ABSTRACT
Influenza A and B viruses are responsible for respiratory infections, representing globally seasonal threats to human health. The 2 viral types often co-circulate and influenza B plays an important role in the spread of infection.
A 6-year retrospective surveillance study was conducted between 2010 and 2016 in 2 large administrative regions of Italy, located in the north (Liguria) and in the south (Sicily) of the country, to describe the burden and epidemiology of both B/Victoria and B/Yamagata lineages in different healthcare settings.
Influenza B viruses were detected in 5 of 6 seasonal outbreaks, exceeding influenza A during the season 2012–2013. Most of influenza B infections were found in children aged ≤ 14 y and significant differences were observed in the age-groups infected by the different lineages. B/Victoria strains prevailed in younger population than B/Yamagata, but also were more frequently found in the community setting. Conversely, B/Yamagata viruses were prevalent among hospitalized cases suggesting their potential role in the development of more severe disease.
The relative proportions of viral lineages varied from year to year, resulting in different lineage-level mismatch for the B component of trivalent influenza vaccine.
Our findings confirmed the need for continuous virological surveillance of seasonal epidemics and bring attention to the adoption of universal influenza immunization program in the childhood. The use of tetravalent vaccine formulations may be useful to improve the prevention and control of the influenza burden in general population.
Introduction
Influenza A and B are major causes of respiratory infections in human and contribute to increase morbidity and mortality globally.Citation1-6 The clinical presentation of influenza A seems to be comparable to that of influenza B;Citation7,Citation8 this latter, like influenza A, can lead to severe complications and death in both pediatric and adult populations.Citation9-11 Despite similar clinical phenotypes, the 2 viral types appear considerably dissimilar in their propensity for genetic reassortment as a consequence of differences in the corresponding host reservoirs.
In fact, influenza A viruses have been isolated from various species including humans and this have contributed to viral heterogeneity, generating various subtypes which have the potential to cause human pandemics.Citation12
Conversely, influenza B virus is supposed to have evolved almost exclusively as human pathogen and this has limited the generation of new strains by reassortment, leading to scarse pandemic potential, although its presence has been confirmed in throat swab obtained from seal,Citation13,Citation14 and detected in nasal swabs from domestic pigs by real-time reverse transcription PCR and sequencing.Citation15
Influenza B viruses are not formally classified into subtypes. However, 2 antigenically and genetically distinct major lineages are universally recognized, which evolved since 1983 from the first isolate B/Lee/40,16 actually referring to B/Victoria/2/87 and B/Yamagata/16/88 strains, henceforth termed the Victoria and Yamagata lineages, respectively.
From a public health point of view, vaccination is the primary measure to prevent influenza and reduce its impact in the population. For decades, licensed trivalent seasonal influenza vaccines have contained 2 type A strains (A/H1N1 and A/H3N2) and one of the 2 known divergent influenza B lineages, which have also been shown to circulate simultaneously. In this context, it has represented a challenge in terms of vaccine efficacy and effectiveness, because of the limited cross protection between the 2 influenza B lineages,Citation17,18 and the degree of mismatch of seasonal vaccines in respect to circulating influenza B virus strains.Citation19,Citation20 These factors make difficult the yearly production of influenza vaccines and force to continuously update the correct B component, determining an increasing interest in production of quadrivalent vaccines that include both antigenic variants of influenza B viruses. Nevertheless, the potential benefits afforded by these vaccines, in terms of reduced burden and outcomes of seasonal influenza illness, are still hampered by different reasons which prevent their adoption to a large-scale context.
Data regarding the circulation in Italy of influenza B strains belonging to different lineages are quite sparse and limited.Citation21-24 The present retrospective surveillance study aimed to improve the knowledge of the burden and epidemiology of influenza B during annual outbreaks among patients with influenza-like illness (ILI) in either community or hospital contexts over the period 2010–2016. The mismatch ratio between seasonal circulating and vaccine included influenza B strains was also analyzed.
Results
Epidemiology of influenza viruses between 2010 and 2016
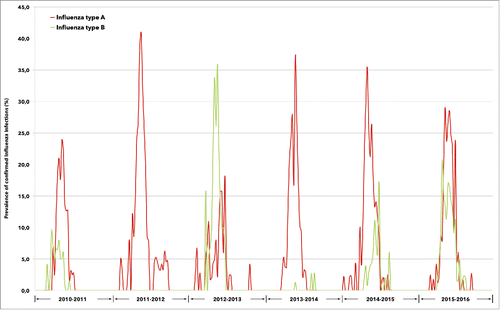
During the 6-year study period, influenza A and B viruses co-circulated in almost all seasonal epidemics, with the exclusion of the season 2011–2012 when influenza B was not detected at all in the regions included in the study. Influenza A strains exceeded influenza B in 4 seasons (2010–2011, 2013–2014, 2014–2015, and 2015–2016), with different timing of peak activity, while influenza B viruses significantly prevailed for most of the season 2012–2013 ().
Figure 1. Prevalence of confirmed influenza A and B infections between 2010 and 2016, according to annual epidemic.
A total of 14,212 specimens were collected and laboratory tested from subjects with ILI symptoms (). Despite the natural fluctuation in ILI incidence rates reported in Italy between 2010 and 2016, the total number of respiratory samples collected in Liguria and Sicily, for influenza detection and genotyping, progressively increased year after year through a significant improvement of the virological surveillance system.
Table 1. Number of specimens tested, influenza cases, and relative percentages attributable to influenza A and B virus subtypes. Period: 2010-2016.
Overall, 13.2% (n = 1,874/14,212) were confirmed influenza cases, of which 70.2% (n = 1,315/1,874) and 29.8% (n = 559/1,874) were influenza A and B infections, respectively.
As described in , roughly 3 quarters of identified influenza B cases (n = 422/559) were from general population (community-based infections), sampled by family practitioners during outpatient visits, whereas 24.5% (n = 137/559) were from hospitalized patients; no gender differences were found (data not shown).
Table 2. Age distribution of influenza B infections, according to community and hospital settings. Period: 2010-2016.
Influenza B infection was widely distributed between age-groups, with a clear predominance in children and teenagers; the median age was 9.0 y and, altogether, subjects aged ≤ 14 y sustained more than 60% of total influenza B infections, recording the highest prevalence in age-group 5–9 y.
Community-based B infections were found in subjects substantially younger than hospitalized patients (median age, years: 8 vs. 51; p < 0.001), and a comparison of age-stratified prevalences showed a significant inverse correlation between the 2 healthcare settings (Figure S1).
Influenza B lineage information was collected during the entire study period. Basing on subtyping pooled data available from the 2 regions, Victoria-lineage viruses were more represented than those belonging to the Yamagata-lineage over the entire study period, accounting for 59.8% (n = 298/498) and 40.2% (n = 200/498) of cases, respectively ().
Table 3. Proportion of influenza B infections, according to viral lineage (pooled data from Liguria and Sicily). Period 2010-2016.
On average, individuals infected with Victoria-lineage viruses were significantly younger than those presenting a Yamagata-lineage infection (median age, years: 8.0 vs. 12.0; p < 0.001), reflecting the age-distribution observed among total B cases.
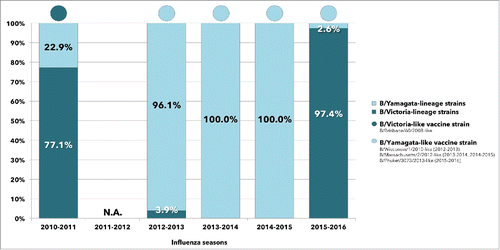
Both influenza B lineages contributed to the 2010–2016 epidemics. Victoria-lineage strains predominated in the seasons 2010–2011 (77.1% vs. 22.9%) and 2015–2016 (97.4% vs. 2.6%), while in 3 consecutive seasonal outbreaks between 2012 and 2015, almost all infections were sustained by viruses belonging to the Yamagata-lineage.
On the whole, according to the different population groups collected in the 2 regions, the proportion of influenza B found in the community was consistently higher than that observed among hospitalized patients. Nevertheless, the relative frequencies of the 2 lineages varied between healthcare settings, showing a wider spread of Victoria strains in the general population (63.9% vs. 36.1% for Victoria- and Yamagata-lineage, respectively), than that documented in ILI subjects with severe respiratory symptoms requiring an hospital admission (37.7% vs. 62.3% for Victoria- and Yamagata-lineage, respectively). In our study, a Yamagata-lineage infection represented an independent risk factor for a complicated influenza outcome (hospital-based vs. community-based management, OR = 2.95; 95%CI: 1.78 - 4.67).
Finally, for each season, the extent of lineage-level mismatch between influenza B strains circulating in our geographic areas and those included in vaccine formulation for the Northern Hemisphere was evaluated.
During the 6 post pandemic seasons reported in the present study, the degree of mismatch was quite similar, when observed at the regional level. Pooled data depicted in , documented a low degree of B mismatch in 5 consecutive seasons (range: 0–22.9%) between 2010 and 2015, while the circulation of B viruses belonging to the opposite lineage of the vaccine strain was exceptionally high (97.4%) during the last analyzed season (2015–2016).
Discussion
In this study, we analyzed the epidemiology and seasonal patterns of 559 influenza B infections identified among 14,212 subjects with ILI symptoms monitored during the surveillance seasons 2010–2016 in Liguria and Sicily, 2 Italian administrative regions located in the northern part and in the southern part of the country, where the influenza virological surveillance is seasonally performed as part of the national network (InfluNet).
Our findings revealed that types A and B influenza viruses almost always co-circulated throughout the study period and confirmed the important role of influenza type B virus in the spread of infection in the population.
On average, influenza B viruses accounted for 29.8% of total laboratory confirmed infections, and it was in the range of other European countries such as FinlandCitation18 and UK.Citation6,Citation25 In our setting, influenza B cases followed year-to-year fluctuations in prevalence and the highest value was observed in 2012–2013 (67.8%), a season characterized by a significant influenza B activity in the whole European region.Citation26
It has been widely highlighted the role of influenza disease as a determinant of excess mortality in the elderly,Citation27-29 irrespective of genotype and subtype.
On the other hand, children and adolescents have been shown to be crucial in the spread of the virus in the community, experiencing some of the highest rates of influenza infection during seasonal epidemicsCitation18,Citation30 and, in this regard, it has been suggested that influenza B viruses could be transmitted with a higher reproductive number (R0) in younger age population.Citation31
It is well known that influenza type B, when present as seasonal circulating virus within a geographic area, mainly occurs among younger persons than influenza A and school-aged children reflect the highest proportion of influenza B cases,Citation18,Citation19,Citation25,Citation32-34 as further confirmed in the present study among children aged 5–9 y.
In accordance, Harvala and colleaguesCitation6 revealed higher rates of influenza B detections in Scottish children under the age of 5 y during the season 2012–2013, although a considerable circulation of B strains were also observed in adults.
In Shanghai, the age distribution of B infections documented between 2009 and 2014 was higher among young outpatients (6–17 years) seeking hospital medical care for ILI,Citation33 and similar findings were reported in a population study conducted in Southern China between 2009 and 2010.Citation35
Our results evidenced that ILI subjects infected by influenza B, in the context of the general population, were significantly younger than those admitted to hospital and an inverse correlation was found between the 2 healthcare settings, by comparison of age-stratified prevalences. Although, to our knowledge, no direct comparisons between the 2 population groups have been previously reported in the literature, studies conducted either in the general populationCitation36 or in the hospital settingCitation6,Citation35 highligthed, on average, an older age of patients in this latter group. According to other authors, no correlation was observed between influenza B infection and gender.Citation34,Citation37,Citation38
During the study period, different patterns of Yamagata- and Victoria-lineage B viruses were observed, albeit with similar trend between the 2 Italian regions. The distinct evolutionary viral variants of influenza B spread as single lineage or co-circulated among each season.
The 2010–2011 influenza outbreak demonstrated the co-circulation of both B lineages in our geographic areas, but with the preponderance of Victoria-lineage viruses, and a similar scenario was depicted in EuropeCitation39 and elsewhere worldwide, with the exception of China where Yamagata-lineage strains predominated.Citation40 Conversely, Victoria-lineage viruses were almost completely replaced by influenza strains belonging to the alternative lineage in the season 2012–2013, reflecting the trend obsderved in other European countries,Citation6,Citation41 as well as in the Southern Hemisphere.Citation42 Notably, a lineage swap was documented in Italy since 2013Citation43 and this epidemiological feature of B lineages was also evidenced in other countries such as Malaysia.Citation44
Moreover, an heterogenous distribution has been reported by age-group. Some authors from China and Malaysia as well as from EuropeCitation35,Citation38,Citation44 revealed stark differences in age among B infected patients, reporting a trend toward a higher proportion of Victoria-lineage sustained infections in children and teenagers than those caused by either Yamagata-lineage viruses, suggesting an intrinsic different transmissibility of the 2 lineages.Citation42 Nevertheless, a limited variation in age susceptibility to different influenza B variants was found in Australia,Citation36 while Mosnier and co-authors,Citation34 in France, and Harvala and colleagues,Citation6 in Scotland, did not find any relationship.
In light of published data, the potential association between viral lineage and age of infected patients is still debated. It may be likely correlated with the local epidemiology of specific geographic region, as a result of a difference in background population immunity. Interestingly, Vijaykrishna and colleaguesCitation42 recently proposed that age difference between Victoria- and Yamagata-lineage infections is thought to be due to differences in the molecular aspects of cellular dynamics which help the viruses to infect the epithelium of the respiratory tract, while more attractive hypotheses are consistent with a higher basic reproductive number (R0) of the Victoria-lineage viruses, which altogether might reduce the mean age of lineage-specific infections.
The distribution of Victoria- and Yamagata-lineage strains differed by healthcare setting. Victoria-lineage viruses were mostly responsible for a milder influenza disease in the general population, while a greater proportion of infections detected in hospital were sustained by viruses belonging to the Yamagata-lineage. Of note, this was not biased by age, suggesting that Yamagata-lineage viruses on average could be responsible of more complicated infections. However, the limited number of patients with these characteristics in our data set and the potential bias due to the different population characteristics and surveillance systems in the 2 regions prevent us from drawing conclusions.
Finally, our findings evidenced that the level of B vaccine mismatch varied during the 6 seasons, with the highest impact observed in 2015–2016.
In a study by Heikkinen et al. (2014) conducted in Finland, a similar proportion of lineage-level mismatched B viruses was observed, although over a different time slot that partially overlapped our study period; in Australia, a mismatch >60% occurred in over one-third seasons between 2001 and 2014,Citation36 while a 10-year influenza surveillance conducted in Northern ItalyCitation23 highlighted the occurrence of B vaccine mismatch in 5 seasons between 2004 and 2014.
The potential impact of vaccine-mismatch on influenza virus epidemiology has been broadly investigated and several findings highlighted the effect of seasonal vaccine mismatch on influenza epidemiology, particularly among those age-groups that preferentially sustain the circulation of influenza B virus. The lack of availability of vaccination data in our study population limit our possibility to draw any inference on this topic. However, as reported by other authors, the magnitude of the impact of seasonal vaccine mismatch on influenza epidemiology depends on several factors, including the annual effectiveness of the vaccine and the annual population vaccine coverage, but also on factors such as the overall burden of influenza during a given season and the proportion of each influenza B virus lineage circulating.Citation28
On the basis of the results shown in this and other studies, it is evident how much important could be the impact of B vaccine mismatch in terms of efficacy of trivalent influenza vaccines in general population, given the global impact of influenza B, the undemonstrated cross-reactivity of trivalent vaccines against the 2 influenza B lineages and, more importantly, the unability to predict the seasonal epidemiology of influenza viruses. It seems quite clear that the real-life efficacy of influenza vaccines could be significant improved by a broader adoption of quadrivalent formulations, expecially in children.Citation18,Citation28,Citation45
Furthermore, it seems a logical consequence that children and adolescents might benefit most from the implementation of specific vaccine-based preventive measures, which may have the potential to reduce the burden of disease in both vaccinated and unvaccinated individuals.
Additionally, benefits may also include contraction in absenteeism due to the need for parents to take time of work to care for sick children,Citation46,47 and reduced pressure on health care services during seasonal peak in influenza activity.Citation48,Citation49
Nevertheless, despite previous assumptions, only USA and Canada among large developed countries, and some rare exceptions in Europe such as Finland, Latvia and United Kingdom, actually recommend the influenza vaccination of healthy children providing the vaccine free of charge.Citation50
Resistance to implementing vaccination programmes on healthy children may found possible explanations in the limited evidence for the field efficacy of inactivated and live attenuated vaccines in younger children, among whom the risk of complication is the greatest,Citation51,Citation52 the level of uptake which programmes would be able to achieve,Citation53 and the additional resources required to expand seasonal influenza vaccination campaigns.
Of course this study suffer of some limitations. First, only 2 Italian regions were included in the virological surveillance campaign and the formal representativeness of this population is unknown. Moreover, different population settings, either from the Northern part or the Southern part of Italy, contributed to the population study and this may have locally biased the results. However, basing on the reports from the National surveillance network, we are reasonably confident that, on average, our settings have adequately represented the epidemiology and burden of influenza B strains in Liguria and Sicily during the 6 seasons studied.
The unpredictability of influenza viruses continues to represent a major challenge to health systems. Nevertheless, vaccination remains the most effective preventive measure in reducing the incidence and severity of disease, although the coverage rates in ItalyCitation54 and other European countriesCitation55 remain suboptimal. In particular, during the study period, coverage rates for influenza vaccination decreased at Italian level from 62.4% to 49.9% and from 17.9% to 13.9% in subjects aged ≥ 65 y and in general population, respectively, and a similar trend was registered in the 2 considered Italian regions.Citation54
The increased use of childhood vaccination is an opportunity for reducing the considerable burden of infection in this age group and it may play a pivotal role in the spread of the virus in the community, also supported by the adoption of quadrivalent vaccines in universal immunization programmes against influenza.
Materials and methods
Case definition
The enrollment criteria for surveillance cases were in accordance to the operative protocol of the Italian Influenza Epidemiological and Virological Surveillance Network.Citation56 A case of ILI was defined as one individual with sudden onset of at least one of the following systemic symptoms: fever (≥ 37.5°C), general discomfort or asthenia, headache, muscle pain, and at least one of the respiratory symptoms between cough, sore throat, and shortness of breath.
Study population and retrospective data collection
We analyzed all available clinical and virological data, collected during 6 consecutive post-pandemic influenza seasons over the period 2010–2016, from week 42 to week 17 of the following year, in 2 different Italian administrative regions located in the South (Sicily) and in the North (Liguria) of the country, where 2 regional reference laboratories for the influenza surveillance belonging to the InfluNet have been active for all the study period.
All surveillance data are aggregated at a regional level, shared at a national level on a weekly basis, and ultimately flow into the World Health Organization's (WHO) global influenza program.
Each year, several pediatricians and general practitioners contribute to the community-based influenza surveillance, while both pediatric and adult hospitals allow the monitoring of ILI patients admitted with severe respiratory distress, potentially correlated with influenza infection.
More specifically, the Ligurian influenza surveillance system essentially collected data from both hospital inpatients and outpatients (99.0%; n = 11,008/11,120), while the influenza virological surveillance in Sicily resulted mostly oriented to general population (70.2%; n = 2,172/3,092).
Anonymised data on birthdate, sex, date of ILI onset, outcomes were gathered from each ILI cases.
Routine testing and influenza virus genotyping
Oropharyngeal samples were obtained from each patient and transported to the regional reference laboratories by using Virocult swabs (MWE, Medical Wire).
Viral RNA was extracted using QIAamp Viral RNA extraction kit (QIAGEN) according to the manufacturer's suggested protocol and the RNA was eluted from the spin column in 60 μL of elution buffer. Eluted RNA was divided into aliquots and stored immediately at -80°C until further use. Each sample was tested by one-step real-time RT-PCR for the presence of influenza virus RNA (protocols available on request), and influenza B positive samples were genotyped using lineage-specific multiplex one-step real-time RT-PCR according the “WHO protocols for molecular diagnosis of influenza virus”Citation57 using a QuantStudio 7 Flex Real-Time PCR system (Applied Biosystem).
Seasonal mismatch with trivalent vaccine B lineage
Data on the circulation of different lineages of influenza B viruses in Sicily and Liguria were retrieved during each season.
The extent of vaccine mismatch against influenza B viruses was estimated by comparing the information concerning the B lineage antigens contained in the trivalent vaccine for the Northern Hemisphere, as recommended by the WHO, and the proportion of circulating B viruses belonging to different lineages.
The level of B vaccine mismatch was defined as the percentage ratio between the proportions of mismatched and matched influenza B viruses per year of surveillance.
Data managements and statistical analysis
Descriptive statistics were used to summarize each of the socio-demographic and clinical variables included in the data set (counts, percentages, median and interquartile range, as appropriate).
The study population was arbitrarily subdivided into 9 different age groups, categorizing children/teenagers into 4 groups (≤ 4, 5–9, 10–14, and 15–19 years) and adults/elderly into 5 groups (20–34, 35–49, 50–64, 65–85, and >85 years).
Median values were compared using the Mann-Whitney U test. All of the analyses with p-values of 0.05 or less were considered to be statistically significant (2 tailed). Data were processed with the STATA MP statistical software package v14.1 for Apple™ (StataCorp).
Abbreviation
| ILI | = | Influenza-like illness |
Disclosure of potential conflicts of interest
The authors declare that they have no conflict of interest.
Authors’ contributions
AO and FT were involved in the conception and design of the studies. AO, GMEC, FP, GC, CA, CT, and PC collected clinical and epidemiological data. AO and FT analyzed and interpreted the results. AO, FT, FV, and FA were involved in drafting the manuscript or revising it critically for important intellectual content. All authors had full access to the data and approved the manuscript before it was submitted by the corresponding author.
Supplemental_Material.zip
Download Zip (64.9 KB)References
- Fischer WA 2nd, Gong M, Bhagwanjee S, Sevransky J. Global burden of influenza as a cause of cardiopulmonary morbidity and mortality. Glob Heart 2014; 9(3):325-36; PMID:25667184; doi: 10.1016/j.gheart.2014.08.004.
- Khieu TQ, Pierse N, Telfar-Barnard LF, Huang QS, Baker MG. Estimating the contribution of influenza to hospitalisations in New Zealand from 1994 to 2008. Vaccine 2015; 33(33):4087-92 pii: S0264-410X(15)00895-6; PMID:26143611; https://doi.org/10.1016/j.vaccine.2015.06.080
- Paul Glezen W, Schmier JK, Kuehn CM, Ryan KJ, Oxford J. The burden of influenza B: a structured literature review. Am J Public Health 2013; 103(3):e43-51; PMID:23327249; doi: 10.2105/AJPH.2012.301137
- Savy V, Ciapponi A, Bardach A, Glujovsky D, Aruj P, Mazzoni A, Gibbons L, Ortega-Barría E, Colindres RE. Burden of influenza in Latin America and the Caribbean: a systematic review and meta-analysis. Influenza Other Respir Viruses 2013; 7(6):1017-32; PMID:23210504; doi: 10.1111/irv.12036
- Haas J, Braun S, Wutzler P. Burden of influenza in Germany: a retrospective claims database analysis for the influenza season 2012/2013. Eur J Health Econ 2016; 17(6):669-79; PMID:26143025; doi: 10.1007/s10198-015-0708-7
- Harvala H, Smith D, Salvatierra K, Gunson R, von Wissmann B, Reynolds A, Frew C, MacLean A, Hunt A, Yirrell D, et al. Burden of influenza B virus infections in Scotland in 2012/13 and epidemiological investigations between 2000 and 2012. Euro Surveill 2014; 19(37)20903; PMID:25259532; https://doi.org/10.2807/1560-7917.ES2014.19.37.20903.
- Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, Belongia EA. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004-2005 through 2007-2008. Influenza Other Respir Viruses 2012; 6(1):37-43; PMID:21668663; https://doi.org/10.1111/j.1750-2659.2011.00263.x
- Cohen JM, Silva ML, Caini S, Ciblak M, Mosnier A, Daviaud I, Matias G, Badur S, Valette M, Enouf V, et al. Striking Similarities in the Presentation and Duration of Illness of Influenza A and B in the Community: A Study Based on Sentinel Surveillance Networks in France and Turkey, 2010-2012. PLoS One 2015; 10(10):e0139431; PMID:26426119; https://doi.org/10.1371/journal.pone.0139431
- Gutiérrez-Pizarraya A, Pérez-Romero P, Alvarez R, Aydillo TA, Osorio-Gómez G, Milara-Ibáñez C, Sánchez M, Pachón J, Cordero E. Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J Infect 2012; 65(5):423-30; PMID:22820034; https://doi.org/10.1016/j.jinf.2012.07.004
- Moon JH, Na JY, Kim JH, Yum MK, Oh JW, Kim CR, Seol IJ. Neurological and muscular manifestations associated with influenza B infection in children. Pediatr Neurol 2013;49(2):97-101; PMID:23859854; https://doi.org/10.1016/j.pediatrneurol.2013.04.004.
- Ak Ö, Biteker F, Cag Y, Öcal G, Benzonana N, Ciblak MA, Özer SJ. Influenza B-associated encephalopathy in two adults. Infect Chemother 2012; 18(6):961-4; PMID:22526386; https://doi.org/10.1007/s10156-012-0413-8
- Yen HL, Webster RG. Pandemic influenza as a current threat. Curr Top Microbiol Immunol 2009; 333:3-24; PMID:19768398; https://doi.org/10.1007/978-3-540-92165-3_1.
- Osterhaus AD, Rimmelzwaan GF, Martina BE, Bestebroer TM, Fouchier RA. Influenza B virus in seals. Science 2000; 288(5468):1051-3; PMID:10807575; doi: 10.1126/science.288.5468.1051.
- Bodewes R, Morick D, de Mutsert G, Osinga N, Bestebroer T, van der Vliet S, Smits SL, Kuiken T, Rimmelzwaan GF, Fouchier RA, et al. Recurring influenza B virus infections in seals. Emerg Infect Dis 2013; 19(3):511-2; PMID:23750359; doi: 10.3201/eid1903.120965
- Ran Z, Shen H, Lang Y, Kolb EA, Turan N, Zhu L, Ma J, Bawa B, Liu Q, Liu H, et al. Domestic pigs are susceptible to infection with influenza B viruses. J Virol 2015; 89(9):4818-26; PMID:25673727; https://doi.org/10.1128/JVI.00059-15
- Nerome R, Hiromoto Y, Sugita S, Tanabe N, Ishida M, Matsumoto M, Lindstrom SE, Takahashi T, Nerome K. Evolutionary characteristics of influenza B virus since its first isolation in 1940: dynamic circulation of deletion and insertion mechanism. Arch Virol 1998; 143(8):1569-83; PMID:9739335; https://doi.org/10.1007/s007050050399
- Belshe RB. The need for quadrivalent vaccine against seasonal influenza. Vaccine 2010; 28 Suppl 4:D45-53; PMID:20713260; https://doi.org/10.1016/j.vaccine.2010.08.028.
- Heikkinen T, Ikonen N, Ziegler T. Impact of influenza B lineage-level mismatch between trivalent seasonal influenza vaccines and circulating viruses, 1999-2012. Clin Infect Dis 2014; 59(11):1519-24; PMID:25139969; https://doi.org/10.1093/cid/ciu664
- Ambrose CS, Levin MJ. The rationale for quadrivalent influenza vaccines. Hum Vaccin Immunother 2012; 8(1):81-8; PMID:22252006; https://doi.org/10.4161/hv.8.1.17623
- Tafalla M, Buijssen M, Geets R, Vonk Noordegraaf-Schouten M. A comprehensive review of the epidemiology and disease burden of Influenza B in 9 European countries. Hum Vaccin Immunother 2016; 12(4):993-1002; PMID: 26890005; https://doi.org/10.1080/21645515.2015.1111494
- Ansaldi F, D'Agaro P, De Florentiis D, Puzelli S, Lin YP, Gregory V, Bennett M, Donatelli I, Gasparini R, Crovari P, et al. Molecular characterization of influenza B viruses circulating in northern Italy during the 2001-2002 epidemic season. J Med Virol 2003; 70(3):463-9; PMID:12767012; https://doi.org/10.1002/jmv.10418
- Puzelli S, Frezza F, Fabiani C, Ansaldi F, Campitelli L, Lin YP, Gregory V, Bennett M, D'Agaro P, Campello C, et al. Changes in the hemagglutinins and neuraminidases of human influenza B viruses isolated in Italy during the 2001-02, 2002-03, and 2003-04 seasons. J Med Virol 2004; 74(4):629-40; PMID:15484280; https://doi.org/10.1002/jmv.20225
- Pariani E, Amendola A, Piatti A, Anselmi G, Ranghiero A, Bubba L, Rosa AM, Pellegrinelli L, Binda S, Coppola L, et al. Ten years (2004-2014) of influenza surveillance in Northern Italy. Hum Vaccin Immunother 2015; 11(1):198-205; PMID:25483536; https://doi.org/10.4161/hv.35863
- Trucchi C, Alicino C, Orsi A, Paganino C, Barberis I, Grammatico F, Canepa P, Rappazzo E, Bruzzone B, Sticchi L, et al. Fifteen years of epidemiologic, virologic and syndromic Influenza surveillance: A focus on type B Virus and the effects of vaccine mismatch in Liguria region, Italy. Hum Vaccin Immunother 2017; 13(2):456-63; PMID:27924684; https://doi.org/10.1080/21645515.2017.1264779
- Caini S, Huang QS, Ciblak MA, Kusznierz G, Owen R, Wangchuk S, Henriques CM, Njouom R, Fasce RA, Yu H, et al. Epidemiological and virological characteristics of influenza B: results of the Global Influenza B Study. Influenza Other Respir Viruses 2015; 9 Suppl 1:3-12; PMID:26256290; https://doi.org/10.1111/irv.12319
- European Centre for Disease Prevention and Control (ECDC). Epidemiological data [accessed 2017 Feb 27]. http://ecdc.europa.eu/en/healthtopics/influenza/epidemiological_data/Pages/epidemiological_data.aspx
- Cohen C, Simonsen L, Kang JW, Miller M, McAnerney J, Blumberg L, Schoub B, Madhi SA, Viboud C. Elevated influenza-related excess mortality in South African elderly individuals, 1998-2005. Clin Infect Dis 2010; 51(12):1362-9; PMID:21070141; https://doi.org/10.1086/657314
- Reed C, Chaves SS, Daily Kirley P, Emerson R, Aragon D, Hancock EB, Butler L, Baumbach J, Hollick G, Bennett NM, et al. Estimating influenza disease burden from population-based surveillance data in the United States. PLoS One 2015; 10(3):e0118369; PMID:25738736; https://doi.org/10.1371/journal.pone.0118369
- Molbak K, Espenhain L, Nielsen J, Tersago K, Bossuyt N, Denissov G, Baburin A, Virtanen M, Fouillet A, Sideroglou T, et al. Excess mortality among the elderly in European countries, December 2014 to February 2015. Euro Surveill 2015; 20(11):21065; PMID:25811643; https://doi.org/10.2807/1560-7917.ES2015.20.11.21065
- Fraaij PL, Heikkinen T. Seasonal Influenza: The Burden of Disease in Children. Vaccine 2011; 29(43):7524-8; PMID:21820476; https://doi.org/10.1016/j.vaccine.2011.08.010.
- Lunelli A, Rizzo C, Puzelli S, Bella A, Montomoli E, Rota MC, Donatelli I, Pugliese A. Understanding the dynamics of seasonal influenza in Italy: incidence, transmissibility and population susceptibility in a 9-year period. Influenza Other Respir Viruses 2013; 7(3):286-95; PMID:22694182; https://doi.org/10.1111/j.1750-2659.2012.00388.x
- Chan PK, Chan MC, Cheung JL, Lee N, Leung TF, Yeung AC, Wong MC, Ngai KL, Nelson EA, Hui DS. Influenza B lineage circulation and hospitalization rates in a subtropical city, Hong Kong, 2000– 2010. Clin Infect Dis 2013; 56(5):677-84; PMID:23074315; https://doi.org/10.1093/cid/cis885
- Zhao B, Qin S, Teng Z, Chen J, Yu X, Gao Y, Shen J, Cui X, Zeng M, Zhang X. Epidemiological study of influenza B in Shanghai during the 2009-2014 seasons: implications for influenza vaccination strategy. Clin Microbiol Infect 2015; 21(7):694-700; PMID:25882368; https://doi.org/10.1016/j.cmi.2015.03.009
- Mosnier A, Caini S, Daviaud I, Nauleau E, Bui TT, Debost E, Bedouret B, Agius G, van der Werf S, Lina B, et al. Clinical Characteristics Are Similar across Type A and B Influenza Virus Infections. PLoS One 2015; 10(9):e0136186; PMID:26325069; https://doi.org/10.1371/journal.pone.0136186
- Tan Y, Guan W, Lam TT, Pan S, Wu S, Zhan Y, Viboud C, Holmes EC, Yang Z. Differing epidemiological dynamics of influenza B virus lineages in Guangzhou, southern China, 2009-2010. J Virol 2013; 87(22):12447-56; PMID:24027322; https://doi.org/10.1128/JVI.01039-13
- Moa AM, Muscatello DJ, Turner RM, MacIntyre CR. Epidemiology of influenza B in Australia: 2001-2014 influenza seasons. Influenza Other Respir Viruses 2017; 11(2):102-9; PMID:27650482; https://doi.org/10.1111/irv.12432
- Roy T, Agrawal AS, Mukherjee A, Mishra AC, Chadha MS, Kaur H, Chawla-Sarkar M. Surveillance and molecular characterization of human influenza B viruses during 2006-2010 revealed co-circulation of Yamagata-like and Victoria-like strains in eastern India. Infect Genet Evol 2011;11(7):1595-601; PMID:21708292; https://doi.org/10.1016/j.meegid.2011.05.022
- Sočan M, Prosenc K, Učakar V, Berginc N. A comparison of the demographic and clinical characteristics of laboratory-confirmed influenza B Yamagata and Victoria lineage infection. J Clin Virol 2014; 61(1):156-60; PMID:25034374; https://doi.org/10.1016/j.jcv.2014.06.018.
- European Centre for Disease Prevention and Control (ECDC). Technical documents. Influenza virus characterisation, Summary Europe, July 2011 [accessed 2017 Feb 27]. http://ecdc.europa.eu/en/publications/Publications/1108_SUR_Influenza_virus_characterisation_July.pdf.
- World Health Organization (WHO). Recommended composition of influenza virus vaccines for use in the 2011-2012 northern hemisphere influenza season [accessed 2017 Feb 27]. http://www.who.int/influenza/vaccines/2011_02_recommendation.pdf?na=1
- Radovanov J, Milošević V, Cvjetković IH, Ristić M, Djilas M, Nikolić N, Patić A, Kovačević G, Galović AJ, Petrović T, et al. Influenza B Viruses in the Population of Province of Vojvodina during the 2012/2013 Season: Differentiation of B/Yamagata and B/Victoria Lineages by Real-time RT-PCR, Antigenic and Phylogenetic Characterization. Srp Arh Celok Lek 2015; 143(7-8):429-37; PMID:26506753; https://doi.org/10.2298/SARH1508429R
- Vijaykrishna D, Holmes EC, Joseph U, Fourment M, Su YC, Halpin R, Lee RT, Deng YM, Gunalan V, Lin X, et al. The contrasting phylodynamics of human influenza B viruses. Elife 2015; 4:e05055; PMID:25594904; https://doi.org/10.7554/eLife.05055
- Tramuto F, Orsi A, Maida CM, Costantino C, Trucchi C, Alicino C, Vitale F, Ansaldi F. The Molecular Epidemiology and Evolutionary Dynamics of Influenza B Virus in Two Italian Regions during 2010-2015: The Experience of Sicily and Liguria. Int J Mol Sci 2016; 17(4):549; PMID:27089319; https://doi.org/10.3390/ijms17040549
- Oong XY, Ng KT, Lam TT, Pang YK, Chan KG, Hanafi NS, Kamarulzaman A, Tee KK. Epidemiological and Evolutionary Dynamics of Influenza B Viruses in Malaysia, 2012-2014. PLoS One 20157; 10(8):e0136254; PMID:26313754; https://doi.org/10.1371/journal.pone.0136254
- Jamotte A, Chong CF, Manton A, Macabeo B, Toumi M. Impact of quadrivalent influenza vaccine on public health and influenza-related costs in Australia. BMC Public Health. 2016;16:630; PMID:27449665; https://doi.org/10.1186/s12889-016-3297-1
- Chow MY, Yin JK, Heron L, Morrow A, Dierig A, Booy R, Leask J. The impact of influenza-like illness in young children on their parents: a quality of life survey. Qual Life Res. 2014;23(5):1651-60; PMID:24370954; https://doi.org/10.1007/s11136-013-0606-3
- Palmer LA, Rousculp MD, Johnston SS, Mahadevia PJ, Nichol KL, 2010. Effect of influenza-like illness and other wintertime respiratory illnesses on worker productivity: The child and household influenza-illness and employee function (CHIEF) study. Vaccine 2010; 28(31):5049-56; PMID:20493819; https://doi.org/10.1016/j.vaccine.2010.05.011.
- Goldstein E, Greene SK, Olson DR, Hanage WP, Lipsitch M. Estimating the hospitalization burden associated with influenza and respiratory syncytial virus in New York City, 2003-2011. Influenza Other Respir Viruses 2015; 9(5):225-33; PMID:25980600; https://doi.org/10.1111/irv.12325
- Poehling KA, Edwards KM, Weinberg GA, Szilagyi P, Staat MA, Iwane MK, Bridges CB, Grijalva CG, Zhu Y, Bernstein DI, et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355(1):31-40; PMID:16822994; https://doi.org/10.1056/NEJMoa054869
- European Centre for Disease Prevention and Control (ECDC). Immunisation schedules by target disease. [accessed 2017 Feb 27]. http://vaccine-schedule.ecdc.europa.eu/Pages/Scheduler.aspx
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev 2012; (8):CD004879; PMID:22895945; https://doi.org/10.1002/14651858.CD004879.pub4
- Heinonen S, Silvennoinen H, Lehtinen P, Vainionpää R, Ziegler T, Heikkinen T. Effectiveness of inactivated influenza vaccine in children aged 9 months to 3 years: an observational cohort study. Lancet Infect Dis 2011; 11(1):23-9; PMID:21106443; https://doi.org/10.1016/S1473-3099(10)70255-3
- Olivier CW. Influenza vaccination coverage rate in children: reasons for a failure and how to go forward. Human Vaccines & Immunotherapeutics 2012; 8(1):107-18; PMID:22252000; https://doi.org/10.4161/hv.8.1.18278
- Italian Ministry of Health. Data on influenza vaccine coverage [accessed 2017 May 5]. http://www.salute.gov.it/portale/influenza/dettaglioContenutiInfluenza.jsp?lingua=italiano&id=679&area=influenza&menu=vuoto
- European Centre for Disease Prevention and Control (ECDC). Seasonal influenza vaccination in Europe – Vaccination recommendations and coverage rates, 2012–13 [accessed 2017 May 5]. http://ecdc.europa.eu/en/publications/Publications/Seasonal-influenza-vaccination-Europe-2012-13.pdf
- Italian Ministry of Health. Epidemiological and virological surveillance, InfluNet operative protocol: influenza season 2016-2017 [accessed 2017 Feb 27]. http://www.salute.gov.it/imgs/C_17_pubblicazioni_2529_allegato.pdf
- World Health Organization (WHO). WHO information for molecular diagnosis of influenza virus - update March 2014 [accessed 2017 Feb 27]. http://www.who.int/entity/influenza/gisrs_laboratory/molecular_diagnosis_influenza_virus_humans_update_201403rev201505.pdf?ua=1, p. 27-9.