ABSTRACT
Background
Clinical trials of vaccines for children to prevent acute otitis media (AOM) infections caused by the bacteria Streptococcus pneumonia (Spn) are in Phase I. The objective of this study was to use serum antibody measurements to pneumococcal purified protein candidate antigens that occurred after natural “immunization” to predict a correlate of protection response needed following an injectable vaccine against AOM in children.
Methods
590 nasal and serum samples were collected from 129 healthy children at 6, 9, 12, 15, 18, 24 and 30–36 months of age and when the child developed AOM. Middle ear fluid to detect Spn was collected at every episode of AOM. Quantitative ELISA was used to determine serum IgG against 7 Spn vaccine antigens: PspA clade 3, PspA clade 5, PhtD, PhtE, LytB, PcpA and Ply. A correlate of protection (COP) was estimated by regressing AOM events against age adjusted antibody levels induced by nasopharyngeal colonization and AOM infections, using logistic regression and generalized estimating equation methods.
Results
A significant COP was found for Spn PhtD (p = 0.0015), PhtE (p = 0.00034), LytB (p = 0.004), PcpA (p = 0.002), and Ply (p = 0.007) between higher antibody levels and reduced frequency of AOM. We estimated that a 2-fold higher antibody level in a child than the mean antibody level induced by NP colonization (after adjusting for subject age) to PhtD, LytB, PcpA, PhtE or Ply reduced the risk of AOM by 14–21%, a 4-fold higher level reduced it by 25–38% and a 10-fold higher level reduced it by 39–54%.
Conclusion
We developed a model to predict the necessary level of serum antibody and fold higher above a threshold to PhtD, PhtE, LytB, PcpA and Ply that would correlate with a reduced likelihood of AOM in children age 6–24 months old if enrolled in a Phase III clinical efficacy trial.
Introduction
Streptococcus pneumoniae (Spn) is a common bacterial pathogen causing pneumonia, acute exacerbations of bronchitis, acute sinusitis, and acute otitis media (AOM). Citation1 The first step of respiratory bacterial infection is nasopharyngeal (NP) colonization, Citation2,Citation3 and NP colonization precedes upper and lower respiratory infections. Citation4,Citation5
Our laboratory has been studying the natural serum antibody response to vaccine candidate antigens of Spn, Nontypeable Haemophilus influenzae and Moraxella catarrhalis in infants and young children during health and AOM visits. Citation6-Citation12 Of these 3 bacterial pathogens, the only licensed vaccine is to Spn and it is limited to the serotype polysaccharides contained in the vaccine. Unfortunately, there are >95 different Spn serotypes and only the pneumococcal conjugate vaccines (PCVs) are given to infants and young children that target at most 13 Spn serotypes. Therefore, we and others, have been evaluating next-generation pneumococcal protein-based vaccine candidates that will target Spn independent of its capsular polysaccharide. Citation13-Citation16 Our group has studied the antibody levels in children age 6 to 30 months of age following natural exposure to Spn after NP colonization and AOM to 5 pneumococcal proteins: histidine triad protein D (PhtD), histidine triad protein E (PhtE) pneumococcal choline binding protein A (PcpA), detoxified pneumolysin D1 (PlyD1) and murein hydrolase (LytB). Citation9 We have also shown that vaccination with monovalent and trivalent vaccines containing PhtD, PcpA or PlyD1 confer protection against pneumonia and sepsis in a mouse model. Citation17-Citation20 Our work has also demonstrated a correlate of natural exposure-induced higher antibody levels to PhtD, PcpA and PlyD1 with protection against Spn-caused AOM in young children. Citation21,Citation22 Sufficient promise for the potential of a trivalent PhtD, PcpA, and PlyD1 vaccine has emerged such that one is in human clinical trials for the prevention of pneumonia. Citation23
The objective of this study was to identify serum antibody levels to pneumococcal purified protein candidate antigens of Spn that correlate with protection against AOM in children. Serum IgG levels against 7 Spn antigens (pneumococcal surface protein A (PspA) clades 3 and 5, PhtD, PhtE, PcpA, PlyD1 and LytB) were compared among cohorts of children during and following NP colonization by Spn.
Results
Serum samples (n = 590) from 129 children between ages of 6 and 36 months were analyzed. The characteristics of the children are shown in .
Table 1. Subject demographics.
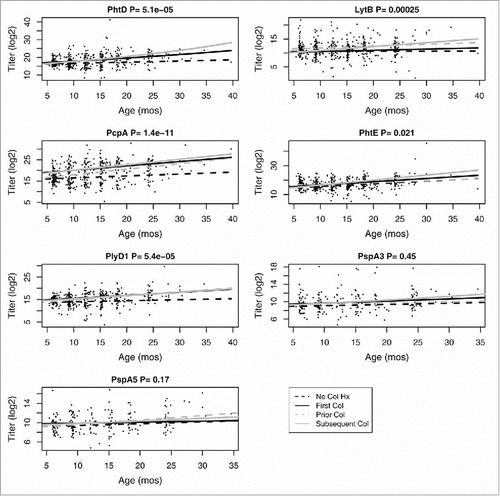
Since NP colonization is a pathogenesis prerequisite to development of Spn-caused AOM, we analyzed natural serum antibody responses associated with NP colonization in the study cohort. shows fitted values for antibody response dependent on colonization history. This dependence was statistically significant for PhtD, PhtE, LytB, PcpA and PlyD1 but not PspA clade 3 or 5 (see Supplemental Information on Statistical Methodology), visually evident by examining the distance between the solid and dashed lines in each component of . That result indicates that natural exposure to PhtD, PhtE, LytB, PcpA and PlyD1 but not PspA clade 3 or 5 as expressed by Spn during NP colonization significantly impacts the production of specific antibodies against them as children age. Antibodies to PhtD, PcpA, and PlyD1 are especially low during uncolonized visits with no history of prior colonization (represented by the dashed black line in ). We conclude that to measure association of antibody levels with AOM risk, it is necessary to determine the NP colonization history for 3 reasons: First, natural antibody level is dependent on colonization; second, AOM does not occur without there first having been NP colonization; and third, in the absence of any NP colonization history, low antibody levels may severely compromise the statistical power in any disease versus control comparison. We therefore evaluate a correlate of protection (COP) by focusing our study on current NP colonization (which includes all AOM events).
Figure 1. Plots of Spn antigen-specific antibody titers vs. age. The 4 colonization states are represented individually: dashed black line represents visits with no history of prior Spn colonization; solid black line represents visits where subjects experienced Spn colonization for the first time; dashed gray line represents visits where subjects had a history of prior colonization but were not currently colonized by Spn; solid gray line represents visits where subjects had a history of prior colonization and were currently colonized by Spn. P-values are given for the effect of colonization history on antigen-specific antibody titers.
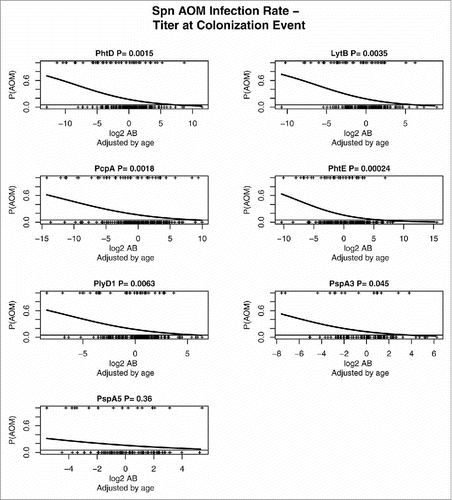
17% of children in the study cohort experienced AOM caused by Spn. shows AOM risk as a function of antibody levels for each protein, in units of log-transformed antibody titers adjusted by age. A significant COP was found for Spn PhtD, PhtE, LytB, PcpA, and Ply, where higher antibody levels are associated with reduced frequency of AOM, visually evident by examining the steepness of the slope of the line depicting probability of AOM.
Figure 2. Estimated Spn AOM infection rates as a function of antibody concentration. The probability P-values, P(AOM), are given for dependence of AOM risk on specific antibody concentrations, log2 AB, adjusted by age of child.
shows the effects of fold increases in each specific antibody titer on the odds ratio for predicted AOM risk. For example, a 2-fold increase in PlyD1 antibody is associated with an odds ratio of 0.79, such that the risk of AOM decreases by the reciprocal value of 21%. A further 2-fold increase (4-fold over the starting titer) reduces the AOM risk by another factor of 0.79, resulting in an odds ratio of 0.62 or a 38% decrease relative to the starting concentration. Since the antibody levels were naturally induced by Spn NP colonization exposure, the specific contributions of each antibody to protection could not be determined. However, antibody levels to PhtD, PcpA and Ply change in synchrony over time whereas those to PhtE and LytB do not. Citation15
Table 2. Odds ratios for AOM risk per fold increase in antibody titer.
allows the derivation of specific values of antibody fold increases above the mean that correlate with increased protection from AOM. For example, a 2-fold higher antibody level to PhtD compared with the mean anti-PhtD antibody level (after adjusting for subject age) correlates with a reduction in AOM odds risk of 0.83 (reciprocal would be 17% for reduced AOM risk). Likewise, a 10-fold higher antibody level to PhtD would result in a reduction in AOM odds risk of 0.53 (47% further reduced risk). For the other 2 components in the trivalent vaccine in development for use in human infants (PcpA and PlyD1) similar reductions in risk of AOM can be calculated.
Discussion
We, Citation8,Citation9 and others Citation24-Citation26 previously found that NP colonization with Spn elicits serum protein-specific IgG responses. It is important to note that the timing of sample collection relative to NP colonization will affect the antibody response to the pathogen such that one would not expect to see an immediate rise in serum antibody level at the moment of colonization. We have also shown that natural serum and mucosal antibodies to the trivalent vaccine candidate proteins PhtD, PcpA and Ply correlate with protection against Spn AOM infections. Citation20,Citation21 This is important preclinical information for vaccine discovery as many vaccine development programs use data from animal model protection studies as to whether to initiate phase I human trials focusing on safety and immunogenicity. If results are favorable, future trials involves additional time and expense. Here for the first time we used statistical modeling of specific natural antibody responses to 7 Spn protein vaccine candidates for prevention of AOM to predict a correlate of protection against AOM that might be used before commencing phase II or III clinical trials. This methodology may be generalizable to other vaccines in development. For the Spn proteins we studied it should now be possible to measure serum antibodies from vaccinated subjects and controls (preferably using an antibody weight-based quantitative assay) and compare the mean antibody levels. From those results, analysis would show how many standard deviations above the mean values of controls and how many fold increases vaccination elevated the antibody levels compared with the controls. Our model would predict that elevations of 2 standard deviations and 2-fold increases would correlate with protection from an infection end point – AOM. The model further quantitates and predicts the anticipated increased likelihood of protection from infection occurring with 10-, 15- or 20- higher vaccine-induced antibody levels in the study population, as might occur with vaccination using purified recombinant proteins with suitable adjuvant.
Because natural immunization elicits antibody to multiple antigens to the pathogen, the modeling cannot be used to predict a specific amount of an antibody to a single antigen that would correlate with protection. Indeed, we have previously shown that serum antibody to PhtD, PcpA and PlyD1 rise in synchrony following NP colonization in children age 6 to 24 months old. Citation15 The predicted correlates of protection therefore must be understood to have interrelationships among the studied antibodies and likely to involve immune responses to additional antigens that correlate with those that were measured. However, this caveat does not compromise the usefulness of the model. For instance, in a phase I trial, if antibody responses to the vaccine components show increases above the control cohort but are lower than the model predicts to provide protection, this would be very useful information in decision-making about proceeding with further investment of time and resources. In contrast, antibody responses after a phase I trial that show increases above control 2 or more standard deviations and 2 or more fold higher than controls would be encouraging. Higher increases measured as standard deviations above the mean and fold rises would be even more encouraging.
This study and model has limitations. Colonization was defined as detection of Spn in the NP at the time when samples were collected. Missed colonization events clearly occurred before and between the 3 month sampling times based on the observation that antibody levels were measurable at the first sampling in the study population at age 6 months and antibody titers rose, albeit much less, even in children who did not have consistently detected NP colonization. We have observed this previously. Citation8,Citation9 The model was developed using samples from children living in a higher income lifestyle. NP colonization density of Spn, upper respiratory viral infections that influence immune responses, environmental factors that influence immune responses such as passive smoke exposure, health status including nutrition, etc., would likely differ in low socioeconomic populations. Thus, testing the model using samples from children in developing countries would be useful. The model does not provide a precise COP antibody level in IU/mL or micrograms/mL nor does it take into consideration differences in antibody avidity (and corresponding functionality) for antibody raised by natural exposure to Spn vs. antibody raised by vaccination with purified recombinant proteins with adjuvant.
In conclusion, NP colonization of Spn produces a serum antibody response to Spn vaccine candidate antigens PhtD, LytB, PcpA, PhtE and Ply, and the antibody levels taken following detected NP colonization by Spn can be used in a model to predict a correlate of protection against AOM in children. Our human preclinical natural antibody model may also support vaccine development for other pathogen targets.
Materials and methods
Subjects and study design
This study was part of a 10-year prospective, longitudinal evaluation of human child immunity to Spn supported by the National Institute of Deafness and Communication Disorders as described previously. Citation8,Citation9,Citation27-Citation30 Data here are from 2006 to 2016, involving healthy children without previous episodes of pneumonia, sinusitis, or AOM. The children were enrolled at age 6 months from a middle class, suburban socio-demographic pediatric practice in Rochester, New York (Legacy Pediatrics). NP swabs or washes, hereafter referred to as NP samples, and serum samples were collected from healthy children at 6, 9, 12, 15, 18, 24 and 30–36 months of age. Tympanocentesis was performed during an AOM event and middle ear fluid collected. The microbiology of NP samples and middle ear fluid was determined by standard culture as described previously. Citation27,Citation29 Serum samples were used for determining antibody responses by quantitative ELISA. All of the children received standard vaccinations including PCV7 (Prevnar, Wyeth Pharmaceuticals, Collegeville, PA) or PCV13 (Prevnar 13, after 2010 when it became available) as appropriate for age. The study was approved by the Institutional Review Board (IRB) of University of Rochester and Rochester General Hospital. Written informed consents were obtained from parents or guardians of all child subjects before enrollment in the study.
Definitions
We define colonization as a non-infection, asymptomatic condition and AOM as a true infection state. The data were grouped into various colonization and infection states based on the time of the child visit as follows: ‘No event’, no identified colonization or AOM with Spn; ‘Prior colonization’, Spn colonization detected previous to current clinical visit; current colonization', Spn colonization detected at current clinical visit; ‘Infection’, AOM caused by Spn.
Quantitative ELISA for antigen-specific antibody
Serum IgG levels against Spn vaccine candidate antigens PspA 3, PspA5, PhtD, PhtE, PcpA, PlyD1 and LytB, were measured using quantitative ELISA in a GLP laboratory of Rochester General Hospital Research Institute. The Spn antigens were recombinant proteins expressed in Escherichia coli (provided by Sanofi Pasteur). An adult serum provided by Sanofi Pasteur, which had high end point ELISA antibody titers of IgG against all 7 Spn antigens was used as a reference serum for Spn antigen-specific ELISA.
The antigen-specific IgG against each individual antigens in the reference serum were quantified using Human IgG ELISA Quantitation Sets (Bethyl Laboratories, Montgomery, TX) according to manufacturer's protocol with some modification. The wells of a 96-well microtiter plate for generating a standard curve were coated with affinity purified human IgG capture antibodies while the wells for measuring specific antibodies were coated with 100 – 500 ng of the corresponding individual antigens in 100 μl of coating buffer (carbonate-bicarbonate, pH 9.6). Citation31 Antigen specific antibody was calculated using the standard curve generated with SoftMax Pro version 5.2 (Molecular Devices Corp., Sunnyvale, CA) using a commercial sera pool containing known amounts of total IgG and total IgA (Bethyl RS10–101).
Serum IgG against the antigens was measured by quantitative ELISA on 384-well plates (Greiner Bio-one) with 20 μl of coating and reaction volume. Plates were coated overnight at 4°C with 100–200 ng of individual antigen in 100 μl /well of coating buffer (carbonate-bicarbonate, pH 9.6). The plates were blocked at 37°C for 1h with 200 μl of PBS containing 4% skim milk and 0.05% Tween 20 (PBST). Then 100 μl /well of 2-fold serially diluted test samples, or reference serum in PBST starting at 1:200 were incubated on the plates at room temperature for 1 hr. Affinity-purified goat anti-human IgG antibody conjugated to horseradish-peroxidase (Bethyl Laboratories, Inc., Montgomery, TX) was diluted 1:5000 in PBST, pipetted at 100 μl /well, and incubated for 1 hr. at room temperature. The plates were developed with 100 μl of TMB Microwell Peroxidase Substrate System (KPL, Gaithersburg, MD) at room temperature for 15 min and then were read with a SpectroMax 340PC at 450-nm filter after color reaction was stopped by stop buffer (1.0 molar phosphoric acid). The specific antibodies were quantified using a 4-parameter logistic-log standard curve generated from reference serum with SoftMax Pro version 5.2 (Molecular Devices Corp., Sunnyvale, CA). The lower limits of detection of each assay were calculated from the lowest concentration in the standard curve whose OD was at least 2 standard deviations above the mean OD of blank controls.
Statistical analysis & model
Generalized estimating equations (GEE) may be used to build linear models with correlated data, and are suited for the analysis of longitudinal data when population level mean responses suffice to resolve hypotheses. Correlation among responses induced by the repeated measure design is modeled by constructing a suitable covariance matrix, permitting accurate calculation of significance levels. Citation13 The R function geeglm from package geepack was used to fit the models described here. Citation14 Indicator function for colonization states are included in the model, permitting inference of a time varying colonization effect on AB level. See ‘Supplemental Information on Statistical Methodology’ for further details.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Supplemental_Material.docx
Download MS Word (26.5 KB)Acknowledgments
The authors thank Dr. Ravinder Kaur, Matt Morris and Robert Zagursky for assistance with manuscript review and revisions and Dr. Janet Casey and other staff of Legacy Pediatrics for sample collection. We also thank Sanofi Pasteur for Spn antigens.
Funding
Supported by NIH NIDCD R01 08671 and Sanofi Pasteur.
References
- Cappelletty D. Microbiology of bacterial respiratory infections. Pediatr Infect Dis J 1998; 17:S55-61; PMID:9727651; https://doi.org/10.1097/00006454-199808001-00002
- Margolis E , Yates A , Levin BR . The ecology of nasal colonization of Streptococcus pneumoniae, Haemophilus influenzae and Staphylococcus aureus: the role of competition and interactions with host's immune response. BMC Microbiology 2010; 10:59; PMID:20178591; https://doi.org/10.1186/1471-2180-10-59
- Bogaert D , De Groot R , Hermans PW . Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis 2004; 4:144-54; PMID:14998500; https://doi.org/10.1016/S1473-3099(04)00938-7
- Garcia-Rodriguez JA , Fresnadillo Martinez MJ . Dynamics of nasopharyngeal colonization by potential respiratory pathogens. J Antimicrob Chemother 2002; 50 Suppl S2:59-73; PMID:12556435; https://doi.org/10.1093/jac/dkf506
- Murphy TF , Bakaletz LO , Smeesters PR . Microbial interactions in the respiratory tract. Pediatr Infect Dis J 2009; 28:S121-6; PMID:19918134; https://doi.org/10.1097/INF.0b013e3181b6d7ec
- Kaur R , Casey JR , Pichichero ME . Serum antibody response to five Streptococcus pneumoniae proteins during acute otitis media in otitis-prone and non-otitis-prone children. Pediatr Infect Dis J 2011; 30:645-50; PMID:21487325; https://doi.org/10.1097/INF.0b013e31821c2d8b
- Kaur R , Casey JR , Pichichero ME . Serum antibody response to three non-typeable Haemophilus influenzae outer membrane proteins during acute otitis media and nasopharyngeal colonization in otitis prone and non-otitis prone children. Vaccine 2011; 29:1023-8; PMID:21129398; https://doi.org/10.1016/j.vaccine.2010.11.055
- Pichichero ME , Kaur R , Casey JR , Sabirov A , Khan MN , Almudevar A . Antibody response to Haemophilus influenzae outer membrane protein D, P6, and OMP26 after nasopharyngeal colonization and acute otitis media in children. Vaccine 2010; 28:7184-92; PMID:20800701; https://doi.org/10.1016/j.vaccine.2010.08.063
- Pichichero ME , Kaur R , Casey JR , Xu Q , Almudevar A , Ochs M . Antibody response to Streptococcus pneumoniae proteins PhtD, LytB, PcpA, PhtE and Ply after nasopharyngeal colonization and acute otitis media in children. Hum Vaccin Immunother 2012; 8:799-805; PMID:22495112; https://doi.org/10.4161/hv.19820
- Ren D , Almudevar AL , Murphy TF , Lafontaine ER , Campagnari AA , Luke-Marshall N , Casey JR , Pichichero ME . Serum antibody response to Moraxella catarrhalis proteins OMP CD, OppA, Msp22, Hag, and PilA2 after nasopharyngeal colonization and acute otitis media in children. Vaccine 2015; 33:5809-14; PMID:26392013; https://doi.org/10.1016/j.vaccine.2015.09.023
- Ren D , Almudevar AL , Pichichero ME . Synchrony in serum antibody response to conserved proteins of Streptococcus pneumoniae in young children. Hum Vaccin Immunother 2015; 11:489-97; PMID:25692218; https://doi.org/10.4161/21645515.2014.990861
- Khan MN , Kaur R , Pichichero ME . Bactericidal antibody response against P6, protein D, and OMP26 of nontypeable Haemophilus influenzae after acute otitis media in otitis-prone children. FEMS ImmunolMedMicrobiol 2012; 65:439-47
- Alderson MR . Status of research and development of pediatric vaccines for Streptococcus pneumoniae. Vaccine 2016; 34:2959-61; PMID:27083428; https://doi.org/10.1016/j.vaccine.2016.03.107
- Moffitt KL , Malley R . Next generation pneumococcal vaccines. Curr Opin Immunol 2011; 23:407-13; PMID:21514128; https://doi.org/10.1016/j.coi.2011.04.002
- Pichichero ME , Khan MN , Xu Q . Next generation protein based Streptococcus pneumoniae vaccines. Hum Vaccin Immunother 2016; 12:194-205; PMID:26539741; https://doi.org/10.1080/21645515.2015.1052198
- Feldman C , Anderson R . Review: current and new generation pneumococcal vaccines. J Infect 2014; 69:309-25; PMID:24968238; https://doi.org/10.1016/j.jinf.2014.06.006
- Khan MN , Xu Q , Pichichero ME . Protection against Streptococcus pneumoniae Invasive Pathogenesis by a protein-based vaccine is achieved by suppression of nasopharyngeal bacterial density during influenza a virus coinfection. Infect Immun 2017; 85:e00530-16; https://doi.org/10.1128/IAI.00530-16
- Verhoeven D , Xu Q , Pichichero ME . Vaccination with a Streptococcus pneumoniae trivalent recombinant PcpA, PhtD and PlyD1 protein vaccine candidate protects against lethal pneumonia in an infant murine model. Vaccine 2014; 32:3205-10; PMID:24731814; https://doi.org/10.1016/j.vaccine.2014.04.004
- Verhoeven D , Perry S , Pichichero ME . Contributions to Protection from Streptococcus pneumoniae Infection Using the Monovalent Recombinant Protein Vaccine Candidates PcpA, PhtD, and PlyD1 in an Infant Murine Model during Challenge. Clin Vaccine Immunol 2014; 21:1037-45; PMID:24850621; https://doi.org/10.1128/CVI.00052-14
- Xu Q , Surendran N , Verhoeven D , Klapa J , Ochs M , Pichichero ME . Trivalent pneumococcal protein recombinant vaccine protects against lethal Streptococcus pneumoniae pneumonia and correlates with phagocytosis by neutrophils during early pathogenesis. Vaccine 2015; 33:993-1000; PMID:25597944; https://doi.org/10.1016/j.vaccine.2015.01.014
- Xu Q , Casey JR , Almudevar A , Pichichero ME . Correlation of Higher Antibody Levels to Pneumococcal Proteins with Protection from Pneumococcal Acute Otitis Media but not Protection from Nasopharyngeal Colonization in Young Children. Clin Microbiol Infect 2017; 23(7):487; PMID:28143785; https://doi.org/10.1016/j.cmi.2017.01.011
- Xu Q , Casey JR , Pichichero ME . Higher levels of mucosal antibody to pneumococcal vaccine candidate proteins are associated with reduced acute otitis media caused by Streptococcus pneumoniae in young children. Mucosal Immunol 2015; 8:1110-7; PMID:25648056; https://doi.org/10.1038/mi.2015.1
- Brooks WA , Chang LJ , Sheng X , Hopfer R , Team PPRS . Safety and immunogenicity of a trivalent recombinant PcpA, PhtD, and PlyD1 pneumococcal protein vaccine in adults, toddlers, and infants: A phase I randomized controlled study. Vaccine 2015; 33:4610-7; PMID:26143615; https://doi.org/10.1016/j.vaccine.2015.06.078
- Prevaes SM , van Wamel WJ , de Vogel CP , Veenhoven RH , van Gils EJ , van Belkum A , Sanders EA , Bogaert D . Nasopharyngeal colonization elicits antibody responses to staphylococcal and pneumococcal proteins that are not associated with a reduced risk of subsequent carriage. Infect Immun 2012; 80:2186-93; PMID:22451514; https://doi.org/10.1128/IAI.00037-12
- Simell B , Ahokas P , Lahdenkari M , Poolman J , Henckaerts I , Kilpi TM , Käyhty H . Pneumococcal carriage and acute otitis media induce serum antibodies to pneumococcal surface proteins CbpA and PhtD in children. Vaccine 2009; 27:4615-21; PMID:19524618; https://doi.org/10.1016/j.vaccine.2009.05.071
- Holmlund E , Quiambao B , Ollgren J , Nohynek H , Kayhty H . Development of natural antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A and pneumolysin in Filipino pregnant women and their infants in relation to pneumococcal carriage. Vaccine 2006; 24:57-65; PMID:16115703; https://doi.org/10.1016/j.vaccine.2005.07.055
- Xu Q , Almudervar A , Casey JR , Pichichero ME . Nasopharyngeal bacterial interactions in children. Emerging Infect Dis 2012; 18:1738-45; PMID:23092680; https://doi.org/10.3201/eid1811.111904
- Xu Q , Casey JR , Chang A , Pichichero ME . When co-colonizing the nasopharynx haemophilus influenzae predominates over Streptococcus pneumoniae except serotype 19A strains to cause acute otitis media. Pediatr Infect Dis J 2012; 31:638-40; PMID:22301480; https://doi.org/10.1097/INF.0b013e31824ba6f7
- Casey JR , Adlowitz DG , Pichichero ME . New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J 2010; 29:304-9; PMID:19935445
- Pichichero ME , Casey JR . Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA 2007; 298:1772-8; PMID:17940232; https://doi.org/10.1001/jama.298.15.1772
- Nabors GS , Braun PA , Herrmann DJ , Heise ML , Pyle DJ , Gravenstein S , Schilling M , Ferguson LM , Hollingshead SK , Briles DE , et al. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine 2000; 18:1743-54; PMID:10699322; https://doi.org/10.1016/S0264-410X(99)00530-7
