ABSTRACT
Tuberculosis (TB) is a significant cause of illness and death worldwide. Immunotherapy has been investigated in the treatment of TB. The purpose of this study was to perform a meta-analysis investigating the effectiveness of the M. vaccae vaccine. Medline, Cochrane, EMBASE, and Google Scholar were searched until November 5, 2015 using the keywords: tuberculosis, pulmonary TB, therapeutic vaccines, immunotherapy, M. vaccae, sputum smear. Randomized controlled trials (RCTs) or 2-arm prospective studies were included. The primary outcome was the sputum smear clearance rate at 1 or 2 months and 6 months after treatment. Secondary outcomes were improvement of chest X-ray findings, sputum culture negative rate at 1 or 2 months and 6 months, erythrocyte sedimentation rate (ESR), hemoglobin, and leukocyte count, weight gain, and mortality. Of 89 records identified, 13 RCTs were included in the meta-analysis. The number of patients ranged from 22 to 1337, and the mean age ranged from 26.4 to 44.3 y. Patients treated with M. vaccae were more likely to have negative sputum smear results at 1–2 months (pooled OR = 2.642, 95% CI: 1.623–4.301, P < .001) and at 6 months (pooled OR = 2.111, 95% CI: 1.141–3.908, P = .017), and have a negative sputum culture at 1 or 2 months (pooled OR = 2.660, 95% CI: 1.978–3.578, P < .001). The results of this meta-analysis suggest that M. vaccae immunotherapy may be effective in the treatment of pulmonary TB.
Introduction
Despite advances in pharmacotherapy, tuberculosis (TB) remains a leading cause of morbidity and death worldwide. In 2014, there were an estimated 9.6 million new TB cases, with an increase of multidrug-resistant (MDR) strains, and there were 1.5 million deaths due to TB.Citation1 To meet the World Health Organization (WHO) 20-year (2016–2035) strategy to end the global TB epidemic, and because of the increased prevalence of MRD-TB, attention has been given to therapies other than traditional pharmacotherapy. One of these alternative therapies is immunotherapy, i.e., TB vaccine to complement pharmacotherapy.
One of the earliest TB vaccines was the bacille Calmette-Guérin (BCG), which is considered to be effective in preventing all forms of TB, including pulmonary disease, in infants, and to be effective in mycobacterial-naïve adults; however, neonatal or childhood BCG vaccination does not appear to offer protection against TB in adults.Citation2 Since the introduction of the BCG vaccine, a large number of approaches to developing an effective vaccine against TB have been examined.Citation2,3 One of the most studied TB vaccines is heat-inactivated M. vaccae.Citation4
M. vaccae is a nonpathogenic species commonly found in soil and water belonging to the same genus as M. tuberculosis that was first described in 1964 by Bonickse and Juhasz.Citation5 M. vaccae was first examined as a TB vaccine in the 1980s by Stanford et al.Citation6 Since that time its safety has been documented,Citation7 and it has been extensively investigated as an immunotherapeutic agent for the treatment of TB and in unrelated disorders including cancer and autoimmune disease.Citation8,9 M. vaccae is available in injectable and oral forms. An injectable form (Vaccae™; Anhui Zhifei Longcom Biologic Pharmacy Co., Ltd, China) is approved in China as an immunotherapeutic agent to help shorten TB drug therapy.Citation10 In one study, the negative sputum conversion rate in MDR-TB patients after 6 months was 43% in patients who received the vaccine compared with 21% of those who received drug therapy alone.Citation11 Used together with drug therapy, M. vaccae has been found to be effective in both drug-sensitive and MDR-TB.Citation12 The drug is currently being examined for its effectiveness in preventing active disease in individuals with latent TB infection in a phase III trial conducted in China that will include 10,000 people aged 15–65 y with a purified protein derivative (PPD) skin test > 15 mm.Citation1
Despite the encouraging results of individual studies, some systematic reviews have not shown that M. vaccae is effective in the treatment of TB,Citation13 while others have shown it to be effective in preventing TB in high risk patients (i.e., those with diabetes mellitus [DM] and human immunodeficiency virus [HIV] infection),Citation14 can improve the sputum conversion rate and X-ray appearance in never-treated TB patients,Citation10 and is most effective when multiple dose regimens are used.Citation4
Thus, the purpose of this study was to perform a meta-analysis investigating the effectiveness of the M. vaccae vaccine in the treatment of TB.
Results
Literature search
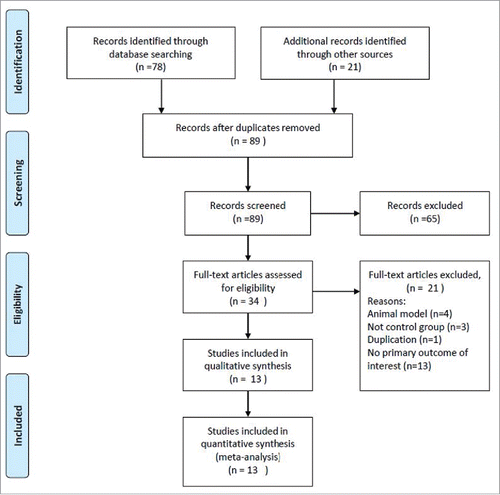
A flow diagram of study selection is shown in . A total of 89 records were identified after duplicates were removed. Sixty-five were excluded after review of the title/abstract and 34 full-text articles were reviewed. Twenty-one were excluded, the reasons for which are shown in . Thus, 13 studies were included in the meta-analysis.Citation7,11,12,15-24
A summary of the basic characteristics of the included studies is shown in . The number of patients in the studies ranged from 22 to 1337, and the mean patient age ranged from 26.4 to 44.3 y. The majority of patients were male (49% to 100%), and the proportion of patients with drug-resistant TB ranged from 2% to 98%.
Table 1. Basic characteristics of included studies.
Primary outcome
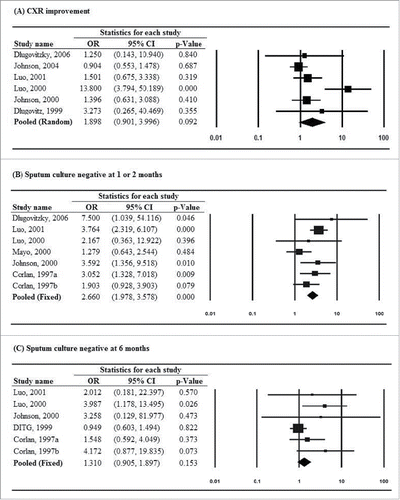
Of 9 studiesCitation7,11,12,15,18,21,22-24 that reported results of sputum smear clearance assessed at 1 or 2 months, all showed that patients treated with M. vaccae immunotherapy had higher odds of sputum smear conversion to negative than patients in the placebo group. Heterogeneity was present across studies, and thus a random-effects model of analysis was used (Q = 17.3, P = .028; I2 = 53.6%). The pooled estimate showed that patients treated with M. vaccae immunotherapy were more likely to have negative sputum smear results at 1–2 months as compared with placebo (pooled OR = 2.642, 95% CI: 1.623–4.301, P < .001) ().
Figure 2. Forest plots for (A) sputum smear conversion to negative at 1 or 2 months and (B) sputum smear conversion to negative at 6 months.
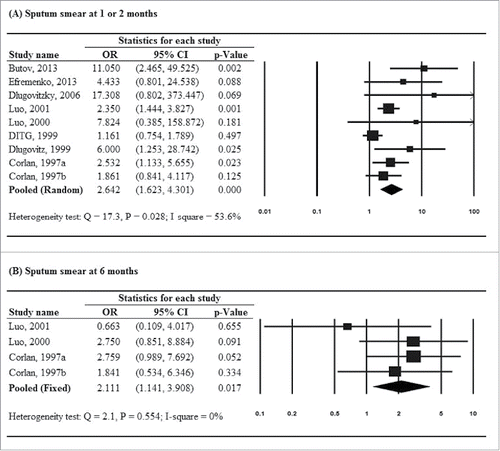
Four studiesCitation11,18,23,24 provided data on sputum smear clearance at 6 months, among which 1 study reported a lower odds of sputum smear conversion to negative in the treatment group than in the placebo group, but the findings was not statistical significant (OR = 0.663, 95% CI: 0.109–4.017, P = .655). A fixed-effects model of analysis was performed as no significant heterogeneity was identified (Q = 2.1, P = .554; I2 = 0%). The pooled estimate showed that patients treated with M. vaccae immunotherapy were more likely to have negative sputum smear results at 6 months as compared with placebo (pooled OR = 2.111, 95% CI: 1.141–3.908, P = .017) ().
Secondary outcomes
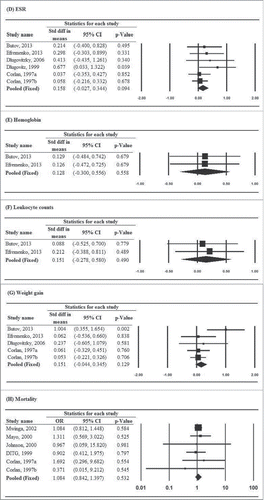
For secondary outcomes, only improvement of chest X-ray findings showed evidence of heterogeneity across studies, and thus a random-effects model was used to generate the pooled estimate (Q = 15.6, P = .008; I2 = 67.9%). Estimates of other secondary outcomes were pooled by using fixed-effects models as no evidence of heterogeneity was identified (data not shown). Findings from 7 studiesCitation11,12,16,18,19,23,24 demonstrated that patients treated with M. vaccae immunotherapy were more likely to have a negative sputum culture at 1 or 2 months (pooled OR = 2.660, 95% CI: 1.978–3.578, P < .001). For other secondary outcomes, no significant treatment effect favoring M. vaccae immunotherapy was found ().
Sensitivity analysis
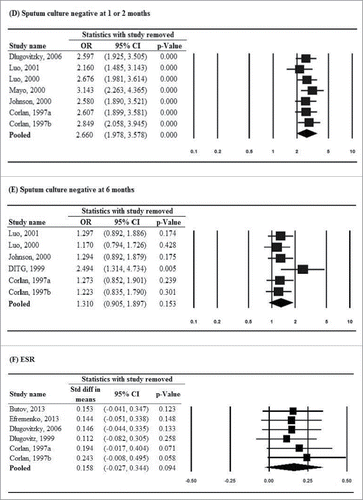
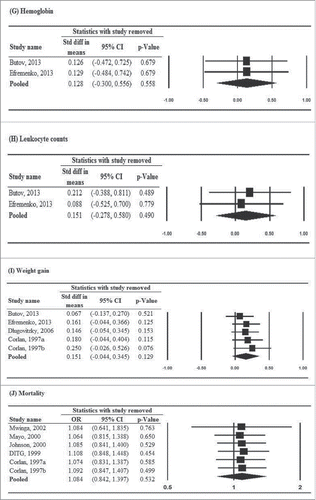
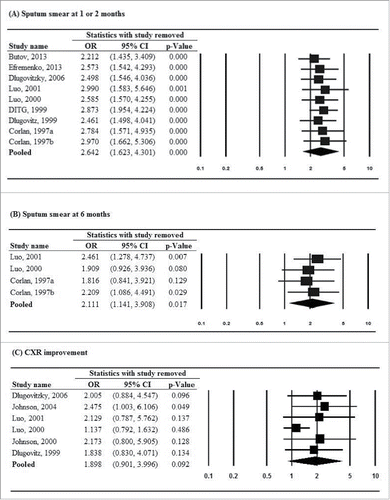
Results of the sensitivity analysis using the leave-one-out approach are shown in . For sputum smear clearance at 1 or 2 months, the direction and magnitude of association remained when any one study was removed, indicating no study had significant influence on the pooled estimate (). However, for sputum smear clearance at 6 months, the significance of pooled estimates changed when 2 studies were removed, respectively (). In addition, 1 study showed a mild influence on the pooled OR of improvement of chest X-ray findings (pooled OR = 2.475, 95% CI: 1.003–6.106, P = .049) when it was removed, while another study had substantial influence on the pooled OR of sputum culture negative at 6 months (pooled OR = 2.494, 95% CI: 1.314–4.734, P = .005) when it was removed ( and , respectively). Other secondary outcomes were not affected by the removal of individual studies in turn.
Figure 4. Sensitivity-analysis for (A) sputum smear conversion to negative at 1 or 2 months, (B) sputum smear conversion to negative at 6 months, (C) improvement of chest X-ray findings, (D) sputum culture negative at 1 or 2 months, (E) sputum culture negative at 6 months, (F) erythrocyte sedimentation rate (ESR), (G) hemoglobin, (H) leukocyte count, (I) weight gain, and (J) mortality.
Publication bias
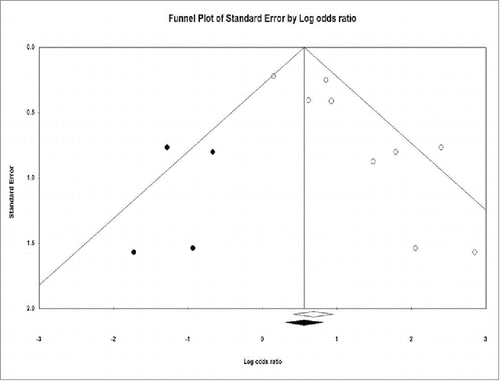
A funnel plot of sputum smear clearance at 1 or 2 months is shown in . Studies included in the meta-analysis are shown by white circles. There was evidence of publication bias given that the plot was not symmetric. No study with negative findings of sputum smear clearance assessed at 1 or 2 months was published or was included in the meta-analysis. The 1-tailed P-value of .004 by Egger's test also supported the observed asymmetry of the funnel plot. According to Duval and Tweedie's trim-and-fill method, 4 studies (shown by black circles) were needed to produce a symmetric funnel plot. Under a random-effects model of analysis, the imputed pooled OR was 1.916 (95% CI: 1.146–3.205) by using the trim-and-fill method if the 4 missing studies were added. Nonetheless, the direction and significance of the pooled OR in the current meta-analysis was consistent with that adjusted by the trim-and-fill method.
Quality assessment
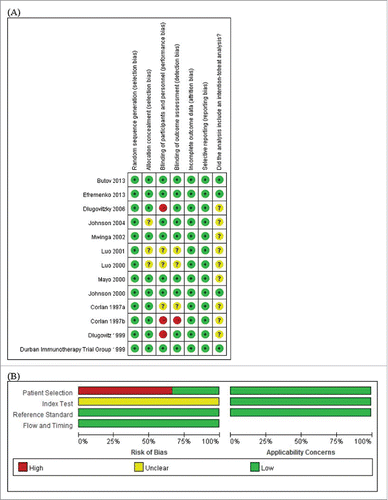
Results of the quality assessment of the included studies are shown in . All trials applied random sequence generation for randomization, indicating that selection bias is a minor concern. Among the 13 trials, 4 scored the highest quality.Citation7,15,20,21 The studies by Dlugovitzky et al.Citation12,22 and Corlan et al.Citation24 did not follow blinding of participants and personnel, which might lead to performance bias. Performance bias is also a major concern in the Corlan et al.Citation24 study because it lacked blinding of outcome assessment.
Discussion
The results of this meta-analysis of RCTs showed that M. vaccae increases the sputum smear conversion rate at 1 or 2 months and 6 months after treatment, and the sputum negative culture rate at 1 or 2 months after treatment in patients with pulmonary tuberculosis. It did not, however, increase the sputum negative culture rate at 6 months or affect any other secondary outcome measures including (ESR), hemoglobin, and leukocyte count, weight gain, and mortality.
The current meta-analysis is the most current that has examined the overall effectiveness of M. vaccae in patients with pulmonary tuberculosis. A Cochrane Database systematic review published in 2003 that included 8 trials and 2,140 adults concluded that M. vaccae immunotherapy did not provide benefits and that most treated patients experienced local adverse reactions.Citation13 Those results, however, may have been biased by the relatively large number of patients in one of the trials.Citation21 The conclusions regarding local reactions, however, was only based on 2 trial with 131 participants. A 2010 meta-analysis by Yang et al.Citation14 examined prevention of TB in high-risk patients and concluded that M. vaccae is effective in preventing TB in PPD strong positive individual, patients with type 2 DM, and aged people with clinical cured pulmonary TB, and is safe and effective in inducing biologically relevant immune response against TB in HIV-infected individuals. A subsequent meta-analysis by Yang et al.Citation10 published in 2011 included 54 studies and reported that M. vaccae improved the sputum conversion rate and X-ray appearance in never-treated TB patients. A systematic review of therapeutic vaccines for TB that did not include a meta-analysis concluded that M. vaccae is effective when multiple doses are administered.Citation4
The effect of M. vaccae is believed to be due to promotion of the T-helper (Th)-1 response and suppression of the Th2 response.Citation6 Murine studies have shown that M. vaccae promotes the production of CD8+ cytotoxic t lymphocytes and kill macrophages infected with M. tuberculosis, and macrophages are also stimulated to produce interleukin (IL)-12 by the CD8+ lymphocytes.Citation25 Interferon (IF)-γ is also believe to play a role in the response to M. vaccae.Citation26
Early clinical trials of M. vaccae immunotherapy for treatment of TB using irradiation-killed and heat-killed preparations showed benefits of therapy including better sputum conversion rates and cure rates and improved of X-ray findings.Citation6 However, the first extensive RCT that was double-blinded conducted in South Africa showed no benefit of M. vaccae immunotherapy; however, a different strain of M. vaccae (Mycobacterium vaccae strain NCTC 11659) was used.Citation21 Other trials performed at around the same time also did not show any benefit of M. vaccae immunotherapy.Citation19,27 The aforementioned trials, however, only used one dose of M. vaccae. On the other hand, a single dose was shown to be effective in other trials including those conducted in Argentina, Romania, Nigeria, and Kuwait.Citation28 It has been suggested that differences seen in the effectiveness of a single dose may be the result of comorbid conditions that alter the Th-1/2 responses or the type/strain of vaccine administered.Citation4 A recent review of the literature that was limited to articles published after 2004 concluded that M. vaccae immunotherapy was effective in patients with TB; however, the available literature supports the use of multiple doses rather than a single dose.Citation4
While early studies suggested a high incidence of adverse local reactions with M. vaccae immunotherapy, the overwhelming majority of the data indicate that a small percentage of patients experience a minor local response similar to that seen with the BCG vaccine that resolves within 24 hours, and an even smaller minority experience headache and/or fever that also resolves with 24 hours.Citation4 Given that the available data indicate that M. vaccae immunotherapy requires multiple doses, there is concern that the vaccine will be less well tolerated. However, M. vaccae immunotherapy comprising as many as 12 doses given at 2-month intervals has been shown to be safe and beneficial in chronic MDR-TB patients.Citation29 Stanford et al.Citation30 reported successful treatment of lung adenocarcinoma with 4 or 5 doses of M. vaccae. However, compliance was relatively poor with only 53% of patients receiving more than 2 injections of M. vaccae. This was attributed to injection site reactivity, which is not unexpected after repeated injections, and was generally superficial and transient. Overall, however, 62% of the subjects experienced an adverse event, 4 of which were classified as serious.
There are limitations to this study that should be considered. There was variability in the dosage, route of administration of the vaccine, and vaccine preparation that was administered between studies, and we did not examine these factors. Results of prior studies have suggested that these factors can affect the effectiveness of the vaccine.Citation4 Although there was moderate heterogeneity among the selected trials, the leave-one-out sensitivity analysis revealed stable results in primary and secondary outcomes. The effect of M. vaccae is relatively weak and transient, the data set is small, and there is the potential the data were skewed by smaller studies with larger effects, although appropriate weighting of the data was performed. Lastly, the results were based on relatively short follow-up periods.
In conclusion, the results of this meta-analysis, as well as that of available literature, suggest that M. vaccae immunotherapy may be effective in the treatment of pulmonary TB. However, the data need to be interpreted with caution given the limitations of the analysis. Further study is necessary to determine the most effective route of administration and dosing schedule, as well as comorbid conditions that may affect its efficacy.
Materials and methods
Literature search strategy and inclusion criteria
This systematic review and meta-analysis was conducted in accordance with PRISMA guidelines.Citation31 Medline, Cochrane, EMBASE, and Google Scholar were searched from inception until November 5, 2015 using the keywords: tuberculosis, pulmonary TB, therapeutic vaccines, immunotherapy, M. vaccae, sputum smear. Reference lists of relevant studies were also hand-searched. Searches were conducted by 2 independent reviewers, and when there was uncertainty regarding eligibility a third reviewer was consulted.
Inclusion criteria for the meta-analysis were: 1) RCTs or 2-arm prospective studies; 2) Patients had sputum acid-fast bacilli smear-positive pulmonary tuberculosis; 3) M. vaccae vaccine was compared with placebo. There was no language limitation for inclusion. Retrospective studies, cohort studies, letters, comments, editorials, case reports, proceedings, and personal communications were excluded. Studies were also excluded if they included pregnant or breast feeding women or individuals with alcohol or substance abuse.
Data extraction
The following information was extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, patient demographic data including age and sex, previous TB treatment and outcome, the presence of significant diseases such as DM or HIV infection, type of M. tuberculosis (drug-sensitive or drug-resistant), the M. vaccae preparation, dosage, and route of administration. Data extraction was also performed by 2 independent reviewers, with a third consulted in instances of uncertainty.
Quality assessment
The quality of each study was assessed using a risk-of-bias assessment tool, outlined in the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0).Citation32 Two reviewers subjectively reviewed all studies and assigned a value of “low risk,” “high risk,” or “unclear” to the following: a) random sequence generation, b) allocation concealment, c) blinding (patients, personnel, and assessor), d) adequate assessment of each outcome, e) avoidance of selective-outcome reporting, and f) inclusion of an intention-to-treat analysis. A third reviewer was consulted for resolution of any disagreement.
Outcome measures and statistical analysis
The primary outcome of the meta-analysis was the sputum smear clearance rate assessed at 1 or 2 months and 6 months after treatment. Secondary outcomes were improvement of chest X-ray findings, sputum culture negative rate at 1 or 2 months and 6 months after treatment, erythrocyte sedimentation rate (ESR), hemoglobin, and leukocyte count, weight gain, and mortality. For sputum smear clearance, improvement of chest X-ray findings, sputum culture negative rate, and mortality, odds ratios (ORs) with 95% confidence intervals (95% CI) were calculated and were compared between the treatment and placebo groups. For ESR, hemoglobin, leukocyte count, and weight gain, standardized differences in means between groups were calculated. Heterogeneity across studies was assessed by the Cochran Q statistic, the weighted sum of the squared deviations of the estimates of all studies, and I2, the percentage of the observed between-study variability due to by heterogeneity. A Cochran Q value of P < .10 or I2 > 50% was considered to indicate significant heterogeneity. Random-effects models according to DerSimonian–Laird method were used if heterogeneity was detected. Otherwise, fixed-effects models using the Mantel-Haenszel approach were used. Sensitivity analysis was performed using the leave-one-out approach. Risk of publication bias for primary outcomes for which at least 5 studies had provided was evaluated by funnel plots as well as Egger's test with a 1-tailed P-value. Absence of publication bias was indicated by the data points forming a symmetric funnel-shaped distribution and an Egger's test p > .05. To reduce the risk of publication bias, Duval and Tweedie's trim-and-fill method was used to make a symmetric distribution of log OR. Pooled ORs and differences in means were calculated, and a 2-sided p -value < .05 was considered statistically significant. All statistical analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- World Health Organization (WHO). Global tuberculosis report 2015. Available at: http://www.who.int/tb/publications/global_report/en/. Accessed: January 20, 2016
- Montagnani C, Chiappini E, Galli L, de Martino M. Vaccine against tuberculosis: what's new? BMC Infect Dis 2014; 14 Suppl 1:S2; PMID:24564340; https://doi.org/10.1186/1471-2334-14-S1-S2
- Ahsan MJ. Recent advances in the development of vaccines for tuberculosis. Ther Adv Vaccines 2015; 3:66-75; PMID:26288734; https://doi.org/10.1177/2051013615593891
- Gröschel MI, Prabowo SA, Cardona PJ, Stanford JL, van der Werf TS. Therapeutic vaccines for tuberculosis–a systematic review. Vaccine 2014; 32:3162-8; PMID:24726245; https://doi.org/10.1016/j.vaccine.2014.03.047
- Bonickse R, Juhasz E. [Description of the new species Mycobacterium vaccae n. sp]. Zentralbl Bakteriol Orig 1964; 192:133-5. [ Article in German]
- Stanford J, Stanford C, Grange J. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis. Front Biosci 2004; 9:1701-19; PMID:14977580; https://doi.org/10.2741/1292
- Efremenko YV, Butov DA, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, Butova TS, Stepanenko AL, Kutsyna GA, Jirathitikal V, et al. Randomized, placebo-controlled phase II trial of heat-killed Mycobacterium vaccae (Longcom batch) formulated as an oral pill (V7). Hum Vaccin Immunother 2013; 9:1852-6; PMID:23782489; https://doi.org/10.4161/hv.25280
- Grange JM, Bottasso O, Stanford CA, Stanford JL. The use of mycobacterial adjuvant-based agents for immunotherapy of cancer. Vaccine 2008; 26(39):4984-90; PMID:18625281; https://doi.org/10.1016/j.vaccine.2008.06.092
- Walker C, Sawicka E, Rook GA. Immunotherapy with mycobacteria. Curr Opin Allergy Clin Immunol 2003; 3:481-6; PMID:14612673; https://doi.org/10.1097/00130832-200312000-00010
- Yang XY, Chen QF, Li YP, Wu SM. Mycobacterium vaccae as adjuvant therapy to anti-tuberculosis chemotherapy in never-treated tuberculosis patients: a meta-analysis. PloS One 2011; 6:e23826; PMID:21909406; https://doi.org/10.1371/journal.pone.0023826
- Luo Y, Lu S, Guo S. [Immunotherapeutic effect of Mycobacterium vaccae on multi-drug resistant pulmonary tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi 2000; 23:85-8. [ Article in Chinese]; PMID:11778496
- Dlugovitzky D, Fiorenza G, Farroni M, Bogue C, Stanford C, Stanford J. Immunological consequences of three doses of heat-killed Mycobacterium vaccae in the immunotherapy of tuberculosis. Respir Med 2006; 100:1079-87; PMID:16278080; https://doi.org/10.1016/j.rmed.2005.09.026
- de Bruyn G, Garner P. Mycobacterium vaccae immunotherapy for treating tuberculosis. Cochrane Database Syst Rev 2003; (1):CD001166; PMID:12535403
- Yang XY, Chen QF, Cui XH, Yu Y, Li YP. Mycobacterium vaccae vaccine to prevent tuberculosis in high risk people: a meta-analysis. J Infect 2010; 60:320-30; PMID:20156481; https://doi.org/10.1016/j.jinf.2010.02.005
- Butov DA, Efremenko YV, Prihoda ND, Zaitzeva SI, Yurchenko LV, Sokolenko NI, Butova TS, Stepanenko AL, Kutsyna GA, Jirathitikal V, et al. Randomized, placebo-controlled Phase II trial of heat-killed Mycobacterium vaccae (Immodulon batch) formulated as an oral pill (V7). Immunotherapy 2013; 5:1047-54; PMID:24088075; https://doi.org/10.2217/imt.13.110
- Johnson JL, Nunn AJ, Fourie PB, Ormerod LP, Mugerwa RD, Mwinga A, Chintu C, Ngwira B, Onyebujoh P, Zumla A. Effect of Mycobacterium vaccae (SRL172) immunotherapy on radiographic healing in tuberculosis. Int J Tuberc Lung Dis 2004; 8:1348-54; PMID:15581204
- Mwinga A, Nunn A, Ngwira B, Chintu C, Warndorff D, Fine P, Darbyshire J, Zumla A; LUSKAR collaboration. Mycobacterium vaccae (SRL172) immunotherapy as an adjunct to standard antituberculosis treatment in HIV-infected adults with pulmonary tuberculosis: a randomised placebo-controlled trial. Lancet 2002; 360:1050-5; PMID:12383985; https://doi.org/10.1016/S0140-6736(02)11141-X
- Luo Y; National Cooperation Group On Clinical Study Of Mycobacterium Vaccae V. [The immunotherapeutic effect of Mycobacterium vaccae vaccine on initially treated pulmonary tuberculosis]. Zhonghua Jie He He Hu Xi Za Zhi 2001; 24:43-7. [ Article in Chinese]; PMID:11802939
- Mayo RE, Stanford JL. Double-blind placebo-controlled trial of Mycobacterium vaccae immunotherapy for tuberculosis in KwaZulu, South Africa, 1991-97. Trans R Soc Trop Med Hyg 2000; 94:563-8; PMID:11132390; https://doi.org/10.1016/S0035-9203(00)90088-9
- Johnson JL, Kamya RM, Okwera A, Loughlin AM, Nyole S, Hom DL, Wallis RS, Hirsch CS, Wolski K, Foulds J, et al. Randomized controlled trial of Mycobacterium vaccae immunotherapy in non-human immunodeficiency virus-infected ugandan adults with newly diagnosed pulmonary tuberculosis. The Uganda-Case Western Reserve University Research Collaboration. J Infect Dis 2000; 181:1304-12; PMID:10753731; https://doi.org/10.1086/315393
- Immunotherapy with Mycobacterium vaccae in patients with newly diagnosed pulmonary tuberculosis: a randomised controlled trial. Durban Immunotherapy Trial Group. Lancet 1999; 354:116-9; PMID:10408487; https://doi.org/10.1016/S0140-6736(98)10448-8
- Dlugovitzky D, Bottasso O, Dominino JC, Valentini E, Hartopp R, Singh M, Stanford C, Stanford J. Clinical and serological studies of tuberculosis patients in Argentina receiving immunotherapy with Mycobacterium vaccae (SRL 172). Respir Med 1999; 93:557-62; PMID:10542989; https://doi.org/10.1016/S0954-6111(99)90155-5
- Corlan E, Marica C, Macavei C, Stanford JL, Stanford CA. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis in Romania. 2. Chronic or relapsed disease. Respir Med 1997; 91:21-9; PMID:9068813; https://doi.org/10.1016/S0954-6111(97)90133-5
- Corlan E, Marica C, Macavei C, Stanford JL, Stanford CA. Immunotherapy with Mycobacterium vaccae in the treatment of tuberculosis in Romania. 1. Newly-diagnosed pulmonary disease. Respir Med 1997; 91:13-9; PMID:9068812; https://doi.org/10.1016/S0954-6111(97)90132-3
- Skinner MA, Yuan S, Prestidge R, Chuk D, Watson JD, Tan PL. Immunizationwith heat-killed Mycobacterium vaccae stimulates CD8+ cytotoxic T cells spe-cific for macrophages infected with Mycobacterium tuberculosis. Infect Immun 1997; 65:4525-30; PMID:9353029
- Zhang L, Ma W, Da J. Mycobacterium vaccae influences the kinetics of Th1/Th2cells and expression of iNOS in a marine model of experimental tuberculosis. Chin J Tuberc Respir Dis 2000; 23:43-6
- Kon OM, Goyal M, Filley E, Gleissberg G, Cunningham D, Rook GA, Shaw RJ. Mycobacterium vaccae: a study of safety and outcome measures. Respir Med 1998; 92:597-8; PMID:9692130; https://doi.org/10.1016/S0954-6111(98)90316-X
- Stanford JL. Immunotherapy with Mycobacterium vaccae in the treatment of mycobacterial disease. J Infect 1999; 39:179-82; PMID:10714790; https://doi.org/10.1016/S0163-4453(99)90044-0
- Stanford JL, Stanford CA, Grange JM, Lan NN, Etemadi A. Does immunotherapy with heat-killed Mycobacterium vaccae offer hope for the treatment of multi-drug-resistant pulmonary tuberculosis. Respir Med 2001; 95:444-7; PMID:11421500; https://doi.org/10.1053/rmed.2001.1065
- Stanford JL, Stanford CA, O'Brien ME, Grange JM. Successful immunotherapy with Mycobacterium vaccae in the treatment of adenocarcinoma of the lung. Eur J Cancer 2008; 44:224-7; PMID:17928219; https://doi.org/10.1016/j.ejca.2007.08.021
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151:W65-94; PMID:19622512; https://doi.org/10.7326/0003-4819-151-4-200908180-00136
- Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Updated March, 2011