ABSTRACT
Tumor cells escape from immune recognition by several mechanisms such as down-regulating of MHC class I molecules, losing of tumor antigens, etc. The purpose of cancer immunotherapy is to robust or reconstruct the capacity of the immune system to recognize and kill tumor cells by overwhelming the mechanisms by which tumors escape the immune response. One of the novel immunotherapeutic strategies were used to potentiate NK- and T cell functions is chimeric antigen receptor (CAR). CARs are composed of an antigen-binding domain of a molecule such as an antibody (that binds to a tumor associated antigens expressed on the surface of tumor cells) and an intracellular T cell activation domain. The CARs provide the recognition of target antigen in a MHC-independent manner. CAR-armed T cells may be unable to kill their targets in the absence of co-stimulators like NK cells. On the other hand, CAR-armed NK cells may also be unable to destroy their targets without receiving help signals from Th cells. Thus, if CAR-armed NK cells use together with CAR-armed T cells, NK cells will be aggregated to the tumor site. Thus, not only CAR T cells will obtain the necessary cytokines/costimulators from NK cells, but also other tumor specific T cells will be primed by recognition of tumor specific antigen (TSA) associated with MHC class I. These new specific primed T cells probably combat against tumor cells which have lost their TAAs that CAR-T cells are redirected to them.
Dear editor,
The immune system plays an essential role in defense against tumor cells so that the patients with compromised or suppressed immune function have an extremely increased incidence of cancer.Citation1 According to the immune surveillance hypothesis, tumor associated antigens (TAA) are considered as “non-self” by the immune system, and an important function of the immune system is to examine carefully the body for the development of malignancy and to eliminate tumor cells as they arise.Citation2 The elements of the both innate and adaptive immunity, such as natural killer (NK) cells, NKT cells, macrophages, neutrophils, eosinophils, specific cytotoxic T lymphocytes (CTLs), antibodies and some cytokines exhibit antitumor activity.Citation1-4 However, the tumor cells escape from immune recognition/killing by several mechanisms such as down-regulating of MHC class I molecules, losing of tumor antigens, defective death receptor signaling, lacking of costimulation, producing of immunosuppressive cytokines and inducing of suppressive cells.Citation5-6
Surgery, chemotherapy and radiotherapy have been used for decades as primary strategies for treatment of cancer patients, however, the development of resistance to medication or radiation led to a subsequent incidence of tumor relapse.Citation7 After many years of disappointing efforts, the application of cancer immunotherapy has recently received a significant consideration.Citation8 The purpose of cancer immunotherapy is to robust or reconstruct the capacity of the immune system to recognize and kill tumor cells by overwhelming the mechanisms by which tumors avoid and inhibit the immune response.Citation9 Some immunotherapeutic strategies have received more attention for treatment of cancers such as cancer vaccines, adoptive transfer of ex vivo activated T and natural killer (NK) cells, genetic engineered NK- and T cells, oncolytic viruses and administration of antibodies or recombinant proteins.Citation8
The natural killer (NK) cells are characterized by the lack of TCR molecules and by the expression of CD16 and CD56 markers. Approximately 90% of circulating NK cells are CD56dim, described by their strong capacity to mediate cytotoxicity against target cells.Citation10 T cells express a T cell receptor (TCR) which binds a peptide originated from pathogens or malignant in association with the major histocompatibility complex (MHC). In this way, T cells are activated, leading to killing of target cells or the releasing of pro-inflammatory cytokines that recruit other immune effector cells.Citation11
The novel immunotherapeutic strategies were used to potentiate NK- and T cell functions and enhance their antitumor activity, including chimeric antigen receptor (CAR)-genetic engineered NK- and T cells.Citation10-11 CARs are composed of an antigen-binding domain of a molecule such as an antibody (that binds to a TAA expressed on the surface of tumor cells) and an intracellular T cell activation domain. The CARs provide the recognition of target antigen in a MHC-independent manner and can be used to improve the outcome of immunotherapy.Citation11
Both T and NK cells expressing CARs have been separately used for cancer immunotherapy. Due to the immune evasion pathways of tumor cells using of the each T cell or NK cell expressing CARs alone, may don't lead to the complete tumor regression. Therefore, CAR-armed T cells may be unable to kill and eliminate their targets in the absence of co-stimulators like NK cells. On the other hand, CAR-armed NK cells may also be unable to destroy their targets without receiving help signals from Th cells. The utilization of the CAR-T or CAR-NK cells in some clinical trials resulted in the partial and transient tumor regression, probably due to the heterogeneity of tumors at the immunological level.Citation12 The massive and repetitive stimulation of T cells may also render them into apoptosis (through Fas-FasL interaction) or make them exhausted or senescent. Moreover, the stimulation of T lymphocytes by tumor specific antigens, without appropriate co-stimulation signals may render them into an anergic situation.Citation13
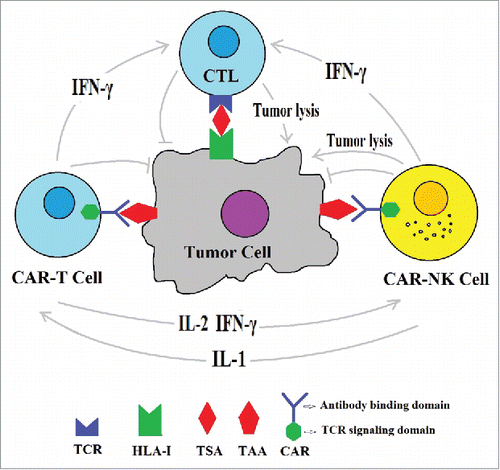
Therefore the synergistic combination strategies by using CAR-armed immune cells should consider in cancer immunotherapy. In addition to CAR-T cells, another important strategy for treatment of cancer can be the concomitant using of the genetic modification of NK cells with CARs that directly recognize tumor associated antigens. The human NK cells not only have direct cytotoxic activity against tumor cells, but also act as antigen presenting cells (APCs) and can present tumor antigen to T cells and activate specific T cells.Citation14 Several different cytokines/co-stimulatory molecules also are expressed by NK cells, which are necessary for long-term T cell activation and effector functions. Thus, if CAR-armed NK cells use together with CAR-armed T cells, NK cells will be aggregated to the tumor site. Thus, not only CAR T cells will obtain the necessary cytokines/costimulators from NK cells, but also other tumor specific T cells will be primed by recognition of tumor specific antigen (TSA) associated with MHC class I (). These new specific primed T cells probably combat against tumor cells which have lost their TAAs that CAR-T cells are redirected to them. In contrast to CAR T cells, CAR-NK cells permit us to use allogeneic NK cell sources, which are less likely result in or even may inhibit graft-versus-host disease, while also potentiating an increase in cytotoxicity due to mismatched killer immunoglobulin-like receptors.Citation15-16
Figure 1. Combinational CAR-T plus CAR-NK cell therapy hypothesis: The CAR-T, CAR-NK and CTL are simultaneously induced to exert synergistic anti-tumor activity.
The Food and Drug Administration of US and Institutional Review Board have confirmed the use of NK-92 cells, which act as potential anti-tumor agents and ideal therapeutic research models. NK-92 cells do not express the main inhibitory receptors. These cells are highly cytotoxic against various types of tumor cells without assaulting non-malignant cells.Citation17-18 Thus, NK92 could be an excellent candidate to be equipped by CAR for therapeutic purposes and it can play as an APC as well.
In conclusion, although combined immunotherapy by using both CAR-NK plus CAR T-cell may be a powerful treatment strategy for malignant diseases, however, some important additional considerations such as defining the target antigen, improvement of CAR signaling and enhancing of the safety the CAR expressing NK-T (the 2 engines!) cell therapy should be consider in future investigations.
Disclosure of potential conflicts of interest
Authors declare no conflict of interest.
References
- Casey SC, Li Y, Felsher DW. An essential role for the immune system in the mechanism of tumor regression following targeted oncogene inactivation. Immunol Res 2014; 58(2-3):282-91; PMID:24791942; https://doi.org/10.1007/s12026-014-8503-6
- Monzavi-Karbassi B, Pashov A, Kieber-Emmons T. Tumor-Associated Glycans and Immune Surveillance. Vaccines 2013; 1(2):174-203; PMID:26343966; https://doi.org/10.3390/vaccines1020174
- Sheikhi A, Saadati K, Jafarzadeh A, Karimi H, Mousavinasab N. Augmenting the expression of NKp44 molecule and the natural killer activity in peripheral blood mononuclear cells from patients with malignant colorectal carcinoma. Drug Res (Stuttg) 2014; 64(6):281-6; PMID:24154937
- Sheikhi AK, Tayade C, Paffaro VA, Croy BA. Are nutural killer cells distributed in relationship to nerve fibers in the pregnant mouse uterus? Pak J Biol Sci. 2007; 10(17):2885-9; PMID:19090193; https://doi.org/10.3923/pjbs.2007.2885.2889
- Sheikhi A, Saadati K, Salmani R, Yahaghi N, Sheikhi A, Siemens DR. In vitro modulation of natural killer activity of human peripheral blood mononuclear cells against prostate tumor cell line. Immunopharmacol Immunotoxicol 2011; 33(4):700-8; https://doi.org/10.3109/08923973.2011.561437
- Spranger S. Mechanisms of tumor escape in the context of the T-cell-inflamed and the non-T-cell-inflamed tumor microenvironment. Int Immunol 2016; 28(8):383-91; PMID:26989092; https://doi.org/10.1093/intimm/dxw014
- Dziadziuszko R, Le AT, Wrona A, Jassem J, Camidge DR, Varella-Garcia M, Aisner DL, Doebele RC. An activating KIT mutation induces Crizotinib Resistance in ROS1-Positive Lung Cancer. J Thorac Oncol 2016; 11(8):1273-81; PMID:27068398; https://doi.org/10.1016/j.jtho.2016.04.001
- Farkona S, Diamandis EP, Blasutig IM. Cancer immunotherapy: the beginning of the end of cancer?. BMC Med 2016; 14:73; PMID:27151159; https://doi.org/10.1186/s12916-016-0623-5
- Disis ML. Mechanism of action of immunotherapy. Seminars Oncol 2014; 41(Suppl 5):S3-13; PMID:25438997; https://doi.org/10.1053/j.seminoncol.2014.09.004
- Rezvani K, Rouce RH. The application of natural killer cell immunotherapy for the treatment of cancer. Frontiers Immunol 2015; 6:578; PMID:26635792; https://doi.org/10.3389/fimmu.2015.00578
- Spear TT, Nagato K, Nishimura MI. Strategies to genetically engineer T cells for cancer immunotherapy. Cancer Immunol Immunotherapy 2016; 65(6):631-49; PMID:27138532; https://doi.org/10.1007/s00262-016-1842-5
- Xu XJ, Zhao HZ, Tang YM. Efficacy and safety of adoptive immunotherapy using anti-CD19 chimeric antigen receptor transduced T-cells: a systematic review of phase I clinical trials. Leuk Lymphoma 2013; 54(2):255-60; PMID:22897728; https://doi.org/10.3109/10428194.2012.715350
- Saff RR, Spanjaard ES, Hohlbaum AM, Marshak-Rothstein A. Activation-induced cell death limits effector function of CD4 tumor-specific T cells. J Immunol 2004; 172:6598-606; PMID:15153474; https://doi.org/10.4049/jimmunol.172.11.6598
- Hanna J, Gonen-Gross T, Fitchett J, Rowe T, Daniels M, Arnon TI, et. al. Novel APC-like properties of human NK cells directly regulate T cell activation. J Clin Invest 2004; 114:1612-23; PMID:15578093; https://doi.org/10.1172/JCI22787
- Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood 2010; 115:4293-301; PMID:20233969; https://doi.org/10.1182/blood-2009-05-222190
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002; 295:2097-100; PMID:11896281; https://doi.org/10.1126/science.1068440
- Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) withphenotypical and functional characteristics of activated natural killer cells. Leukemia 1994; 8:652-8; PMID:8152260
- Suck G. Novel approaches using natural killer cells in cancer therapy. Semin Cancer Biol 2006; 16:412-8; PMID:16914327; https://doi.org/10.1016/j.semcancer.2006.07.006
