulator molecule, B7 on macrophages,Citation33 which then inhibit cytokine production by Thl cells.Citation34 IL-10 production is not confined to Th2 cells, as it has been reported that IL-I0 could be produced by melanoma cells.Citation35 Melanoma cells, in turn, then have the potential to use IL-10 as a means to modulate immune responses such as induction of M2 polarized macrophages.Citation35 Moreover, it has been shown that M2 macrophages also release IL-10.Citation36
Adrenomedullin (ADM)
ADM is a multifunctional molecule involved with tumor angiogenesis and widely expressed in a variety of tumor types,Citation37-39 such as melanoma.Citation40 Levels of ADM and its receptor are increased in human melanoma, suggesting a role in melanomagenesis. Tumor-associated macrophages (TAM) are identified as the major source of ADM in melanoma.Citation41 TAM-derived ADM can induce the phosphorylation of endothelial nitric oxide synthesis in endothelial cells via a paracrine mechanism and polarize macrophages to an M2 phenotype via autocrine mechanisms to enhance melanoma tumor angiogenesis and tumor growth.Citation41 Based upon data generated from a mouse melanoma study,Citation41 a mathematical model has been derived to assess these interactions among mouse melanoma cells, Th2/Th1 cells and M2/M1 macrophages. With this model it is possible to investigate the role of re-polarization between M1 and M2 macrophages on tumor growth, and the findings obtained indicate that melanoma growth is associated with a type-II immune response as it can result from large numbers of Th2 and M2 cells.Citation42
CD73
Nucleotidase CD73 expression is upregulated in melanoma.Citation43 Tumor macrophage infiltration can be dramatically decreased and the microenvironment substantially altered following inhibition or knockdown of tumor CD73, due to their effects upon the polarization of M1 or M2 macrophages.Citation44 Yegutkin et al reported that host CD73 knockout did not affect B16F10 melanoma infiltration by macrophages in B16 melanoma.Citation45 However, suppression of tumor cell CD73 or chemical inhibition of CD73 decreases macrophage infiltration. One conclusion from such results is that CD73 plays a role in the regulation of macrophage infiltration. Pro-neoplastic and pro-angiogenic M2 phenotypes of tumor-associated macrophages are often observed in hypoxic regions of a tumorCitation46; and, a downregulation of pro-M1 cytokines are found in response to any reductions in CD73.Citation44 Such changes in the microenvironment contribute to macrophage polarization resulting in a pro-neoplastic M2 phenotype that can then regulate the progression of a tumor.Citation46
Arginine metabolism
Arginine metabolism plays a role in macrophage polarization. This relationship follows from findings which show that macrophages using arginine can induce nitric oxide synthase to produce nitric oxide (NO) as M1 types and ornithine through arginase as M2 types.Citation47 The number of M1 type macrophages varies as a function of tumor progression and location.Citation48 Mostly within peritumoral locations, considerable numbers of M1-type macrophages were reported to be present in situ and in thin melanomas. In contrast, within tumors of advanced stages and in melanoma metastases decreased numbers of these macrophages were found in peritumoral, as well as in intratumoral locations.Citation48 In both peritumoral and intratumoral locations, the percent of M2 type macrophages (arginase-positive) was lower than that of M1 type macrophages in thin melanomas. Macrophages -induced NO release was shown to be dependent on tumor microenvironment, with high levels being observed as associated with IFN-γ while low levels associated with more advanced tumors.Citation48
The macrophage mannose receptor (MR)
MR is upregulated in the alternative anti-inflammatory/pro-tumoral M2 macrophage and has been shown to be essential for cytokine production.Citation49 In the mouse melanoma model with lung metastasis, recruitment of CD68+CD11b+CD11c− monocytes was abrogated in C57BL/6 mice without MR (i.e., MR−/−) and fewer lung colonies were observed in MR−/− mice as compared with that in the wild type.Citation49
Exosome
Exosomes are microvesicles of 20–100 nm diameters which can be released by tumor cells.Citation50 As a result of their nanoscale size, exosomes readily penetrate and interact with local tumor cells. Moreover, these microvesicles can affect other cell types distal to the advancing tumor cell front.Citation51 Melanoma cells release exosomes which influence the tumor immuno-microenvironment,Citation50 via effects upon the cytokine and chemokine profiles of the macrophages.Citation52 Tumor cells treated with melanoma cell-derived exosomes respond vastly different from those induced by either LPS or IL-4.Citation52
Tregs
Tregs can promote the differentiation of monocytes to tumor-promoting M2 macrophages. The interaction of Tregs and M2 macrophages represents a mutually beneficial effect, as the M2 macrophages directly induce Tregs, which then suppresses tumor specific cytotoxic T-cells.Citation53 Human malignant melanoma cells with monosomy of chromosome 3 can produce chemokines such as macrophage-derived chemokine, thymus- and activation-regulated chemokine and MCP-1, all of which contribute to Treg migration and can also be produced by M2 macrophages.Citation54
Macrophages promote tumorigenesis of melanoma by cytokines
Macrophages recruited to the melanoma can, in turn, produce melanoma-stimulating molecules such as IFN-γ, angiotensin, cyclooxygenase-2 (COX-2) and IL-1β to support the growth and metastasis of melanoma.
IFN-γ
Interferon gamma (IFNγ) is a dimerized soluble cytokine that is the only member of the type II class of interferons.Citation55 IFN-γ has also been reported that IFN-γ may have pro-tumorigenic effects in solid tumors under certain conditions.Citation55,56 Although interferon γ, reduces cellular growth in vitro, when inoculated with B16 melanoma cells intravenously, it stimulates lung colonization along with an enhanced expression of class I major histocompatibility complex antigens.Citation55,56 These effects are more frequently observed in advanced melanoma and are related to an increased risk of metastasis in primary melanoma.Citation56 Elevated levels of IFN-γ show promise as being an independent predictor of disease recurrence. In addition, they may serve as a means for identifing early-stage melanoma patients that are more vulnerable to disease recurrence and who may then benefit from adjuvant therapies, such as immunotherapies.Citation57 Indeed, a randomized clinical trial by the Southwest Oncology Group observed an adverse effect of IFN-γ on melanoma relapse and mortality rates.Citation58 Moreover, in a mouse skin cancer model, as induced by ultraviolet B, macrophage-produced IFN-γ promoted melanoma growth by inhibiting apoptosis.Citation59 Pro-tumorigenic effects of IFN-γ may, in part, be due to a pro-expression of CD74 in melanoma.Citation60
Angiotensin
Macrophages express angiotensin II type 1 and type 2 receptors during the process of monocyte differentiation to macrophages.Citation61 Tumors produce angiotensin II to enhance the amplification of macrophages to stimulate cancer-promoting immunity.Citation62 Angiotensin-converting enzyme (ACE) is a peptidase which is responsible for the cleavage of angiotensin I. Mice with enhanced macrophage ACE levels show increased production of interleukin-12 and nitric oxide but reduced interleukin-10, and are resistant to melanoma.Citation63 However, ACE inhibitors as a pharmacological tool to inhibit tumor angiogenesis is controversial.Citation64 Angiotensin II type 1 receptor expression on TAM is related with increased melanoma tumor growth.Citation64 It is possible that the angiotensin II type 1 receptor pathway may play an important role in promoting tumor angiogenesis and growth via a macrophage and VEGF-dependent mechanism.Citation64
COX-2
Cyclooxygenase-2 (COX-2), also known as prostaglandin-endoperoxide synthase 2, is involved in the conversion of arachidonic acid to prostaglandin H2.Citation65 As shown with use of immunohistochemical analysis, COX-2-positive macrophages, are rare in common nevi and “dysplastic nevi,” but found in high levels in situ and in thin melanoma. COX-2-positive macrophages were also found in more advanced tumors and metastatic melanoma, although at much lower levels than that observed in situ or in thin melanoma. As demonstrated in vitro, COX-2 has been shown to be expressed in peritoneal macrophages when exposed to B16 murine melanoma cells, but not following exposure to normal murine fibroblasts. Taken together, results obtained from both in vivo and in vitro studies indicate that not only may COX-2 expressed in macrophages have the potential to provide a valid and reliable biomarker of melanoma progression, but also the possibility that melanoma cells themselves might stimulate COX-2 in macrophages.Citation65
IL-1β
IL-1β as a pleiotropic pro-inflammatory cytokine contributes to cell growth, differentiation and regulation of immune responses.Citation66 The IL-1β gene or its protein expression are associated with the degree of invasiveness and metastasis of melanoma.Citation67 Interestingly, although metastatic melanoma cell lines do not secrete IL-1β, they do promote IL-1β production from macrophages.Citation68 Therefore, more work directed toward revealing the mechanisms and consequences of IL-1β production by infiltrating macrophages may be of interest for the development of IL-1β targeted therapy, such as an anti-IL-1β antibody (Canakinumab) of metastatic melanoma.Citation68 Moreover, IL-1β as generated from tumor cells may be considered as a threat to the host's immune system. In this regard, IL-1β-producing melanoma cells can induce reduced tumor growth by recruiting immune cells.Citation69
The cancer cell fusion theory
The cancer cell fusion theory initially proposed by Prof. Aichel,Citation70 and currently accepted by many investigators, states that the fusion of cancer cells with macrophages or other phagocytes could underlie cancer metastasis. The Aichel's hypothesis can be simplified by the model — white blood cell + non-metastatic cancer cell = metastatic cancer cells.Citation70 Cancer cells, especially non-adherent ones, favor destruction by white blood cells, preferably by phagocytes like macrophages or neutrophils. If a captured cancer cell escapes to be digested by a predator phagocyte, the 2 cells would fuse, pooling their chromosomes to form a white blood cell–tumor cell hybrid. At least some of these hybrids become metastatic, exhibiting both motility and continuous cell division.Citation70 It has been demonstrated that hybrids of weakly metastatic Cloudman S91 mouse melanoma cells and normal mouse or human macrophages can be created in vitro with use of polyethylene glycol -induced fusion.Citation71 Compared to the parental melanoma cells, most daughters of the 35 hybrids tested were found to be more aggressive, with the result being that metastases onset was more rapid and observed in greater numbers of mice.Citation71 The majority of these hybrid clones showed markedly enhanced chemotactic motility toward a variety of attractants in 2-chambered culture systems, a hallmark of metastatic cells.Citation72 Most notably, the expression of macrophage-like glycosylation patterns showed an increase in oligosaccharide chains conjugated with β1,6-branched oligosaccharides and the responsible glucosyltransferase, β1,6-N-acetylglucosaminyltransferase (GNT-V).Citation73 The significance of this finding is twofold: 1) β1,6-branched oligosaccharides and GNT-V are highly associated with malignant transformation as has been shown in rodent and human cells and 2) these melanoma patients show a poor prognosis.Citation74
Conclusion
Whether macrophages in the tumoral microenvironment are anti- or pro-tumor has been enigmatic. The primary reason for this controversy regarding macrophages in tumor progression can be traced to the contrasting results obtained regarding the effects of macrophages in the literature. It remains, however, widely accepted that macrophages, owing to their malleability, can be domesticated as a tumor's handyman, as summarized in . Interactions between 2 categories of cells often can offer mutual benefits and achieve the common goal of melanoma progression. It is anticipated that the application of such interactions could be used to develop a feasible anti-melanoma strategy which incorporates a combination of macrophage recruitment inhibition and the “re-education” of macrophage polarization.
Figure 1. Melanoma progression and pro-tumor activities of macrophages. ① Macrophage recruitment to melanoma. Melanomas release many different types of macrophage-recruiting molecules, such as MCP-1 and VEGF-C, to attract macrophage migration to melanoma sites. ② Polarization to pro-tumor M2 type. Macrophages can be induced and educated to adopt a protumoral phenotype (M2) in the text of melanoma, which is co-made up by both melanoma cells and macrophages. ③ Cytokines by macrophages promote tumorigenesis of melanoma. Macrophages recruited to the melanoma can produce melanoma-stimulating molecules such as IFN-γ, angiotensin, COX-2, IL-1β and S100A4 to support the growth and metastasis of melanoma. ④ The cancer cell fusion theory. Macrophages in the melanoma microenvironment can devour melanoma cells, if digestion fails, then would likely form a hybridoma of the macrophage-melanoma cell, that results in metastasis of the melanoma.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by One College One Policy Project, Modern College of Arts and Science, Shanxi Normal University (2016KJYJ-6); Scientific Research Foundation for Doctor, Shanxi Normal University (0505/02070293); Shanxi Provincial University Science and Technology Innovation Project (20161107); National Natural Science Foundation of China (31600730); Key Discipline Construction of Shanxi Normal University (0505/02100030).
References
- Organization WH. World cancer report, 2014. WHO Report. Geneva: WHO; 2014; Chapter 5.14.
- committee; Cme. China's guidelines of diagnosis and treatment of melanoma (2011). Chinese Clin Oncol 2012(02):159-71.
- Han Y, Qu X. The current situations and advances in cutaneous malignant melanoma. Modern J Integrated Traditional Chinese Western Med 2015(30):3416-20.
- Kaufmann SH. Elie Metchnikoff's and Paul Ehrlich's impact on infection biology. Microbes Infect 2008; 10(14):1417-9; PMID:18824243; https://doi.org/10.1016/j.micinf.2008.08.012
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8(12):958-69; PMID:19029990; https://doi.org/10.1038/nri2448
- Brigati C, Noonan DM, Albini A, Benelli R. Tumors and inflammatory infiltrates: friends or foes? Clin Exp Metastasis 2002; 19(3):247-58; PMID:12067205; https://doi.org/10.1023/A:1015587423262
- Kozłowska K, Cichorek M. Heterogeneity of peritoneal macrophages in hamsters-bearing transplantable melanomas in relation to their transglutaminase. Folia Histochem Cytobiol 1990; 29(4):141-7.
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000; 164(12):6166-73; PMID:10843666; https://doi.org/10.4049/jimmunol.164.12.6166
- Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 2014; 41(1):14-20; PMID:25035950; https://doi.org/10.1016/j.immuni.2014.06.008
- Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Seminars in Immunopathology 2013; 35(5): 585-600.
- Wang H, Zhang L, Yang L, Liu C, Zhang Q, Zhang L. Targeting macrophage anti-tumor activity to suppress melanoma progression. Oncotarget 2017; 8(11):18486-96.
- Mantovani A, Allavena P, Sozzani S, Vecchi A, Locati M, Sica A. Chemokines in the recruitment and shaping of the leukocyte infiltrate of tumors. Semin Cancer Biol 2004; 14(3): 155-60.
- Koga M, Kai H, Egami K, Murohara T, Ikeda A, Yasuoka S, Egashira K, Matsuishi T, Kai M, Kataoka Y. Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of malignant melanoma in mice. Biochem Biophys Res Commun 2008; 365(2):279-84; PMID:17991428; https://doi.org/10.1016/j.bbrc.2007.10.182
- Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol 2007; 127(8):2031-41; PMID:17460736; https://doi.org/10.1038/sj.jid.5700827
- Eubank T, Bailey M, Gross A, Sumner L, Marsh C, Glaser R, Yang E. 7. CCL2 down-regulation from macrophages by stress-induced β-adrenergic receptor activation is protective in a model of malignant melanoma. Brain Behavior Immunity 2011; 25:S180-1; https://doi.org/10.1016/j.bbi.2011.07.010
- Nesbit M, Schaider H, Miller TH, Herlyn M. Low-level monocyte chemoattractant protein-1 stimulation of monocytes leads to tumor formation in nontumorigenic melanoma cells. J Immunol 2001; 166(11):6483-90; PMID:11359798; https://doi.org/10.4049/jimmunol.166.11.6483
- Salven P, Lymboussaki A, Heikkila P, Jaaskela-Saari H, Enholm B, Aase K, von Euler G, Eriksson U, Alitalo K, Joensuu H. Vascular endothelial growth factors VEGF-B and VEGF-C are expressed in human tumors. Am J Pathol 1998; 153(1):103-8; PMID:9665470; https://doi.org/10.1016/S0002-9440(10)65550-2
- Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol 2001; 159(3):893-903; PMID:11549582; https://doi.org/10.1016/S0002-9440(10)61765-8
- Marquardt H, Todaro GJ. Human transforming growth factor. Production by a melanoma cell line, purification, and initial characterization. J Biol Chem 1982; 257(9):5220-5.
- Lázár-Molnár E, Hegyesi H, Tóth S, Falus A. Autocrine and paracrine regulation by cytokines and growth factors in melanoma. Cytokine 2000; 12(6):547-54; PMID:10843728; https://doi.org/10.1006/cyto.1999.0614
- Krasagakis K, Krüger‐Krasagakes S, Fimmel S, Eberle J, Thölke D, Von Der Ohe M, Mansmann U, Orfanos CE. Desensitization of melanoma cells to autocrine TGF‐β isoforms. J Cell Physiol 1999; 178(2):179-87; PMID:10048582; https://doi.org/10.1002/(SICI)1097-4652(199902)178:2%3c179::AID-JCP7%3e3.0.CO;2-5
- Reed JA, McNutt NS, Prieto VG, Albino AP. Expression of transforming growth factor-beta 2 in malignant melanoma correlates with the depth of tumor invasion. Implications for tumor progression. Am J Pathol 1994; 145(1):97; PMID:8030760
- Van Belle P, Rodeck U, Nuamah I, Halpern AC, Elder DE. Melanoma-associated expression of transforming growth factor-beta isoforms. Am J Pathol 1996; 148(6):1887; PMID:8669474
- Moretti S, Pinzi C, Berti E, Spallanzani A, Chiarugi A, Boddi V, Reali U, Giannotti B. In situ expression of transforming growth factor [beta] is associated with melanoma progression and correlates with Ki67, HLA-DR and [beta] 3 integrin expression. Melanoma Res 1997; 7(4):313-21; PMID:9293481; https://doi.org/10.1097/00008390-199708000-00006
- Hazelbag S, Fleuren GJ, Baelde J, Schuuring E, Kenter GG, Gorter A. Cytokine profile of cervical cancer cells. Gynecologic Oncol 2001; 83(2):235-43; PMID:11606077; https://doi.org/10.1006/gyno.2001.6378
- Ortegel JW, Staren ED, Faber L, Warren WH, Braun DP. Modulation of tumor-infiltrating lymphocyte cytolytic activity against human non-small cell lung cancer. Lung Cancer 2002; 36(1):17-25; PMID:11891029; https://doi.org/10.1016/S0169-5002(01)00472-X
- Gratchev A, Kzhyshkowska J, Utikal J, Goerdt S. Interleukin‐4 and dexamethasone counterregulate extracellular matrix remodelling and phagocytosis in type‐2 macrophages. Scandinavian J Immunol 2005; 61(1):10-7; PMID:15644118; https://doi.org/10.1111/j.0300-9475.2005.01524.x
- Gratchev A, Kzhyshkowska J, Kannookadan S, Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E, Muller-Molinet I, Gooi L. Activation of a TGF-β-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-β receptor II. J Immunol 2008; 180(10):6553-65; PMID:18453574; https://doi.org/10.4049/jimmunol.180.10.6553
- Vodovotz Y, Bogdan C, Paik J, Xie Q, Nathan C. Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med 1993; 178(2):605-13; PMID:7688028; https://doi.org/10.1084/jem.178.2.605
- Berking C, Takemoto R, Schaider H, Showe L, Satyamoorthy K, Robbins P, Herlyn M. Transforming growth factor-β1 increases survival of human melanoma through stroma remodeling. Cancer Res 2001; 61(22):8306-16; PMID:11719464
- Ma D, Niederkorn J. Transforming growth factor-beta down-regulates major histocompatibility complex class I antigen expression and increases the susceptibility of uveal melanoma cells to natural killer cell-mediated cytolysis. Immunology 1995; 86(2):263; PMID:7490128
- de Waal Malefyt R, Haanen J, Spits H, Roncarolo M-G, Te Velde A, Figdor C, Johnson K, Kastelein R, Yssel H, De Vries JE. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 1991; 174(4):915-24; PMID:1655948; https://doi.org/10.1084/jem.174.4.915
- Ding L, Linsley P, Huang L, Germain R, Shevach E. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol 1993; 151(3):1224-34; PMID:7687627
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol 1991; 146(10):3444-51; PMID:1827484
- Chen Q, Daniel V, Maher DW, Hersey P. Production of IL‐10 by melanoma cells: Examination of its role in immunosuppression mediated by melanoma. Int J Cancer 1994; 56(5):755-60; PMID:8314354; https://doi.org/10.1002/ijc.2910560524
- Liu C-Y, Xu J-Y, Shi X-Y, Huang W, Ruan T-Y, Xie P, Ding J-L. M2-polarized tumor-associated macrophages promoted epithelial–mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest 2013; 93(7):844-54; PMID:23752129; https://doi.org/10.1038/labinvest.2013.69
- Wimalawansa SJ. Amylin, calcitonin gene-related peptide, calcitonin, and adrenomedullin: a peptide superfamily. Crit Rev™ Neurobiol 1997; 11(2–3):167-239
- Zudaire E, Martınez A, Cuttitta F. Adrenomedullin and cancer. Regulatory Peptides 2003; 112(1):175-83; PMID:12667640; https://doi.org/10.1016/S0167-0115(03)00037-5
- Nikitenko L, Fox S, Kehoe S, Rees M, Bicknell R. Adrenomedullin and tumour angiogenesis. Br J Cancer 2006; 94(1):1-7; PMID:16251875; https://doi.org/10.1038/sj.bjc.6602832
- MartÍnez A, Elsasser TH, Muro-Cacho C, Moody TW, Miller MJ, Macri CJ, Cuttitta F. Expression of adrenomedullin and its receptor in normal and malignant human skin: a potential pluripotent role in the integument. Endocrinology 1997; 138(12):5597-604; https://doi.org/10.1210/endo.138.12.5622
- Chen P, Huang Y, Bong R, Ding Y, Song N, Wang X, Song X, Luo Y. Tumor-associated macrophages promote angiogenesis and melanoma growth via adrenomedullin in a paracrine and autocrine manner. Clin Cancer Res 2011; 17(23):7230-9; PMID:21994414; https://doi.org/10.1158/1078-0432.CCR-11-1354
- den Breems NY, Eftimie R. The re-polarisation of M2 and M1 macrophages and its role on cancer outcomes. J Theoretical Biol 2016; 390:23-39; PMID:26551154; https://doi.org/10.1016/j.jtbi.2015.10.034
- Sadej R, Spychala J, Skladanowski AC. Expression of ecto-5′-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res 2006; 16(3):213-22; PMID:16718268; https://doi.org/10.1097/01.cmr.0000215030.69823.11
- Koszalka P, Golunska M, Stanislawowski M, Urban A, Stasilojc G, Majewski M, Wierzbicki P, Skladanowski AC, Bigda J. CD73 on B16F10 melanoma cells in CD73-deficient mice promotes tumor growth, angiogenesis, neovascularization, macrophage infiltration and metastasis. Int J Biochem Cell Biol 2015; 69:1-10; PMID:26545615; https://doi.org/10.1016/j.biocel.2015.10.003
- Yegutkin GG, Marttila-Ichihara F, Karikoski M, Niemela J, Laurila JP, Elima K, Jalkanen S, Salmi M. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol 2011; 41(5):1231-41; PMID:21469131; https://doi.org/10.1002/eji.201041292
- Eljaszewicz A, Wiese M, Helmin-Basa A, Jankowski M, Gackowska L, Kubiszewska I, Kaszewski W, Michalkiewicz J, Zegarski W. Collaborating with the enemy: function of macrophages in the development of neoplastic disease. Mediators Inflamm 2013; 2013:831387; PMID:23576856; https://doi.org/10.1155/2013/831387
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11(10):889-96; PMID:20856220; https://doi.org/10.1038/ni.1937
- Massi D, Marconi C, Franchi A, Bianchini F, Paglierani M, Ketabchi S, Miracco C, Santucci M, Calorini L. Arginine metabolism in tumor-associated macrophages in cutaneous malignant melanoma: evidence from human and experimental tumors. Hum Pathol 2007; 38(10):1516-25; PMID:17640716; https://doi.org/10.1016/j.humpath.2007.02.018
- Gal A, Tapmeier T, Balathasan L, Muschel R. 304 Myeloid response and macrophage polarization in mouse melanoma lung metastasis. European J Cancer Supplements 2010; 8(5):78-9; https://doi.org/10.1016/S1359-6349(10)71108-3
- Iero M, Valenti R, Huber V, Filipazzi P, Parmiani G, Fais S, Rivoltini L. Tumour-released exosomes and their implications in cancer immunity. Cell Death Different 2008; 15(1):80-8; https://doi.org/10.1038/sj.cdd.4402237
- Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res 2011; 71(11):3792-801; PMID:21478294; https://doi.org/10.1158/0008-5472.CAN-10-4455
- Marton A, Vizler C, Kusz E, Temesfoi V, Szathmary Z, Nagy K, Szegletes Z, Varo G, Siklos L, Katona RL. Melanoma cell-derived exosomes alter macrophage and dendritic cell functions in vitro. Immunol Letters 2012; 148(1):34-8; PMID:22898052; https://doi.org/10.1016/j.imlet.2012.07.006
- Savage ND, de Boer T, Walburg KV, Joosten SA, van Meijgaarden K, Geluk A, Ottenhoff TH. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFβ-1. J Immunol 2008; 181(3):2220-6; PMID:18641362; https://doi.org/10.4049/jimmunol.181.3.2220
- Kimpfler S, Sevko A, Ring S, Falk C, Osen W, Frank K, Kato M, Mahnke K, Schadendorf D, Umansky V. Skin melanoma development in ret transgenic mice despite the depletion of CD25+ Foxp3+ regulatory T cells in lymphoid organs. J Immunol 2009; 183(10):6330-7; PMID:19841169; https://doi.org/10.4049/jimmunol.0900609
- Taniguchi K, Petersson M, Höglund P, Kiessling R, Klein G, Kärre K. Interferon gamma induces lung colonization by intravenously inoculated B16 melanoma cells in parallel with enhanced expression of class I major histocompatibility complex antigens. Proc Natl Acad Sci 1987; 84(10):3405-9; https://doi.org/10.1073/pnas.84.10.3405
- Garbe C, Krasagakis K, Zouboulis CC, Schröder K, Krüger S, Stadler R, Orfanos CE. Antitumor activities of interferon alpha, beta, and gamma and their combinations on human melanoma cells in vitro: changes of proliferation, melanin synthesis, and immunophenotype. J Invest Dermatol 1990; 95:231S-7S; https://doi.org/10.1111/1523-1747.ep12875837
- Porter GA, Abdalla J, Lu M, Smith S, Montgomery D, Grimm E, Ross MI, Mansfield PF, Gershenwald JE, Lee JE. Significance of plasma cytokine levels in melanoma patients with histologically negative sentinel lymph nodes. Ann Surg Oncol 2001; 8(2):116-22; PMID:11258775; https://doi.org/10.1007/s10434-001-0116-3
- Meyskens FL, Kopecky KJ, Taylor CW, Noyes RD, Tuthill RJ, Hersh EM, Feun LG, Doroshow JH, Flaherty LE, Sondak VK. Randomized trial of adjuvant human interferon gamma versus observation in high-risk cutaneous melanoma: a Southwest Oncology Group study. J Natl Cancer Inst 1995; 87(22):1710-3; PMID:7473820; https://doi.org/10.1093/jnci/87.22.1710
- Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, Feigenbaum L, Fuchs E, Lyakh L, Young HA. Interferon-[ggr] links ultraviolet radiation to melanomagenesis in mice. Nature 2011; 469(7331):548-53; PMID:21248750; https://doi.org/10.1038/nature09666
- Tanese K, Hashimoto Y, Berkova Z, Wang Y, Samaniego F, Lee JE, Ekmekcioglu S, Grimm EA. Cell Surface CD74–MIF Interactions Drive Melanoma survival in response to Interferon-γ. J Invest Dermatol 2015; 135(11):2901.
- Okamura A, Rakugi H, Ohishi M, Yanagitani Y, Takiuchi S, Moriguchi K, Fennessy PA, Higaki J, Ogihara T. Upregulation of renin‐angiotensin system during differentiation of monocytes to macrophages. J Hypertens 1999; 17(4):537-45; PMID:10404956; https://doi.org/10.1097/00004872-199917040-00012
- Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, Kuswanto W, Rauch PJ, Chudnovskiy A, Iwamoto Y. Angiotensin II drives the production of tumor-promoting macrophages. Immunity 2013; 38(2):296-308; PMID:23333075; https://doi.org/10.1016/j.immuni.2012.10.015
- Shen XZ, Li P, Weiss D, Fuchs S, Xiao HD, Adams JA, Williams IR, Capecchi MR, Taylor WR, Bernstein KE. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am J Pathol 2007; 170(6):2122-34; PMID:17525278; https://doi.org/10.2353/ajpath.2007.061205
- Egami K, Murohara T, Shimada T, Sasaki K-i, Shintani S, Sugaya T, Ishii M, Akagi T, Ikeda H, Matsuishi T. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest 2003; 112(1):67-75; PMID:12840060; https://doi.org/10.1172/JCI16645
- Bianchini F, Massi D, Marconi C, Franchi A, Baroni G, Santucci M, Mannini A, Mugnai G, Calorini L. Expression of cyclo-oxygenase-2 in macrophages associated with cutaneous melanoma at different stages of progression. Prostaglandins Other Lipid Mediat 2007; 83(4):320-8; https://doi.org/10.1016/j.prostaglandins.2007.03.003
- Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol Rev 2011; 243(1):136-51; PMID:21884173; https://doi.org/10.1111/j.1600-065X.2011.01046.x
- Elaraj D, Weinreich D, Varghese S, Puhlmann M, Hewitt S, Carroll N, Feldman E, Turner E, Alexander H. The role of interleukin 1 in growth and metastasis of human cancer xenografts. Clin Cancer Res 2006; 12(4):1088-96; PMID:16489061; https://doi.org/10.1158/1078-0432.CCR-05-1603
- Gehrke S, Otsuka A, Huber R, Meier B, Kistowska M, Fenini G, Cheng P, Dummer R, Kerl K, Contassot E. Metastatic melanoma cell lines do not secrete IL-1β but promote IL-1β production from macrophages. J Dermatol Sci 2014; 74(2):167-9; PMID:24581590; https://doi.org/10.1016/j.jdermsci.2014.01.006
- Björkdahl O, Wingren A, Hedhund G, Ohlsson L, Dohlsten M. Gene transfer of a hybrid interleukin-1 beta gene to B16 mouse melanoma recruits leucocyte subsets and reduces tumour growth in vivo. Cancer Immunol Immunother 1997; 44(5):273-81; PMID:9247562; https://doi.org/10.1007/s002620050383
- Pawelek JM. Fusion of bone marrow-derived cells with cancer cells: metastasis as a secondary disease in cancer. Chin J Cancer 2014; 33(3):133-9; PMID:24589183; https://doi.org/10.5732/cjc.013.10243
- Rachkovsky M, Sodi S, Chakraborty A, Avissar Y, Bolognia J, McNiff JM, Platt J, Bermudes D, Pawelek J. Melanoma × macrophage hybrids with enhanced metastatic potential. Clin Exp Metastasis 1998; 16(4):299-312; https://doi.org/10.1023/A:1006557228604
- Rachkovsky M, Pawelek J. Acquired Melanocyte Stimulating Hormone-inducible Chemotaxis following Macrophage Fusion with Cloudman S91 Melanoma cells. Cell Growth Differ 1999; 10(7):517-24.
- Chakraborty AK, Pawelek J. Beta1,6-branched oligosaccharides regulate melanin content and motility in macrophage-melanoma fusion hybrids. Melanoma Res 2007; 17(1):9-16; PMID:17235237; https://doi.org/10.1097/CMR.0b013e3280114f34
- Handerson T, Camp R, Harigopal M, Rimm D, Pawelek J. Beta1,6-branched oligosaccharides are increased in lymph node metastases and predict poor outcome in breast carcinoma. Clin Cancer Res 2005; 11(8):2969-73; PMID:15837749; https://doi.org/10.1158/1078-0432.CCR-04-2211
