ABSTRACT
Cellular immunity is important for protection against the serious complications of influenza in older adults. As it is unclear if newer influenza vaccines elicit greater cellular responses than standard vaccines, we compared responses to 2 standard and 2 newer licensed trivalent inactivated vaccines (TIVs) in a randomized trial in older adults. Non-frail adults ≥ 65 y old were randomly assigned to receive standard subunit, MF59-adjuvanted subunit, standard split-virus or intradermal split-virus TIV. Peripheral blood mononuclear cells (PBMC) harvested pre- and 3-weeks post-vaccination were stimulated with live A/H3N2 virus. PBMC supernatants were tested for interleukin 10 (IL-10) and interferon gamma (IFN-γ), and lysates for granzyme B (GrB). Flow cytometry identified CD4+ and CD8+ T- cells expressing intracellular IL-2, IL-10, IFN-γ, GrB, or perforin. Differences following immunization were assessed for paired subject samples and among vaccines. 120 seniors participated, 29-31 per group, which were well matched demographically. Virus-stimulated PBMCs were GrB-rich before and after vaccination, with minimal increases evident. Immunization did not increase secretion of IFN-γ or IL-10. However, cytolytic effector T-cells (CD8+GrB+perforin+) increased significantly in percentage post-vaccination in all groups, to similar mean values across groups. CD4+GrB+perforin+ T-cells also increased significantly after each vaccine, to similar mean values among vaccines. Vaccination did not increase the low baseline percentages of CD4+ or CD8+ T-cells expressing IFN-γ, IL-2 or IL-10 . In conclusion, participants had pre-existing cellular immunity to H3N2 virus. All 4 vaccines boosted cellular responses to a similar but limited extent, particularly cytolytic effector CD8+ T-cells associated with clinical protection against influenza.
Introduction
Influenza virus is responsible for a substantial disease burden in older adults. The need for more effective vaccines in the older population is well recognized but there have been significant challenges in optimizing vaccine-mediated protection in this age group. Antibody responses have been used to evaluate new vaccines but have limitations as a sole predictor of vaccine efficacy.Citation1-4 For example, we showed that serum antibody titers against different influenza strains did not differentiate between those older individuals who subsequently developed influenza illness and those who did not.Citation5,6 Age-related changes in T-cell responses are associated with a decline in the antibody response to influenza vaccination.Citation7,8 In fact, T-cell mediated mechanisms of protection are increasingly being recognized as relevant for influenza vaccine efficacy in older adults.Citation9 Specifically, levels of the cytolytic mediator granzyme B (GrB) and the interferon-gamma:interleukin-10 (IFN-γ:IL-10) ratio predict protection against influenza illnessCitation5,6 and are inversely correlated with influenza illness severityCitation10 in older adults. T cell correlates of protection using flow cytometric methods have been established in young adults based on experimental influenza A/H3N2 challengeCitation11 and natural infection with A/H1N1pdm.Citation12 These studies showed that in the absence of protective levels of antibody, correlates of protection in experimental influenza A/H3N2 challenge or natural H1N1pdm infection in young adults were, respectively, based on the IFN-γ+ CD4 T cell responseCitation11 or IFN-γ+IL-2− CD8 T cell responseCitation12 to matrix (M)- and nucleoprotein (NP)-derived peptides.
Prior exposure to influenza through infection or vaccination affects antibody titers and antibody responses to vaccination more so than aging does, while the decline in cell-mediated immune responses to influenza is related more to aging than to virus exposure.Citation13 Standard trivalent inactivated influenza vaccines (TIVs) provide a weak stimulus to T cell mediated immunity, especially in CD8+ T cells.
Although T cell memory from previous exposure to natural infection with influenza virus can be re-stimulated by vaccination, virtually all of the epitopes that drive the CD8 T cell response in humans are contained within the internal proteins of the virusCitation14,15 not specifically included in current vaccines. Influenza-specific CD8+ T-cells cannot protect against influenza infection but are critical for virus clearance, particularly from the lungs, and for preventing serious complications.Citation16 These cells are highly specific for protein sequences conserved across different influenza strains and do not depend on an exact match of the vaccine strain with the circulating strain of influenza virus. Since the content of influenza internal proteins such as matrix and nucleoprotein varies widely among the available split-virus influenza vaccines and are virtually absent in subunit vaccines, differences between these vaccine formulations in the CD8 vs. CD4 T- cell response to influenza vaccination would be predicted.Citation17 Specifically, in vitro studies have suggested that a greater cellular response is elicited by split-virus vaccines due to higher matrix and nucleoprotein concentrations.Citation17 Likewise, use of an adjuvant or the intradermal route of immunization might engage cellular immune responses to a greater extent than after standard vaccines.Citation18-20 Given these potential differences to elicit immune responses, we evaluated the antibodyCitation21 and cell-mediated immune responses to vaccination in a randomized study of 4 seasonal influenza vaccines available in Canada including standard subunit, MF59 adjuvanted subunit and spilt-virus vaccines given intramuscularly or intradermally. This report presents the results for cell-mediated immune responses.
Results
In total, 120 participants were enrolled and immunized, 29–31 individuals per group, in autumn of 2011. All subjects provided blood samples at both visits but one baseline and 3 post-immunization samples were of insufficient volume for all intended assays (). All post-immunization samples were obtained per protocol.
Figure 1. Subject disposition summary. Altogether 120 participants were enrolled and each group of 29 to 31 subjects was immunized with TIV1, ADV, IDV or TIV2 influenza vaccines. TIV1 = subunit vaccine; ADV = subunit vaccine with MF59 adjuvant; TIV2 = split-virus vaccine; IDV = split-virus vaccine given intradermally.
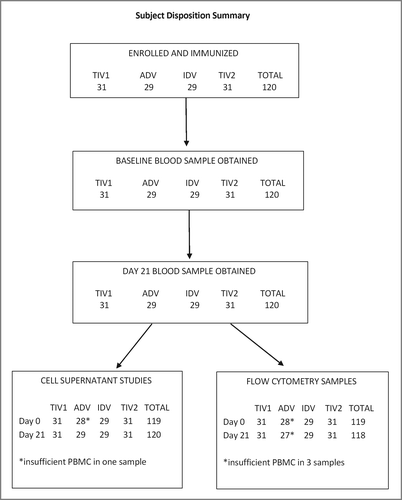
The 4 groups were well-matched in terms of sex and age distribution, ethnicity, body mass index and frequency of co-morbid conditions (). Nearly all participants had received TIV in both pre-study years. The frequency of HAI titers ≥ 40 to the H3N2 virus in the 2011-2012 vaccine was similar across the 4 groups, averaging 33% ().
Table 1. Subject demographics by vaccine group.
Table 2A. Estimated sample size based on 0.80 power and 25% difference between vaccine groups.
Table 2B. Estimated sample size based on 0.80 power and 25% difference between vaccine groups (for percentage positive T-cells expressing GrB and perforin).
Global assessment of secreted effectors
GrB production by freshly isolated, virus-stimulated PBMCs was substantial before vaccination, with similar geometric mean concentrations (GMCs) evident among the study groups (). Three weeks after vaccine administration, a marginal increase in GMC was evident in all vaccine groups except in the TIV split-virus group, which had a marginally higher pre-vaccination GMC. However, post-vaccination GMCs were not significantly different among the 4 groups. When individual responses were explored, considerable diversity was evident within and among the groups. The proportion of participants who had a substantial increase in GrB concentration after vaccination varied by group: split-virus 16.1% (5/31), split-virus intradermal 24.1% (7/29), subunit 38.7% (12/31) and adjuvanted subunit 46.4% (13/28). More participants had a GrB response to the subunit vaccines than to the split-virus vaccines (25/59 vs 12/60, respectively; p = 0.022, chi-square test). However, our outcome measure was the group GMC of inducible GrB activity following influenza vaccination,Citation6 rather than individual GrB response rates, the significance of which are uncertain.
Figure 2. Geometric mean concentrations (GMC) of effectors produced by stimulated fresh PBMCs. PBMCs from pre- (light circles) and post-immunized (dark circles) elderly individuals were stimulated with live A/H3N2 influenza virus. Shown are individual levels and GMCs (horizontal bars) in PBMC lysates of GrB (panel A), IFN-γ (panel B) and IL-10 (panel C) and IFN-γ:IL-10 ratio (panel D) in PBMC culture supernants. Paired t-tests were used to compare GMC of pre- and post-vaccinated groups of individuals and one-way ANOVA was used for cross-vaccine comparisons. All comparisions appeared non-significant. TIV1 = subunit vaccine; ADV = subunit vaccine with MF59 adjuvant; TIV2 = split-virus vaccine; IDV = split-virus vaccine given intradermally.
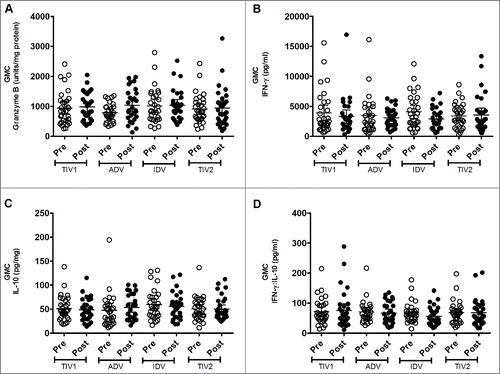
Baseline production of IFN-γ by virus-stimulated PBMCs was substantial in each vaccine group, with no significant intergroup differences (). No post-immunization increase in IFN-γ production by stimulated PBMCs was evident in any group, nor did post-immunization values differ significantly among the 4 groups. A similar phenomenon was evident with IL-10 production by stimulated PBMCs: baseline GMCs were similar among groups and did not increase significantly after vaccination (). Likewise, geometric mean ratios of IFN-γ:IL-10 did not increase after immunization or differ among groups (). Exploration of individual responses did not identify differences in the small proportions of subjects in each group who had an appreciable increase in amounts of these cytokines following vaccination (data not shown).
Cell-specific assessment of intracellular effectors
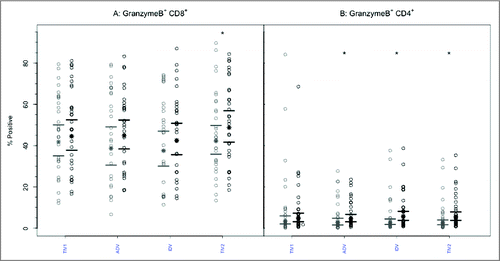
Similar to the above observations, GrB expression was frequently detectable in CD8+ T lymphocytes obtained pre-vaccination: group geometric mean (GM) percentages ranged from 43.9–46.5, with no significant intergroup differences (). No significant increases in GrB expression in CD8+ T- cells were evident post-immunization based on GM percentages except after split-virus vaccine (TIV2), nor were differences evident among the vaccine groups (p = 0.675, ANOVA). Within the CD4+ T-cell population (), the proportions positive for GrB before vaccination were much smaller, with group GMPs ranging from 5.5%–9.9%. After vaccination, the proportion of CD4+ T lymphocytes positive for GrB increased slightly (GMP range 7.3%-9.3%), the increase being statistically significant (p < 0.005) after all but subunit TIV vaccine. However, the geometric mean percentages of CD4+GrB+ lymphocytes observed post-vaccination did not differ significantly (p = 0.862, ANOVA) among the 4 vaccine groups ().
Figure 3. CD8+ and CD4+ T-cells mediated GrB responses to 4 commercial vaccines. PBMCs from pre- (light circles) and post-immunized (dark circles) elderly individuals were stimulated with live A/H3N2 influenza virus. Phenotype of the stimulated T-cells was measured by flow cytometry and percentage of CD8+ (panel A) and CD4+ T-cells (panel B) expressing GrB was then measured by immunocytochemistry (ICC). Individual values are expressed as percentages of CD4+ and CD8+ cells expressing GrB, while the group geometric mean percentage is denoted with a bold star (*). Horizontal bars represent 95% confidence intervals. Paired t-tests were used to compare means of pre- and post-vaccinated individuals in each group (*significant difference p ≤ 0.005). In panel A, only TIV2 induced an increase in GrB+ CD8+ T-cells while in panel B, ADV, IDV and TIV2 vaccines induced significant increases in proportions of GrB+CD4+ T-cells after vaccination. TIV1 = subunit vaccine; ADV = subunit vaccine with MF59 adjuvant; TIV2 = split-virus vaccine; IDV = split-virus vaccine given intradermally.
Perforin expression was infrequently present at baseline within CD8+ or CD4+ T lymphocytes, with no intergroup differences ( and , p-values > 0.85). Following vaccination, the GM percentage of CD8+ T-cells positive for perforin increased significantly (p < 0.005) after each vaccine except split-virus (TIV2)(). The GM percentages post-vaccination did not differ significantly among the vaccine groups (p = 0.954) and remained <3%. Among CD4+ T cells (), the proportion of perforin+ cells increased significantly after each vaccine (2.15–2.61 fold) but the GM post-vaccination values remained <0.8% and did not differ among the vaccine groups (p = 0.785).
Figure 4. CD8+ T-cells mediated perforin and GrB responses to 4 commercial vaccines. PBMCs from pre- (light circles) and post-immunized (dark circles) elderly individuals were stimulated with live A/H3N2 influenza virus. Phenotype of the stimulated T-cells was measured by flow cytometry and percentages of CD8+ T-cells expressing perforin (panel A) or both perforin and GrB (panel B) were then measured by ICC. Individual values are expressed as percentages of CD8+ cells expressing perforin alone or with GrB, while the group geometric mean percentage is denoted with a bold star (*). Horizontal bars represents 95% confidence of intervals. Paired t-tests were used to compare means of pre and post vaccinated individuals in each group (*statistical significance p < 0.005). Panel A shows significant increases after 3 vaccines and Panel B after all 4 vaccines. TIV1 = subunit vaccine; ADV = subunit vaccine with MF59 adjuvant; TIV2 = split-virus vaccine; IDV = split-virus vaccine given intradermally.
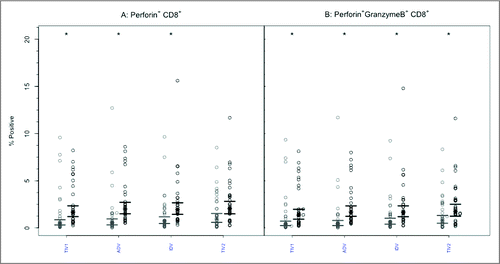
Figure 5. CD4+ T-cells mediated perforin and GrB responses to 4 commercial vaccines. PBMCs from pre- (light circles) and post-immunized (dark circles) elderly individuals were stimulated with live A/H3N2 influenza virus. Phenotype of the stimulated T-cells was measured by flow cytometry and percentages of CD4+ T-cells expressing perforin (panel A) or both perforin and GrB (panel B) were then measured by ICC. Individual values are expressed as percentage of CD4+ cells expressing perforin alone or with GrB, while group geometric mean percentage is denoted with a bold star (*) . Horizontal bars represent 95% confidence intervals. Paired t-tests were used to compare means of pre- and post-vaccinated individuals in each group (*statistical significance p < 0.005). All 4 vaccines induced significant increases in perforin expression (Panel A) and dual perforin-GrB expression (Panel B). TIV1 = subunit vaccine; ADV = subunit vaccine with MF59 adjuvant; TIV2 = split-virus vaccine; IDV = split-virus vaccine given intradermally.
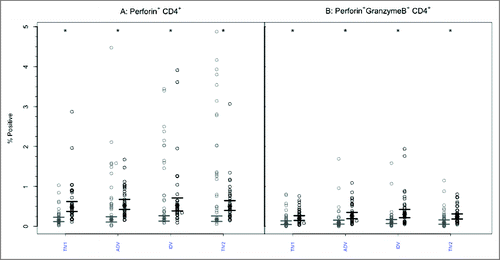
Prior to vaccination, the GM percentage of polyfunctional CD8+ T cells expressing both GrB and perforin was <2% in all 4 groups (). After vaccination, the GM percentage of cells expressing both effectors increased significantly (p < 0.005) after each vaccine but the GM percentages achieved did not differ among the vaccine groups (p = 0.938) and remained <3% in all. A similar pattern was evident with CD4+ T lymphocytes (): the GM percentage of cells with dual GrB and perforin expression increased significantly (p < 0.005) in each study group. The achieved GM proportions remained <0.5% in all groups, with no intergroup differences (p = 0.100).
With respect to CD4+ T cells expressing IFN-γ or IL-2, the GM proportions of each remained <0.2% at both time points, with no increase following vaccination or differences among groups (data not shown) . Likewise, within the corresponding CD8+ sub-populations, GMPs were <0.22% for IFN-γ or IL-2expression at baseline, with no increase after vaccination or differences among groups. These results are consistent with previous studies of the response to a similar TIV vaccine preparation albeit with PR8 influenza virus used to stimulate PBMC.Citation22
Vaccine internal protein content
By liquid chromatography- mass spectrometry, the influenza A M1 protein content in the subunit TIV vaccine was 0.59 µg/ml ( ± 0.09SD) and 2.57 µg/ml ( ± 0.12SD) in the split-virus TIV vaccine. Influenza A NP content in the subunit TIV vaccine was 0.18 µg/ml (0.04 ± SD) and 31.03 µg/ml (3.44 ± SD) in the split-virus TIV vaccine. On closer analysis, NP and M1 proteins from H1N1 and B vaccine viruses were detected, with M2 protein present in only trace amounts, while no H3N2 internal proteins were identified. Adjuvanted and intradermal vaccines were not tested.
Discussion
This randomized clinical trial was designed to compare antibody and cell-mediated responses to 4 influenza vaccines available in Canada for the 2011-2012 influenza season. We previously published the immunogenicity, safety and tolerability results showing modestly enhanced antibody responses after MF59-adjuvanted subunit TIV compared with the other 3 vaccines, with no between-group differences in safety or tolerability.Citation21 Current reliance on antibody responses for licensing new influenza vaccines may fail to recognize important contributions of the cell-mediated immune response to protecting older adults against the serious complications of influenza. In addition, adjuvants such as MF59 and AS03Citation23 may further enhance protection by broadening the antibody response to neutralize drift variants of circulating influenza viruses not covered by the seasonal vaccine strains. Thus, measures of both humoral and cell-mediated immunity are needed to predict protection against influenza in older adults. We used previously established measures of the T-cell response to influenza virus challenge in vitro as correlates of protection to assess differences in cell-mediated immunity in subjects recruited at the Vancouver site of this multi-center trial.
The principal findings of this study were that seniors had pre-existing cellular immunity to H3N2 influenza virus that was boosted to a similar but limited extent by each of the 4 study vaccines. It was expected that seniors would have established cellular immunity to H3N2 influenza virus from previous infections and immunizations. It was reassuring to observe significantly increased numbers of cytolytic CD8+ T cells (p < 0.005) following administration of each vaccine. Differences in the magnitude of responses among the vaccine groups resulting from compositional differences in the vaccine products were not evident. Tests of analogous but more recent vaccine products revealed substantial differences: the split-virus TIV had 4-fold greater M1 protein content and 17-fold greater NP content than the subunit TIV vaccine. However, none of these proteins appeared to be derived from the H3N2 component of the vaccines, possibly explaining the limited responses seen in the present study which focused on H3N2 responses.
Strengths of this study design included substantial uniformity among the volunteers, who were typical of seniors living independently and receiving annual influenza immunization. Such a study population was considered most likely to reveal any important differences in immune responses to the various vaccines, unlike frail seniors with reduced response capacity. Limiting participation to one center favored high compliance with study procedures and rapid and uniform processing of PBMCs, including their consistent stimulation with live influenza virus. Measuring cellular responses to include both secreted effectors from PBMCs and intracellular effectors in CD4+ and CD8+ T lymphocytes increased the chances of observing significant differences among the vaccinated groups. The threshold set for statistical significance (p < 0.005) of most comparisons was adopted to achieve a Bonferroni-like correction for multiple comparisons.
Our study had several limitations. For example, group sizes were small; larger group sizes might have revealed response differences among the products tested. Other limitations included the use of a single virus (A H3N2 strain) as the response probe, so results may not reflect responses to H1N1 and B components of influenza vaccines, with their potentially greater content of internal proteins. Quantifying cellular responses as percentages of cells expressing identified markers may be less accurate than with methods allowing quantitation of target cell numbers. Observations were limited to a single, commonly-used time point following immunization: earlier or later sampling might have provided different results. Results may not be broadly generalizable given the ethnic uniformity and general good health of the study population.
In this study, pre-immunization PBMCs from older adults showed high levels of GrB+Perf− CD8+ T cells. Recently, we showed that GrB activity in unstimulated T-cells is associated with CMV seropositivity and accumulation of late or terminally differentiated CD8+ T-cells.Citation24 This activity contributes to the level of GrB activity measured in influenza-stimulated PBMC but has demonstrated toxicity in the extracellular environment when produced in the absence of perforin.Citation25 Thus, GrB activity in influenza-stimulated PBMC adjusted for the CMV effect,Citation24 or the frequency of GrB+ CD8+ T cells that are also Perf+26 are the more reliable measures of the CD8 T-cell response to influenza vaccination, the latter being the measure selected for this study.Citation26 We were not able to include measurement of cytomegalovirus seropositivity in the present study. In addition, GrB, IFN-γ and IL-10 production in influenza-stimulated PBMC before vaccination reflects previous exposure to influenza virus through natural infection and immunization, and previously established T-cell memory that can be re-stimulated with vaccination. Unexpectedly, no post immunization increase in IFN-γ and IL-10 levels in influenza-stimulated PBMC was evident for any of these vaccines. Our results contrast with our previous studies showing a significant increase in both IFN-γ and IL-10 following vaccination such that the IFN-γ:IL-10 ratio did not change in response to vaccinationCitation5,10 but does decline with agingCitation27 and is a correlate of protection.Citation5,10 It is important to point out that these previous studies included individuals who had laboratory-confirmed influenza where the IFN-γ:IL-10 ratio could be demonstrated as a correlate of protection.Citation5,10 Since this study did not include surveillance for influenza illness, a similar analysis of the correlates of protection could not be performed. Interestingly, Co et al. evaluated the ability of 3 commercial TIVs from the 2007-2008 season in the USA to elicit T-cell responses in healthy individuals.Citation17 All 3 vaccines showed a significant difference in IFN-γ producing activated CD4+ and CD8+ T-cells that varied according to the internal protein content of the vaccines, consistent with the role of these virus proteins in activating different T-cell responses. Our results may be consistent with low levels of internal proteins in the vaccines used in our study.
Significantly higher levels of GrB and IFN-γ:IL-10 were observed at 4 weeks post-vaccination in our previous studies.Citation5,6 In the present study, we found that all 4 vaccines induced a slight increase (non-significant) in GrB activity at 21 d post-vaccination, when levels measured in PBMC lysates were similar across all vaccine groups. In contrast, the geometric mean percentage of CD4+ T lymphocytes positive for GrB increased significantly after 3 vaccines (p < 0.005) except subunit TIV but again there was no difference in the mean percentages of CD4+GrB+ lymphocytes observed post-vaccination across the 4 vaccine groups. Our results are in line with a previous study in which Couch et al compared CD4+ T-cell mediated responses with or without AS03 (similar to MF59) adjuvant in TIV vaccine.Citation18 The study concluded that inclusion of AS03 in TIV significantly enhanced antigen-specific CD4+ T cell responses in older adults on day 21 after vaccination. A positive effect of AS03 adjuvant system on the CD4 T-cell response to vaccination and protection against A/H3N2 influenza in older adults were demonstrated in a large randomized clinical trial.Citation28 Other measures of the cell-mediated immune response in our study using flow cytometry and ICC to measure T-cell frequencies and contents were similar across the vaccine groups, particularly within the CD8 T-cell subset depending upon which cytokine or cytolytic mediator was measured. Overall the response to vaccination was modest compared with our previous studies and may reflect low/absent levels of H3N2 influenza internal proteins in the vaccines used in this study.
In summary, our study demonstrated similar in-vitro T-cell responses following administration of 4 commercially available influenza vaccines despite differences in composition (split-virus vs subunit}, presence of adjuvant and route of administration. A randomized controlled comparison was important for reducing the chance of confounding in the interpretation of the results and highlighted the variability of the response to vaccination depending on the selected measure of cell-mediated immunity. A variety of mechanisms can be postulated for our observed results but vaccine composition analyses including specific internal proteins and larger studies including surveillance for laboratory-confirmed influenza in older adults are needed to understand how these measures of the cell-mediated immune response translate to clinically relevant protection and the proposed mechanism of protection for each of these vaccines.
Participants and methods/materials
Study design and participants
This single center study was nested within a larger, prospective, multicenter trialCitation21 comparing the safety and immunogenicity of influenza vaccines in seniors and was conducted between September, 2011, and January, 2012. The institutional research review board of each center provided ethics approval. The nested study added investigations of cellular immune responses to the standard protocol, described previously.Citation21 The study was registered at ClinicalTrials.gov (NCT01368796) and conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) and Good Clinical Practice Guidelines of the International Committee on Harmonization.
Eligible subjects were non-frail adults ≥ 65 y of age, in good health or with stable health conditions, living independently either in the community or in seniors' residences. Volunteers were required to have had TIV vaccination in at least one of the 2 previous seasons. Exclusion criteria were previously reported.Citation21 Written informed consent was obtained before enrollment.
Participants in the nested study were centrally randomized to receive one of 4 study vaccines, with stratification of the process by age sub-group and sex. Assignments were arranged in balanced blocks of 6, determined by computer-generated random number lists. Following enrolment, detailed information was obtained from participants regarding their health, prior TIV vaccinations, medication use and general fitness. A baseline blood sample (20 mL) was obtained, following which the assigned vaccine was administered with subject and evaluator blinding. Follow-up blood samples (20 mL) were obtained 3 weeks (window 20-28 days) after vaccination.
Study vaccines
Single commercial lots of 4 TIVs for 2011-2012 were obtained: a subunit vaccine (Agriflu, Novartis Vaccines, lot #112104)(TIV1); a formulation of the subunit vaccine with MF59 adjuvant (Fluad, Novartis Vaccines, lot #117703)(ADV); a split-virus vaccine (Vaxigrip, Sanofi Pasteur, lot # C4109AA)(TIV2); and an intradermal preparation of the split-virus vaccine (Intanza 15, Sanofi Pasteur, lot #H8187-1)(IDV). Each formulation delivered 15 µg of hemagglutinin of each component strain per dose. Each product was approved for use in adults ≥ 65 y of age and was supplied in pre-filled syringes. Constituent strains in these products were A/California/7/2009 (H1N1)-like, A/Perth/16/2009 (H3N2)-like and B/Brisbane/60/2008. Vaccines were stored and transported at 2–8 °C, avoiding freezing.
Injections were given in the deltoid area, using a 1” safety needle for IM injections or the micro-needle supplied for intradermal injections.
Immunoassays
The heparinized venous blood samples were promptly (< 4 hours) processed to harvest peripheral blood mononuclear cells (PBMCs) by Ficoll gradient purification.Citation29 A portion of the unstimulated cells were cryopreservedCitation30 and stored in liquid nitrogen for subsequent simultaneous analysis of paired pre- and post-immunization samples (see below). Aliquots (3.0 × 106 PBMC/mL) of fresh PBMC were stimulated with influenza A/H3N2 virus at a MOI (multiplicity of infection) of 2 in AIM V medium (Gibco, Grand Island, NY) as described previously.Citation31 The specific virus was sucrose-gradient purified, live influenza A/Victoria/3/75, which we have shown stimulates equivalent T- cell responses to H3N2 vaccine strains related to the cross-reactivity of the T-cell epitopes among the different strains of influenza virus. PBMC were harvested after 20 hours of culture, with cells and medium then frozen at −80 °C. Unstimulated aliquots of PBMCs were processed in parallel as negative controls.
Total GrB activity was measured in PBMC lysates by cleavage of the substrate IEPDpna (Calbiochem, Billerica, MA) as described previously and validated.Citation5,29 GrB activity was measured against a commercially available GrB standard (Biomol, Enzo Life Sciences, Ann Arbor, MI), adjusted for the amount of protein in the lysate and reported as units per mg protein (BCA assay, Pierce, Rockford, IL) in the PBMC lysates. Interferon gamma (IFN-γ) and interleukin 10 (IL-10) secreted by stimulated PBMCs into the culture medium were measured using Bio-plex assay kits from Bio-Rad Laboratories (Mississauga, ON). Briefly, 50 µL culture medium was incubated with antibody-coupled beads, complexes were washed, then incubated with biotinylated detection antibody and, finally, with streptavidin-phycoerythrin before assessing cytokine concentrations. Human recombinant cytokine standards were provided by the vendor (Bio-Rad Laboratories). Cytokine levels were determined using a multiplex array reader and software from Luminex™ Instrumentation System (Bio-Plex Workstation from Bio-Rad Laboratories). Minimum level of detection (MLD) for the cytokines (pg/mL) was 0.5 for IL-10 and 1.5 for IFN-γ. Undetectable cytokine levels were assigned a value of ½ of the MLD for determination of geometric mean values.
Upon completion of the study visits, paired pre- and post-vaccination samples of cryopreserved PBMCs were thawed, tested for viability (by trypan blue dye exclusion), with live cells then adjusted in number and stimulated by incubation with live A/H3N2 virus, as above. For the last 6 hours of incubation, samples were treated with the secretion inhibitor brefeldin to permit identification of effector molecule production at the single cell level by intracellular flow cytometry as described previously.Citation29 Aliquots of stimulated and unstimulated cells from pre- and post-immunization samples of the same subject were studied side-by-side. During the cytometric analysis, CD3+ T lymphocytes were further divided on the basis of surface marker expression for CD4 and CD8. Within each of the CD4+ and CD8+ T cell subsets, the percentage of cells expressing intracellular effector molecules such as IFN-γ, interleukin 2 (IL-2), GrB or perforin was then identified by immunocytochemistry (ICC). We also assessed if cells were able to express more than one of the effector molecules (polyfunctionality) such as the dual presence of GrB and perforin.
Vaccine internal protein quantification
Two vaccines were tested (split-virus and subunit TIV), corresponding with the products used in the trial but produced for the 2014-2015 season. Strains included in the vaccines were A/California/7/2009 (H1N1) pdm09-like, A/Texas/50/2012 (H3N2)-like and B/Massachusetts/2/2012-like. All vaccine samples were prepared in triplicate as follows: vaccine samples were solubilized using RapiGest and digested for 2 hrs at 37°C using an excess amount of sequencing grade trypsin. Each sample was spiked with a standard protein before digestion to enable absolute protein quantification. Following proteolysis, the prepared vaccine sample was analyzed using a C-18 reversed phase capillary column directly coupled to a mass spectrometer. A Thermo Orbitrap™ Fusion™ Tribrid mass spectrometer (Milford, MA, USA) was operated in Data Dependent ‘Top Speed' mode to collect the MS and MS/MS data. Progenesis QI for Proteomics software was used to identify peptides associated with the nucleoproteins and matrix proteins in the vaccine samples by searching against an in-house custom influenza database. The proteins were then quantified by integrating the mass spectral response from the 3 most abundant peptides for each protein and comparing to those of the internal standard. Results were expressed in ug/mL of vaccine.
Statistical analysis
Group size was set at 30 participants per group , based on locally achievable enrolment numbers and previously validated power calculations for GrB levels in influenza-challenged PMBC, which demonstrated that 12-18 subjects per group were needed to detect a 25% difference in GrB levels.Citation24
Groups were compared for participant characteristics and completion of the relevant visits. In each study group, responses of fresh PBMCs to virus stimulation were compared between baseline and post-vaccination samples by calculating and comparing the geometric mean concentrations (GMC) and 95% confidence intervals, for each measured effector. We calculated mean GrB activity in lysates of influenza-stimulated PBMC. In addition, the IFN-γ:IL-10 ratio was calculated and compared among groups. To analyze the effect of the pre and post-immunization time points and different vaccine groups, one-way ANOVA test was applied. For analysis of the flow cytometry data, the geometric mean percentage (GMP) of CD4+ and CD8+ cells expressing the targeted effector molecules was calculated for each vaccine group, with 95% confidence intervals. Paired t-tests were used to compare pre- and post-immunization data within each group of individuals. Forcomparisons spanning the 4 vaccine groups, one-way ANOVA tests were applied. Statistical significance level was set as α = 0.005 to provide a Bonferroni-like adjustment for multiple comparisons. Statistical analyses were performed using SAS 9.3; graphs were created using R 3.2.1.
Disclosure of potential conflicts of interest
Janet McElhaney has participated on advisory boards for GlaxoSmithKline, Sanofi Pasteur and Pfizer and on data monitoring boards for Sanofi Pasteur; she has participated in clinical trials sponsored by GlaxoSmithKline and has received honoraria and travel and accommodation reimbursements for presentations sponsored by Merck, GlaxoSmithKline, Sanofi Pasteur and Pfizer, and travel reimbursement for participation on a publication steering committee for GlaxoSmithKline. Scott Halperin has served on ad hoc advisory boards for Sanofi Pasteur, GlaxoSmithKline and Novartis Vaccines and has received funding for undertaking clinical trials from all 3 companies. Arun Kumar, Tobias Kollmann, David Scheifele, Lisa Walrond, Terry D. Cyr, and Shahzma Merani report no funding from commercial sources in the past 3 y or other commercial interests.
Authors' contributions
DS, JM, SH developed the protocol and secured funding. JM and DS oversaw the clinical activities. JM, TK, SM, LW and TDC performed the laboratory analyses. DS oversaw data assembly and analysis. AK and SM assisted with data analysis and interpretation. All authors contributed to development of the manuscript and approved the final version.
Acknowledgments
We gratefully acknowledge the excellent support of the staff of the Vaccine Evaluation Center in Vancouver including Ed Fortuno, Carol LaJeunesse, Bing Cai and Shu Yu Fan. We additionally want to thank the staff at the VITALiTY Research Center and the VCHRI Clinical Research Unit including Gale Tedder, Lynn Cunada, and Connie Feschuk for their outstanding commitment to study coordination, recruitment, and vaccine administration; and Dominica Kwok, Stephanie Hughes, and Jee Lee for their excellent technical assistance.
Funding
This study was funded by a grant from the PHAC/CIHR Influenza Research Network (PCIRN). Additional funding for the laboratory analyses was provided by Sanofi Pasteur and Novartis Vaccines, which also supplied the vaccines used in the study.
References
- Bernstein E, Kaye D, Abrutyn E, Gross P, Dorfman M, Murasko DM. Immune response to influenza vaccination in a large healthy elderly population. Vaccine 1999; 17:82-94; PMID:10078611; https://doi.org/10.1016/S0264-410X(98)00117-0
- Murasko DM, Bernstein ED, Gardner EM, Gross P, Munk G, Dran S, Abrutyn E. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol 2002; 37:427-39; PMID:11772530; https://doi.org/10.1016/S0531-5565(01)00210-8
- McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev 2011; 10:379-88; PMID:21055484; https://doi.org/10.1016/j.arr.2010.10.008
- McElhaney JE, Effros RB. Immunosenescence: What does it mean to health outcomes in older adults? Curr Opin Immunol 2009; 21:418-24; PMID:19570667; https://doi.org/10.1016/j.coi.2009.05.23
- McElhaney JE, Xie D, Hager WD, Barry MB, Wang Y, Kleppinger A, Ewen C, Kane KP, Bleackley RC, Mcelhaney JE, et al. T cell responses are better correlates of vaccine protection in the elderly 1. J Immunol 2006; 176:6333-9; PMID:16670345; https://doi.org/10.4049/jimmunol.176.10.6333
- McElhaney JE, Ewen C, Zhou X, Kane KP, Xie D, Hager WD, Barry MB, Kleppinger A, Wang Y, Bleackley RC. Granzyme B: Correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine 2009; 27:2418-25; PMID:19368783; https://doi.org/10.1016/j.vaccine.2009.01.136
- Goronzy JJ, Fulbright JW, Crowson CS, Poland G a, O'Fallon WM, Weyand CM. Value of immunological markers in predicting responsiveness to influenza vaccination in elderly individuals. J Virol 2001; 75:12182-7; PMID:11711609; https://doi.org/10.1128/JVI.75.24.12182-12187.2001
- Saurwein-Teissl M, Lung TL, Marx F, Gschösser C, Asch E, Blasko I, Parson W, Böck G, Schönitzer D, Trannoy E, et al. Lack of antibody production following immunization in old age: Association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol 2002; 168:5893-9; PMID:12023394; https://doi.org/10.4049/jimmunol.168.11.5893
- Effros RB. Role of T lymphocyte replicative senescence in vaccine efficacy. Vaccine 2007; 25:599-604; PMID:17014937; https://doi.org/10.1016/j.vaccine.2006.08.032
- Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine 2010; 28:6145-51; PMID:20646987; https://doi.org/10.1016/j.vaccine.2010.07.036
- Wilkinson TM, Li CK., Chui CSC, Huang AKY, Perkins M, Liebner JC, Lambkin-Williams R, Gilbert A, Oxford J, Nicholas B, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med [Internet] 2012; 18:276-82; PMID:22286307; https://doi.org/10.1038/nm.2612
- Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, Bean T, Barclay W, Deeks JJ, Lalvani A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med [Internet] 2013; 19:1305-12; PMID:24056771; https://doi.org/10.1038/nm.3350
- Mosterín Höpping A, McElhaney J, Fonville JM, Powers DC, Beyer WEP, Smith DJ. The confounded effects of age and exposure history in response to influenza vaccination. Vaccine 2016; 34:540-6; PMID:26667611; https://doi.org/10.1016/j.vaccine.20105.11.058
- Jameson J, Cruz J, Terajima M, Ennis FA. Human CD8+ and CD4+ T lymphocyte memory to influenza a viruses of swine and avian species. J Immunol [Internet] 1999; 162:7578-83; PMID:10358215
- Wang M, Lamberth K, Harndahl M, Røder G, Stryhn A, Larsen MV, Nielsen M, Lundegaard C, Tang ST, Dziegiel MH, et al. CTL epitopes for influenza a including the H5N1 bird flu; genome-, pathogen-, and HLA-wide screening. Vaccine 2007; 25:2823-31; PMID:17254671; https://doi.org/10.1016/j.vaccine.2006.12.038
- Grant EJ, Quiñones-Parra SM, Clemens EB, Kedzierska K. Human influenza viruses and CD8(+) T cell responses. Curr Opin Virol [Internet] 2016; 16:132-42; PMID:26974887; https://doi.org/10.1016/j.coviro.2016.01.016
- Co M, Orphin L, Cruz J, Pazoles P, Green K, Potts J, Leporati A, Babon J, Evans J, Ennis F, et al. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine [Internet] 2009; 27:319-27; PMID:18977404; https://doi.org/10.1016/j.vaccine.2008.09.092
- Couch RB, Bayas JM, Caso C, Mbawuike IN, Lopez CN, Claeys C, El Idrissi M, Herve C, Laupeze B, Oostvogels L, et al. Superior antigen-specific CD4+ T-cell response with AS03-adjuvantation of a trivalent influenza vaccine in a randomised trial of adults aged 65 and older. BMC Infect Dis 2014; 14:425; PMID:25078387; https://doi.org/10.1186/1471-2334-14-425
- Moris P, van der Most R, Leroux-Roels I, Clement F, Dramé M, Hanon E, Leroux-Roels GG, Van Mechelen M. H5N1 influenza vaccine formulated with AS03(A) induces strong cross-reactive and polyfunctional CD4 T-cell responses. J Clin Immunol [Internet] 2011; 31:443-54; PMID:21174144; https://doi.org/10.1007/s10875-010-9490-6
- Nougarede N, Bisceglia H, Rozières A, Goujon C, Boudet F, Laurent P, Vanbervliet B, Rodet K, Hennino A, Nicolas JF. Nine µg intradermal influenza vaccine and 15 μg intramuscular influenza vaccine induce similar cellular and humoral immune responses in adults. Hum Vaccines Immunother 2014; 10:2713-20; PMID:25483667; https://doi.org/10.4161/hv.29695
- Scheifele DW, McNeil SA, Ward BJ, Dionne M, Cooper C, Coleman B, Loeb M, Rubinstein E, McElhaney J, Hatchette T, et al. Safety, immunogenicity, and tolerability of three influenza vaccines in older adults: Results of a randomized, controlled comparison. Hum Vaccin Immunother 2013; 9:2460-73; PMID:23839537 ; https://doi.org/10.4161/hv.25580
- Wagar LE, Gentleman B, Pircher H, McElhaney JE, Watts TH. Influenza-specific T cells from older people are enriched in the late effector subset and their presence inversely correlates with vaccine response. PLoS One [Internet] 2011; 6:e23698; PMID:21887299; https://doi.org/10.1371/journal.pone.0023698
- Del Giudice G, Rappuoli R. Inactivated and adjuvanted influenza vaccines. Curr Top Microbiol Immunol 2015; 386:151-80; PMID:25038938; https://doi.org/10.1007/82.2014.406
- Haq K, Fulop T, Tedder G, Gentleman B, Garneau H, Meneilly GS, Kleppinger A, Pawelec G, McElhaney JE. Cytomegalovirus seropositivity predicts a decline in the t cell but not the antibody response to influenza in vaccinated older adults independent of type 2 diabetes status. J Gerontol A Biol Sci Med Sci 2016; 00:1-8; PMID:27789617; https://doi.org/10.1093/gerona/glw216
- McElhaney JE, Zhou X, Talbot HK, Soethout E, Bleackley RC, Granville DJ, Pawelec G. The unmet need in the elderly: How immunosenescence, CMV infection, co-morbidities and frailty are a challenge for the development of more effective influenza vaccines. Vaccine 2012; 30:2060-7; PMID:22289511; https://doi.org/10.1016/j.vaccine.2012.01.015
- Zhou X, McElhaney JE. Age-related changes in memory and effector T cells responding to influenza A/H3N2 and pandemic A/H1N1 strains in humans. Vaccine 2011; 29:2169-77; PMID:22289511; https://doi.org/10.1016/j.vaccine.2012.01.015
- Skowronski DM, Hottes TS, McElhaney JE, Janjua NZ, Sabaiduc S, Chan T, Gentleman B, Purych D, Gardy J, Patrick DM, et al. Immuno-epidemiologic correlates of pandemic H1N1 surveillance observations: Higher antibody and lower cell-mediated immune responses with advanced age. J Infect Dis 2011; 203:158-67; PMID:21288814; https://doi.org/10.1093/infdis/jiq039
- McElhaney JE, Beran J, Devaster JM, Esen M, Launay O, Leroux-Roels G, Ruiz-Palacios GM, van Essen GA, Caplanusi A, Claeys C, et al. AS03-adjuvanted versus non-adjuvanted inactivated trivalent influenza vaccine against seasonal influenza in elderly people: A phase 3 randomised trial. Lancet Infect Dis 2013; 13:485-96; PMID:23518156; https://doi.org/10.1016/S1473-3099(13)70046-X
- Blimkie D, Fortuno ES, Yan H, Cho P, Ho K, Turvey SE, Marchant A, Goriely S, Kollmann TR. Variables to be controlled in the assessment of blood innate immune responses to Toll-like receptor stimulation. J Immunol Methods 2011; 366:89-99; PMID:21277305; https://doi.org/10.1016/j.jim.2011.01.009
- Smolen KK, Gelinas L, Franzen L, Dobson S, Dawar M, Ogilvie G, Krajden M, Fortuno ES, Kollmann TR. Age of recipient and number of doses differentially impact human B and T cell immune memory responses to HPV vaccination. Vaccine 2012; 30:3572-9; PMID:22469863; https://doi.org/10.1016/j.vaccine.2012.03.051
- Behzad H, Huckriede ALW, Haynes L, Gentleman B, Coyle K, Wilschut JC, Kollmann TR, Reed SG, McElhaney JE. GLA-SE, a synthetic toll-like receptor 4 agonist, enhances T-cell responses to influenza vaccine in older adults. J Infect Dis 2012; 205:466-73; PMID:22147791; https://doi.org/10.1093/infdis/jir769
