ABSTRACT
Spray drying is a promising method for the stabilization of vaccines, which are usually formulated as liquids. Usually, vaccine stability is improved by spray drying in the presence of a range of excipients. Unlike freeze drying, there is no freezing step involved, thus the damage related to this step is avoided. The edge of spray drying resides in its ability for particles to be engineered to desired requirements, which can be used in various vaccine delivery methods and routes. Although several spray dried vaccines have shown encouraging preclinical results, the number of vaccines that have been tested in clinical trials is limited, indicating a relatively new area of vaccine stabilization and delivery. This article reviews the current status of spray dried vaccine formulations and delivery methods. In particular it discusses the impact of process stresses on vaccine integrity, the application of excipients in spray drying of vaccines, process and formulation optimization strategies based on Design of Experiment approaches as well as opportunities for future application of spray dried vaccine powders for vaccine delivery.
Introduction
Many vaccines are inherently unstable in liquid form because they are prone to chemical and physical degradation, Citation1 making it difficult to achieve an adequate shelf life or prevent the need for a cold chain. Although many factors contribute to vaccine degradation, temperature instability is likely to be the most relevant. Citation2 For this reason, almost all liquid vaccines require the cold chain to ensure vaccine stability. This usually requires keeping vaccines at 2–8 °C during storage and transport. Citation3 Maintenance of the cold chain is challenging, especially in developing countries, where vaccines are needed the most. Citation4 The cold chain also contributes to the financial burden of vaccination programs. According to the World Health Organization, approximately half of supplied vaccines are wasted due to cold chain disruption, which has a detrimental effect on vaccination programs. Citation5
The stability of vaccines may be improved by optimizing the composition of the vaccine matrix, i.e. the formulation. Through removal of water, vaccine stability can be increased due to decreased mobility and the prevention of degradation pathways that are facilitated by water. Citation6 Dry vaccine formulations are generally less sensitive to temperature induced degradation. This makes these vaccines less dependent on the cold chain, thereby improving cost effectiveness of vaccination programs by reducing vaccine wastage. Citation4 Additionally, dried vaccines may attain an extended shelf life, which holds great potential for stockpiling in case of pandemics or bioterrorism threats. Citation7
There are several methods available to dry vaccines. Citation8 Freeze-drying is commonly used for drying of vaccines on an industrial scale. It involves freezing of a liquid solution followed by removal of water by sublimation of ice and thereafter by desorption of remaining water at low pressure and higher temperature. This results in a dried cake in the final container and requires reconstitution before administration. Citation9
Spray drying, an alternative to freeze-drying, is well established to produce dried biologics. Spray drying has the advantage over freeze-drying that no freezing or high vacuum is involved. As a result of being a one-step drying process, spray drying consumes less energy compared with lyophilization which results in lower operating costs. Citation10 Spray drying results in a dispersed fine powder compared with a dry cake as obtained by conventional freeze-drying. This may enable further powder handling and can be delivered without reconstitution to for example mucosal routes of administration. Mucosal powder vaccine delivery, e.g. via the respiratory tract, may induce mucosal immunity at the port of entry of the pathogen, potentially providing additional protection compared with parenteral vaccine delivery. Spray drying being a continuous drying process, serves as an attractive method to produce bulk powder vaccines. Yet it does have some drawbacks. Antigen is exposed to shear stress during atomization, and elevated temperatures during drying, further formation of air-water interfaces during droplet formation might lead to antigen denaturation. This is addressed in further section. Additionally, a secondary drying step may be required when a very low residual moisture content is desired in the end product. This may reduce the time and energy savings for spray drying as compared with freeze-drying.
In this review, we discuss the current status and novel developments of spray drying as a method for drying vaccines. Furthermore, spray drying process optimization strategies based on Design of Experiment approaches are addressed. Finally, the potential and limitations of delivery routes for powder vaccines are discussed.
Spray drying vaccines
Process principle
Spray drying is a single step drying process that converts a liquid feed into fine dispersible particles, with controlled physiochemical and morphological characteristics. Citation11 It has gained significant attention in formulating dried vaccines for its ease of use and potential for simple scale-up. Citation12
The drying process can be divided into 3 phases ( ). The process begins with the nebulization of liquid feed (liquid containing vaccine and excipients), generating an aerosol, into a heated gaseous drying medium. There are 3 types of spraying flow patterns that can be applied depending on the direction in which the air and liquid enter the drying chamber: counter current, co-current and mixed flow. Considering most vaccines are heat sensitive biologics, it is crucial to use the co-current drying mode. In this mode, the wettest aerosol droplet comes in contact with highest air temperature and driest particles with the lowest temperature, minimizing the risk of heat damage to vaccine. The drying temperature is determined by the inlet air temperature which ranges from 60 to 220 °C for a laboratory scale dryer. During the initial phase of drying, solvent starts to evaporate immediately. As the microenvironment surrounding the droplet gets saturated an equilibrium state is attained between vaccine droplet and drying air. The evaporation at this point is characterized by constant drying rate, where the temperature of the particle is defined by the wet bulb temperature. Once saturation conditions on the vaccine droplet surface can no longer be maintained due to diminishing water content, the secondary drying phase begins that is marked by a falling water content of the droplet/particle. As the droplet shrinks, the dissolved material concentrates at the surface, forming a solid layer around the droplet. Following this, further solvent evaporation occurs through the dried surface layer.
Figure 1. Overview of spray drying process [Adapted from Kanojia et al. (reference 11)] . The liquid vaccine excipient mixture enters the nozzle along with nitrogen via 2 different inlets. The mixing of liquid and nitrogen occurs just before end of the nozzle resulting in formation of aerosols. The heating mantle is located around the nozzle and the actual temperature is displayed on the equipment control panel (not shown). The dried particles enter the cyclone, following the airflow as depicted due to the special design of the cyclone. The nitrogen is separated, filtered and dehumidified before re-circulating back into the system and powder ends up in the collection vessel.
![Figure 1. Overview of spray drying process [Adapted from Kanojia et al. (reference 11)] . The liquid vaccine excipient mixture enters the nozzle along with nitrogen via 2 different inlets. The mixing of liquid and nitrogen occurs just before end of the nozzle resulting in formation of aerosols. The heating mantle is located around the nozzle and the actual temperature is displayed on the equipment control panel (not shown). The dried particles enter the cyclone, following the airflow as depicted due to the special design of the cyclone. The nitrogen is separated, filtered and dehumidified before re-circulating back into the system and powder ends up in the collection vessel.](/cms/asset/80ce2ea4-873a-4bac-9544-2aca414aedd8/khvi_a_1356952_f0001_c.jpg)
Heating and evaporation of water from the vaccine containing droplets could reversibly or irreversibly affect the antigen due to alteration in secondary structure, pH shifts and precipitation of active ingredient while exceeding the solubility limit as also observed in lyophilization. Citation13 However, the self-cooling effect of droplets during evaporation prevents the temperature increase of droplet surface above the wet bulb temperature. Citation14 For a more detailed description on evaporation and particle formation, the reader is referred to the publication of Vehring. Citation15 It is a good practice to use a lower inlet air temperature to reduce potential thermal stress to the vaccines. Depending on the capacity of spray drier and airflow rate, the drying process may take between 0.2 and 30s per droplet. Citation16 The large surface area of aerosol formed during spray drying and large volume of drying gas ensures such a rapid drying process.
Finally, the dried particles are separated from the process gas stream using either cyclone separators or baghouse filters. Most commonly used are the cyclone separators, the principle of which is based on the density difference between particle and drying gas. Bag filters use the concept of impaction onto a filter or electrostatic precipitation. The drying gas is released into the environment in an open system, or filtered, dehumidified and returned to the drying unit in a closed system. The separated bulk vaccine powder can be stored as bulk powder or aliquoted in different single or multidose containers under controlled conditions. One can further process bulk vaccine powder for additional drying (if required to reduce the residual moisture content), encapsulation or coating with other excipients. Citation17
Variants in spray drying technique
Spray drying technology exists as several variations, each with pros and cons. The variants includes spray freeze drying and supercritical drying using CO2 assisted nebulization.
Spray freeze drying
Spray freeze drying technique incorporates aspects of both spray drying and freeze-drying. The process includes atomization (droplet generation), freezing and sublimation drying. In Spray freeze drying a liquid feed (excipient vaccine mixture) is directly atomized into a cryogenic medium, instead of a heated gaseous medium as in spray drying. This leads to rapid freezing of the droplets, which are later dried by sublimation, identical to the freeze-drying process. Citation18 This allows processing of extremely heat sensitive antigens. In addition, spray freeze drying has an advantage over freeze-drying in preparation of dry alum adjuvant containing vaccines. Alum is sensitive to freezing and tends to agglomerate during freeze-drying. Maa et al. Citation19 showed that by spray freeze drying it is possible to limit the alum particles aggregation with no loss in adjuvant activity. However, during the freezing step, degradation of antigen due to exposure to ice surfaces is possible.
Several vaccine candidates have been successfully spray freeze-dried. This includes spray freeze-dried powder for Influenza vaccine Citation20-Citation22 ; alum adsorbed diphtheria, tetanus and hepatitis B vaccine; Citation19 , Citation23 , Citation24 anthrax vaccine, Citation25 , Citation26 and plague vaccine. Citation27
Carbon dioxide-assisted nebulization with bubble drying
Super critical drying involves the use of carbon dioxide or nitrous oxide in their supercritical fluid state to aid the drying process. Generally, the protein solution is mixed with the supercritical fluid before atomization and then sprayed under atmospheric condition. The setup is similar to that used for a typical spray drying process. The supercritical fluid is used as an antisolvent that causes precipitation of the protein. The liquid evaporation occurs in the supercritical region, where the distinction between gas and liquid ceases to apply. Jovanovic et al. Citation28 have summarized the narrower literature regarding the stabilization of proteins and drying by supercritical drying. Carbon dioxide-assisted nebulization with bubble drying (CAN-BD®) is based on the concept of supercritical drying, patented by Sievers et al.. Citation29 , Citation30 It involves mixing and dissolution of CO2 within the aqueous vaccine excipient solution under supercritical conditions (pressure and temperature usually between 8–10 MPa and 30–50°C). Citation31 , Citation32 The pressurized mixture is released as a spray through a nozzle, the rapid decompression of liquid mixture and expansion of compressed CO2 results in fine spray of droplets. This aerosol is dried rapidly by heated gas (generally nitrogen, around 25 to 65 °C) into micron size particles. CO2 is used as an aerosolizing aid that permits drying at lower temperature unlike spray drying, which may favor drying of thermosensitive vaccines. However, the high pressure requirement may impact the antigen stability and dissolution of CO2 may result in pH fluctuations (decrease toward acidic pH), if not properly controlled. Vaccines that are produced by CAN-BD include live attenuated measles vaccine, Citation33 , Citation34 and hepatitis B surface antigen protein. Citation31
Impact of process stress on vaccine quality
Shear stress may occur when the vaccine-excipient liquid mixture is atomized into small droplets, resulting in possible reduction or loss of antigen activity. Thompson et al. Citation35 observed a loss of 2 log titers when increasing the atomization pressure from 250 to 450 Ls/hour for a spray dried adenoviral vector vaccine. In addition, the choice of nozzle used for atomizing during spray drying can play a crucial role in causing shear stress. Grasmeijer et al. Citation36 demonstrated that during spray drying of a shear sensitive protein lactate dehydrogenase (LDH), the choice of nozzle had a significant influence on recovery of functional protein. A loss of 37% of LDH activity was observed during atomization with ultra-sonification nozzle compared with 9% loss in activity using a 2-fluid nozzle. The shear stress experienced by vaccines if needed, could be overcome by modulating the atomization pressure, solution feed rate, solution viscosity or use of stabilizers. Citation37 Optimal selection of these parameters can help in minimizing shear stress induced degradation.
Dehydration stress in combination with adsorption to air-liquid interface may result in protein denaturation and subsequent aggregation, resulting in partial or complete loss of activity. Citation38 , Citation39 The hydrophobic protein residues align themselves toward the air-liquid interface, formed after atomization. Interaction among these protein residues during drying, might result in aggregation and finally precipitation. Use of surfactants or proteins can reduce process loss and may increase the stability of vaccines during spray drying. Citation40
The temperatures experienced by the particle containing antigen in the late drying phase is close to the outlet temperature Citation41 and can vary based upon the selected inlet temperature of the drying gas, feed flow rate and drying airflow rate. The inlet air temperature, being the central component, directly influences the outlet temperature. Moreover, the evaporation rate has an impact on particle characteristics and thus variables important for evaporation are also important for powder end product characteristics. Citation42 As described previously in the process principle, the vaccine droplets experience temperatures equivalent to the wet bulb temperatures during the constant drying phase. Nevertheless, the thermal stability is antigen dependent. Therefore, one needs to carefully optimize the drying conditions to attain a dry powder with low moisture content without adversely affecting its activity. Apart from the above mentioned factors, individual process parameters may be critical and can be adjusted in the spray drying process. These include drying air temperature, atomization process (e.g., droplet size, spray rate, spray mechanism) and airflow rate. Citation11 , Citation15 , Citation43 , Citation44 Considering the heterogeneous nature (live attenuated, inactivated or subunit) of vaccines, optimal drying requires a careful optimization of the drying process parameters of each individual vaccine. In addition, the presence of oxygen, the storage temperature, the water activity, the relative humidity and exposure to light are factors that may influence the shelf life of dried vaccine powders and they require careful consideration. summarizes the role of different process parameters and their influence on product characteristics in context of different stress factors. For a more detailed description on influence of process parameters on spray dried vaccine powder characteristics, the reader is referred to work of Kanojia et al. Citation11
Table 1. Spray drying process parameters affecting product characteristics. The process parameters influence different stress factors experienced by the antigen. Shear stress (‡), heat stress (*) and dehydration stress (†).
Impact of formulation parameters on vaccine quality
Sugars and polysaccharides
Sugars (trehalose, sucrose, inulin etc.) are the most commonly used stabilizing excipients for spray drying of vaccines (summarized in ). There are 2 major theories explaining their protective mode of action. Citation66 Immobilization of vaccine antigen in an amorphous sugar glass matrix during drying is portrayed by the vitrification theory. Citation67 Drying of vaccines in the presence of sugars can yield both amorphous and crystalline powders, based on their glass transition temperature (Tg). Citation68 In a glassy state, sugars exhibit high viscosity and as a result molecular mobility of protein is restricted. Citation69 The transition from this glassy state to the undesirable rubbery state occurs at the Tg. Excipients with low Tg tend to crystallize upon spray drying and absence of an amorphous matrix destabilizes the protein. Thus, selection of right excipient with appropriate Tg can influence antigen stability during drying and further storage. As a rule of thumb the Tg should be well above the storage temperature to accommodate storage stability of proteins. Citation66 The other hypothesis is based on the theory that integrity of proteins in hydrated state is sustained by hydrogen bonding with water molecules. Upon drying, the bonding is replaced with hydrogen bonds between sugar and protein, thereby maintaining protein integrity. Citation70 This also may be the case for viral or bacterial membranes. Research from Muttil et al. Citation71 suggested formation of hydrogen bonds between hydroxyl group of the sugars and the phosphate group in the lipid bilayer of live attenuated Listeria monocytogenes during spray drying. Furthermore, to maximize the hydrogen bonding with a protein, the sugar molecule should firmly fit the irregular surface of the protein. This can be achieved more easily in the amorphous state of the sugar rather than the crystalline state. Additionally, the molecular weight of the sugar influences the hydrogen bonding ability between protein and sugar molecules during drying. Grasmeijer et al. Citation66 described that trehalose, with its lower molecular weight, formed more hydrogen bonds and fitted better to the irregular surface of the protein compared with inulin, a higher molecular weight sugar, during spray drying. The relevance of the carbohydrate flexibility was further described by Tonnis et al.. Citation72 Nevertheless, considering the heterogeneous nature of the vaccines, it is difficult to envisage stabilization with only one of above illustrated theories and interaction between these mechanisms may be relevant for stabilization by sugars and polysaccharides. In addition, often other type of excipients, such as surfactants and divalent ions, have to be included to provide enough stabilization.
Surfactants
Surfactants may reduce the surface tension of atomized droplets during drying and compete with the vaccine antigen for the surface at the air liquid interface. They are composed of hydrophilic and hydrophobic regions, and their action is presumed to be mediated by direct interaction with both the proteins and interfaces. Citation73-Citation75 They are used to prevent and reduce the formation of protein aggregates. Pluronic F68, a mild non-ionic surfactant, was successfully used for spray drying of a live attenuated measles vaccine Citation40 and a live attenuated influenza vaccine. Citation46 These studies illustrate that surfactants compete with the antigen for the surface to reduce shear stress and providing stability during atomization and drying. However, the amount of surfactants should be carefully optimized. Indiscriminate use of surfactants with vaccines containing bacteria or viruses could disrupt the membrane. This was observed with hepatitis B virus inactivation Citation76 and various bacterial strains Citation77 by using Tween 80 or Tween 20.
Divalent ions
Divalent cations improve the stability of several viruses. MgCl2 (divalent magnesium ion) has been used as an effective stabilizer of the liquid live attenuated oral polio vaccine. Citation78 A study from Chen et al. Citation79 describes that MgCl2 stabilizes poliovirus conformation by specific ionic interaction with capsid and by preventing water penetration into the capsid, thus reducing capsid swelling. Other studies have also suggested the stabilization of rotavirus, Citation80 , Citation81 and adenovirus Citation82 by divalent cations occur through stabilization of the viral capsid. A combination of Zn2+ and Ca2+ improved the storage stability of a spray dried live attenuated measles vaccine by 1 log TCID50 when stored for 1 week at 37°C. Citation40 Ohtake et al. Citation40 hypothesized that, Zn2+ and Ca2+ interact with the membrane lipids and proteins. The exact nature of this cation-viral molecule interaction is not clearly understood. However, the suggested interaction may preserve the conformation of the proteins, thereby aiding integrity of viral structure during processing. Since the core genetic material is surrounded by a viral envelope instead of a capsid in measles virus, as opposed to rotavirus and adenovirus, which are non-enveloped viruses, the exact nature of interaction needs further investigation.
Proteins
Proteins such as albumin have been regularly used to stabilize vaccines. Human serum albumin (HSA) was used in the formulation of spray dried measles vaccine. Citation40 HSA improved the storage stability by 0.8 log TCID50 compared with the formulation without HSA, when stored for 4 weeks at 37°C. The mode of action could be explained by different mechanisms. Large molecular components like proteins may slow down the migration of antigen to the air-liquid interface during droplet drying. Thus, after drying the vaccine component is concentrated into the core of the dried particle, minimizing the interaction with moisture during storage. Moreover, inclusion of protein components elevates the Tg of the formulation, Citation83 , Citation84 thereby improving storage stability. In addition, the stabilizing effect could be explained by increased interaction of the stabilizing protein with the vaccine particle, i.e., particle coating and surfactant like surface enrichment of antigen with protein. Citation85
Polymers
Besides the excipient groups mentioned above, various studies have reported the use of enteric coating polymers such as Eudragit L30 D-55 and FS 30D, cellulose acetate phthalate, hydroxyl propyl methyl cellulose acetate succinate and poly lactic-co-glycolic acid as an encapsulating polymer for spray dried vaccines. Citation51 , Citation54 , Citation59 , Citation64 These polymers are soluble at pH 5.5 and above; thus, can provide protection to antigens in the enteric environment. This provides a great potential for vaccines to be delivered via the oral route, which is further discussed in the delivery section.
Implementing design of experiment approach to spray dried vaccines
As outlined above, spray drying consists of a substantial number of both process and product variables that can be fine-tuned for optimal results. A broader application of spray drying, would require a thorough understanding of critical process parameters and critical product characteristics of the dried vaccine products. Optimization through an OFAT (one-factor-at-a- time) approach is resource and time consuming when attempting to establish the (sub) optimum. Moreover, the presence of complex biologics like vaccines makes the process optimization more arduous. Citation86 A Design of Experiments (DoE) approach can be used instead to systematically study the effects of multiple factors on a certain parameter using strategically planned combinations of variables and subsequent statistical analysis. DoE can be used to identify critical and non-critical parameters, and their respective interactions, of a production process. Furthermore, it can be used to quantify the impact of raw materials (excipient combination and vaccine) and process parameters on the product characteristics and quality. Therefore, with DoE one can obtain more valuable information with fewer experiments, compared with an OFAT approach. Several studies have used a DoE approach to investigate and optimize the spray drying process for vaccines, including influenza, Citation11 human papillomavirus Citation55 and human type 5 adenoviral vector (AdHu5 encoding LacZ). Citation35 Kanojia et al. Citation11 obtained a design space for spray dried inactivated influenza vaccine, substantiating the interplay of process parameters (e.g., inlet air drying temperature, liquid feed flow rate, atomization pressure) and how they affect the dried product characteristics (e.g., vaccine antigenicity, powder particle size, residual moisture content, process yield). Muttil et al. Citation55 used a DoE approach to optimize the excipient composition for a spray dried formulation containing virus like particles against human papillomavirus. The powder containing virus like particle stored at 37 °C for 12 months when intramuscularly (i.m.) administered in mice elicited comparable IgG titers as liquid vaccine administered by the i.m. route. The design space was used to understand the interaction of excipients concentration and process parameters (inlet air drying temperature, liquid feed flow rate, atomization pressure) to optimize powder yield, moisture content and particle size. Other studies from Thompson et al. Citation35 optimized spray drying process conditions, to decrease loss in viral activity for an adenoviral vector vaccine during drying.
Utilization of DoE in spray dried vaccines is limited to only the few aforementioned studies. However, an increase in use of DoE approach is expected, driven by both regulatory authorities and industry. There are FDA guidelines for quality management of biologicals like vaccines described in Q10. Citation87 Compliance with these regulations and implementing DoE during early stages of the development of a spray dried vaccine would help regulatory agencies expedite the approval process. Citation88 , Citation89 Additionally, DoE can provide a robust process, which can lead to fewer manufacturing deviations or failures. Citation90
Current state of experimental spray dried vaccines
A number of publications have described spray drying as a suitable method for drying vaccines (). The table describes vaccines and their subtypes (live attenuated or inactivated), key excipients used, process conditions and the key outcomes. To date, there are no marketed spray dried vaccines. However, several spray dried vaccines are in the pipeline and some are in early clinical trials.
Ohtake et al. Citation40 produced a relatively stable spray dried live attenuated measles vaccine, with only a minor process loss. The formulation contained trehalose and sucrose, surfactant Pluronic F68 along with L-arginine, human serum albumin and combinations of divalent ions as the key stabilizers. However, the formulation without Pluronic performed better during storage at 37 °C for 8 weeks in terms of vaccine stability (0.9 log loss without surfactant and 1.2 log loss with surfactant. The measles spray dried (carbon dioxide assisted nebulization with a bubble dryer) vaccine for pulmonary administration (particle size: 3–5µm), was the first spray dried vaccine to enter phase I clinical trial showing promising results. Citation91 The study used a spray dried live attenuated measles vaccine with myo-inositol as the stabilizer, substituting sorbitol used in the lyophilized marketed formulation (keeping all other excipients). The vaccine experienced 0.6 log loss in its viral activity during drying and surpassed the WHO stability requirement for freeze-dried measles vaccine (less than 1 log loss in viral activity after 1 week storage at 37°C). This formulation has also been previously prepared by Burger et al.. Citation33 The clinical study in healthy adults showed a comparable immune response to that of subcutaneously administered (same dose of) licensed vaccine (reconstituted lyophilized powder). Two experimental dry powder inhalers PuffHaler® and Solovent™ were used to administer the vaccine powder. Powder delivery through these devices has been described in a chapter from Weninger et al.. Citation92 Measles powder vaccine has shown the potential to be the first spray dried vaccine in the market in future.
Table 2. Summary of spray dried vaccine formulations with the process parameters and key findings (inlet: inlet drying temperature, outlet: outlet temperature, fr: feed flow rate into the system, atom: atomization pressure and n.a: not available).
There are several spray dried vaccine candidates in pre-clinical studies (). Lovalenti et al. Citation46 showed that formulations containing low concentrations of Pluronic F68 had lower process loss than formulation without (0.8 ± 0.4 versus 1.2 ± 0.4 log TCID50, respectively) suggesting that surfactant in the formulation could provide a shielding effect from destabilizing stresses on live attenuated influenza vaccine during spray drying. The immunogenicity was comparable to liquid control when intranasally administrated (after reconstitution) in ferrets. Sou et al. Citation47 prepared spray dried whole inactivated virus (WIV) influenza vaccine with trehalose and leucine as stabilizing excipients. The results indicate that administration of powder by the pulmonary route showed stronger induction of mucosal and systemic immune response compared with that of subcutaneously administered liquid vaccine. Saluja et al. Citation21 describe a relatively simple formulation for influenza subunit vaccine that provides full stabilization of the vaccine for at least 3 y at room temperature. Another study from Kanojia et al. Citation11 describes spray drying of thermostable whole inactivated influenza vaccine described the use of Design of Experiment approach to optimize the spray-drying process. The main finding of this study are summarized in the Design of Experiment section. These results underscore the potential of spray dried influenza vaccines to pitch a thermostable, effective and affordable influenza vaccine candidate for future clinical studies.
Immunization with inhalable BCG vaccine powder has shown to significantly reduce the bacterial burden in guinea pigs, compared with animals immunized with parenteral BCG when challenged with Mycobacterium tuberculosis. Citation48 A recent phase I clinical study detected antigen specific CD4 T cells in bronchoalveolar lavages after immunization with an pulmonary aerosol liquid TB vaccine candidate (MVA85A), showing no systemic adverse events. Citation93 These results reassured the safety and better efficacy of the pulmonary route for the delivery of MVA85A vaccines, and thus powder MVA85A vaccine Citation51 , Citation52 could be a potential candidate for future clinical studies. Another study with a spray dried bacterial vaccine used a polymer encapsulated Vibrio cholera. Citation59 Heat inactivated Vibrio cholera was spray dried with cellulose acetate phthalate and alginate, and was administered as an oral suspension in rats eliciting IgG and IgM responses comparable to orally administrated liquid Vibrio cholera. In another study, Citation94 Eudragit® encapsulated Vibrio cholera microparticles, were shown to be antigenically stable when stored for 6 months at 40°C. These are promising results for the development of an oral spray dried vaccine with an extended shelf life. Several other studies on spray dried diphtheria, anthrax and hepatitis B vaccine are outlined in , showing the potential of spray drying in vaccine development.
Delivery of spray dried vaccines
Dry powder vaccine formulations produced by spray drying provide an opportunity for the combination of both improved antigen stability and alternative routes of administration for the vaccine. Various groups are researching the delivery of spray dried vaccines via pulmonary, mucosal, skin and oral routes. Citation17 , Citation45 , Citation48 , Citation53 , Citation91 Production of controlled engineered particles of desirable size range gives spray drying the flexibility to produce powder antigens that can be administered via diverse routes of administration. The scope of delivery via different routes is described in the following sections.
Pulmonary delivery
Inhalable dried particles are distributed in the respiratory tract based on their aerodynamic particle size. In humans particles with aerodynamic diameter between 5–3 µm are best suited for delivery to the airways. Smaller particles, with an aerodynamic diameter of 1–3 µm are used for deep lung delivery. Citation10 Minne et al. Citation95 showed the influence of the site of deposition of the antigen on the immune response in mice. They observed better systemic, local and cellular immune responses when the influenza split virus vaccine was deposited to the deeper lung areas than when the vaccine was targeted to the upper airways. Given that many pathogens like influenza, tuberculosis or measles invade the host via mucosal membranes, non-invasive delivery of vaccine antigens would provide more local and direct protection at the site of infection. Citation96 In addition, the large surface area, extensive vascularization and thin epithelium in alveolar region facilitates efficient delivery of antigen. Moreover, it has been suggested that dry particulate antigens, as opposed to dissolved antigens, are better taken up by the antigen presenting cells leading to a more powerful immune response. Citation97-Citation99
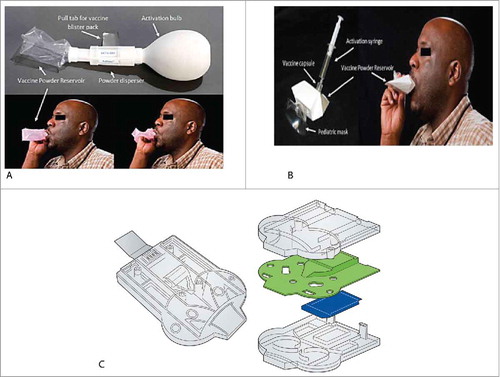
Dry powder inhalers incorporate the advantage of stable vaccine formulations with rapid delivery and high lung deposition depending on the particle dynamics and fluid dynamics in the respiratory tract. Citation100 Two experimental dry powder inhalers, PuffHaler® and Solovent (Becton Dickinson) ( and ) have been used for delivery of powder measles vaccine in adults males (18 to 45 y age). Citation91 Delivery by either PuffHaler® or Solovent had a safety (no serious adverse events) and immunogenicity profile (measles IgG antibodies and measles specific neutralizing antibody titers) comparable to that of licensed measles vaccine delivered by the subcutaneous route. Each device disperses powder vaccine into an inexpensive, single-use, disposable reservoir from which the patient inhales, eliminating the risk of cross-contamination. Citation92 Another prototype device is a single use disposable inhaler, the Twincer (University of Groningen, ). The simple design reduces the production costs, as the 3 plate-like parts (with blister) can simply be stacked and clicked together. Citation101 Boer et al. Citation102 have shown that due to high de-agglomeration efficacy of Twincer, a powder dose of 25 mg can be effectively de-agglomerated and dispersed for inhalation (when tested in vitro). Cyclops, a modified version of the Twincer inhaler, was used to evaluate safety and pharmacokinetic parameters of powder tobramycin in patients with cystic fibrosis. Citation103 This study illustrated the efficacy of the device to deliver powder efficaciously via pulmonary route and can be potentially used to deliver powder vaccines via the pulmonary route, with a future to enter the market. Thus, the use of these portable single-use devices and possibility of self-administration of powder vaccine reduces the need of trained healthcare workers. Furthermore, it offers a solution for vaccination of people who suffer from needle-phobia.
Figure 2. Pulmonary delivery of spray dried powders (Adapted from references 89 and 99, with permission from Elsevier). A. PuffHaler® Device (AktivDry LLC, USA): Air from the activation bulb lofts vaccine powder from the disperser into the reservoir once the pressure threshold of the burst valve is exceeded. The reservoir filled with powders are directly inhaled through a mouthpiece with adults and adolescents or mask (not shown) placed over the nose and mouth with infants and young children. B. Solovent™ Device (Becton, Dickinson & Company, USA): Air from the activation syringe ruptures the membrane of the vaccine capsule, releasing vaccine powder in to the reservoir. Patient inhales it through the mask. C. Twincer (University of Groningen, The Netherlands): A dry powder inhaler for pulmonary delivery. The vaccine powder is placed between the plates in an aluminum blister for moisture protection. The powder can be made available for inhalation by removal of a pull off blister strip.
Intranasal delivery
The nasal cavity and its associated lymphoid tissue, are an excellent site for vaccine delivery, Citation104 although there are concerns with regard to possible interaction with the olfactory bulb and other neuronal tissues. Citation105 Because there is a non-ciliated area in the anterior part and ciliated area in the posterior part of the nasal cavity, the site of deposition of vaccine in the nose is crucial considering the mucociliary clearance of the vaccine from the nose. Citation106 The deposition site is determined by the size of the dried particle and velocity at which the delivery device releases the particles in the cavity. Citation107 For aerosols or particles larger than 50 µm, intra nasal (i.n.) delivery is reproducible and independent of the vaccine recipient's breathing. This is because the site of deposition is governed by inertial impaction (bigger particles collide with the nasal mucosa rather than follow the streamline direction of the inhaled air). Citation108 Chitosan, an additive with mucoadhesive properties, facilitates antigen binding with the mucosal epithelial surfaces. Citation109 Huang et al. Citation110 showed that a chitosan containing anthrax vaccine powder formulation with 10 µg of recombinant Protective Antigen (rPA), when delivered intranasally with a specially designed device, as described previously, Citation111 elicited protective immune responses in rabbits. The protection against the spore challenge was improved for powder vaccinated rabbits when compared with one with liquid formulation (10 µg rPA) administered by MicroSprayer® devices (PennCentury).
In general, particles designed for nasal delivery should be large enough to impact in the nasal cavity with minimal deposition in the pulmonary airway. A study from Garmise et al. Citation112 produced powder with a target volume median diameter of 26.9 µm for i.n. influenza vaccination in rats. Intra nasal immunization with vaccine powder generated equivalent serum IgG titers as liquid vaccine administered intranasally. Also, the nasal IgA titers were comparable between powder and liquid formulations. An investigational device, is developed by CDC and Creare, an engineering services company, for administration of dry-powder vaccine via nasopharyngeal tissues. Citation113 It operates by exhalation through the mouth, blowing the powder into the nose while simultaneously generating air flow that limits entry to the lower respiratory tract. While several studies have shown the proof of concept for intranasal powder vaccination, clinical studies are needed for demonstrating safety and efficacy of i.n. powder vaccination in humans. The device cannot be used by very young children, limiting its use in pediatric vaccination programs.
Buccal and sublingual delivery
The buccal region is an attractive site for delivery of vaccine antigens. Langerhans and dendritic cells which are superficially present under the oral mucosal epithelium, act as an important target for induction of immune responses. Citation114 Also, it is suggested that the efficiency of vaccine delivery via this route is directly related to the permeability of the mucosal membranes (thickness buccal mucosa around 500–800 µm and sublingual region around 100–200 µm). Citation115 Several groups are developing dosage forms for optimizing sublingual and buccal delivery of vaccines. Citation116 Gala et al. Citation17 produced spray dried microparticles (particle size ∼3 µm) using bovine serum albumin and glutaraldehyde, containing live attenuated measles vaccine. These particles were incorporated into an oral disintegrating film (ODF) and administered to pigs via the buccal route, eliciting IgG responses in serum when compared with pre-dosing IgG responses. ODF with vaccine containing microparticles in which the polymer matrix protects the antigen in a stable form, offer a great platform for efficient and non-invasive delivery of vaccines. Adjuvants can also be co-encapsulated, in the microparticles along with vaccine, during spray drying. These microparticles were found to induce IL-8, IL-1 and TNF in an in vitro system. Citation117
Skin delivery
Ease of access and existence of a large number of antigen presenting cells make the skin an attractive organ for delivery of vaccines. Citation118 The top layer of the skin consists of the stratum corneum, an effective barrier preventing penetration of foreign molecules with molecular weights larger than 500 Da. Citation92 Therefore invasive or minimally invasive methods are needed to administer vaccines to the skin. Many epidermal vaccination strategies are currently developed, some of them based on powder vaccine formulations and devices. The aim is to deliver antigen directly into the epidermis because of the presence of an extensive immune network. Citation20 , Citation119
Most preclinical studies with powder epidermal vaccination were done with the Powder Ject device. It operates on compressed helium to deliver powder vaccine into the epidermis.. Citation120 Powders used conventionally for delivery by epidermal powder immunization (EPI) are spray dried with a suitable size (20 to 70 µm) and density. Citation121 There have been several pre-clinical studies that performed epidermal powder delivery using formulations that were spray freeze-dried.
The powder vaccines were immunogenic in animals when delivered as an epidermal powder for influenza, Citation121 hepatitis B surface antigen Citation23 and diphtheria toxoid. Citation23 Mice receiving influenza vaccine through EPI (average particle size 46 µm) showed approximately 3-fold higher serum Hemagglutination Inhibition (HI) titers compared with the liquid influenza vaccine administered by i.m route. EPI in guinea pigs (average particle size 40 µm), with powder hepatitis B surface antigen adsorbed to alum elicited comparable serum IgG response to liquid via i.m route. A phase I clinical trial with spray freeze-dried influenza vaccine showed that inclusion of adjuvants might improve the vaccine efficacy when delivered with PowderJect device. Citation121 Despite these encouraging results, it may be difficult to efficiently deliver powders to the skin because the density of spray freeze-dried particles is relatively low. The required kinetic energy to penetrate the skin is difficult to achieve unless more dense particles Citation101 for instance spray dried particles (which are generally denser than spray freeze-dried particles) or gold particles are used.
Oral delivery
Oral delivery of vaccines has always been an interesting alternative to parenteral injection because of its ease of administration, higher compliance and low production cost. However, the hostile environment of the gastrointestinal tract (GI) and oral tolerance are major obstacles associated with oral vaccine delivery. Citation122-Citation124 A study from Xiang et al. Citation125 reported that positively charged particles with a size of less than 5 µm can preferentially enter Peyer's patch of the small intestine and maybe transported to antigen presenting cells. Nevertheless, other authors found that a broader size range of less than 10 µm was also suitable to elicit a good mucosal immune response. Citation126 To negate the destructive effect of the GI tract, enteric coating polymers are used as a delivery vehicle for oral spray dried vaccines. Citation127 These include Eudragit S 100, L 100, cellulose acetate phthalate and hydroxyl propyl methyl cellulose acetate succinate (HPMC AS). Pastor et al. Citation59 studied a spray dried powder heat inactivated cholera vaccine (particle size 6.0 µm), which was given orally. Rats showed serum IgG responses with powder vaccine suspension comparable to liquid suspension, when administered by oral gavage. Another study Citation94 demonstrated the thermostability of the cholera vaccine powder with no loss in antigenicity (either with or without alginate), when stored at 40 °C for 9 months (liquid vaccine control absent). Shastri et al. Citation128 formulated an oral whole inactivated influenza vaccine powder with Eudragit and trehalose (particle size between 1 and 6 µm). After oral administration (with 20 µg vaccine) in mice, antigen specific immunoglobulins were induced and protection was demonstrated against a viral challenge when compared with naïve mice controls. Another study reported formulation of enteric coated spray dried microparticles containing a tumor cell lysate against breast cancer Citation129 and administered as oral suspension in mice followed by tumor challenge. It was observed that vaccinated mice developed significantly smaller tumors compared with naive controls. Oral powder vaccine enables tableting or granulation and thus facilitate storage and transport. This could be of immense potential for mass vaccination. Thus, delivery of vaccine powder by the oral route is promising, but the only known licensed dry oral vaccine is Vivotif, based on lyophilized live attenuated Salmonella typhi.
Challenges
Aside from the clear benefits of spray dried vaccines, several challenges still need to be overcome before spray dried vaccines can be marketed for commercial use. A successful marketed spray dried vaccine would require a commercial scale setup. Since spray drying is a continuous process, it can be readily scaled up by adjusting parameters of an individual production unit. Depending on the batch size (that needs to be atomized and dried), an increased thermodynamic input would be needed to remove the additional solvent to produce a dried powder matching the profile from the laboratory or pilot scale unit. Therefore, extensive engineering would be required to ensure that the dried vaccine product created at higher production rates, meets the properties of the initially designed product. Zhu et al. Citation12 developed a stable spray dried recombinant influenza vaccine and scaled it up, producing 3 consistent batches of ∼50 g on a pilot scale dryer. In past, a commercial spray dried inhaled insulin Exubera® was marketed by Pfizer for treatment of Type 1 and Type 2 diabetes, but was withdrawn from the market for reason unrelated to spray drying manufacturing. Thus, scale-up of spray-dried vaccine is challenging but feasible with additional considerations.
Asceptic process is essential for producing vaccines meant for administration via parenteral route. Terminal sterilization of spray dried vaccine by irradiation is an option, however it may adversely affect the structure and activity of the product. Scherlieβ et al. Citation130 showed that by using a formulation containing chitosan and glycyrrhizinic acid with trehalose matrix for a spray dried influenza A (H1N1) vaccine, terminal irradiation is feasible. With the current technology sterile spray dried product without post production sterilization is not achievable. Yet, the emerging availability of asceptic spray drying technology and additional practical experience would help extending spray drying of vaccines to the commercial domain.
With the current technology, powder filling and accurate dosing is challenging when compared with lyophilized vaccines, which are accurately dosed as liquid before drying. The formulation choice, powder handling procedures and flow properties of the powder play an important role in dose distribution. It is necessary to handle powder under control humidity conditions to avoid any unwanted moisture uptake. Additional consideration are required when handling vaccine powder containing hygroscopic excipients (e.g., sorbitol, citric acid). Despite the challenges, it is possible to achieve consistent powder dosing. Exubera®, a spray dried insulin was filled into 1–3 mg dose containers on a commercial scale.
Future perspectives
Spray drying is a promising platform for drying vaccines. The growing scientific interest in the field of particle engineering and new nano and micro technologies would add to the advancement of spray drying and needle free approaches for vaccination. The current pharmaceutical drying technology for vaccines is constructed around freeze drying. However, the recent advances with aseptic spray drying of biopharmaceuticals Citation131 has attracted a strong interest from the industry to expand spray drying process for vaccines. For future expansion in this field, one needs to address concerns regarding scaling up, regulatory, and biosafety (and legislation) aspects. The potential of spray dried vaccines to be stored and transported outside the cold chain would simplify vaccine delivery to remote areas and reduce the economic burden of vaccination programs. Within the next 5 years, the results from clinical studies and evidence from pharmaceutical production would clarify the position of spray drying as a viable competitor to conventional drying method for vaccines.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Kumru OS , Joshi SB , Smith DE , Middaugh CR , Prusik T , Volkin DB. Vaccine instability in the cold chain: Mechanisms, analysis and formulation strategies. Biologicals. 2014;42(5):237–59. doi:10.1016/j.biologicals.2014.05.007. PMID:24996452
- Brandau DT , Jones LS , Wiethoff CM , Rexroad J , Middaugh CR . Thermal stability of vaccines. J Pharm Sci. 2003;92(2):218–31. doi:10.1002/jps.10296. PMID:12532371
- Zaffran M . Vaccine transport and storage: Environmental challenges. Dev Biol Stand. 1996;87:9–17. PMID:8853997
- Chen D , Kristensen D . Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines. 2009;8(5):547–57. doi:10.1586/erv.09.20. PMID:19397412
- Organization WHO . Monitoring vaccine wastage at country level: Guidelines for programmme managers 2005. http://www.who.int/iris/handle/10665/68463
- Maltesen MJ , van de Weert M . Drying methods for protein pharmaceuticals. Drug Discov Today Technol. 2008;5(2–3):e81–8. doi:10.1016/j.ddtec.2008.11.001. PMID:24981095
- Geeraedts F , Saluja V , ter Veer W , Amorij JP , Frijlink HW , Wilschut J , Hinrichs WL , Huckriede A . Preservation of the immunogenicity of dry-powder influenza H5N1 whole inactivated virus vaccine at elevated storage temperatures. AAPS J. 2010;12(2):215–22. doi:10.1208/s12248-010-9179-z. PMID:20195930
- Amorij JP , Huckriede A , Wilschut J , Frijlink HW , Hinrichs WL . Development of stable influenza vaccine powder formulations: Challenges and possibilities. Pharm Res. 2008;25(6):1256–73. doi:10.1007/s11095-008-9559-6. PMID:18338241
- Adams G . The principles of freeze-drying. Methods Mol Biol. 2007;368:15–38. doi:10.1007/978-1-59745-362-2_2. PMID:18080460
- Greb E . Is spray drying a viable alternative to lyophilization? Equipment and Processing Report; 2009. Epub Dec 16, 2009.
- Kanojia G , Willems GJ , Frijlink HW , Kersten GF , Soema PC , Amorij JP . A Design of Experiment approach to predict product and process parameters for a spray dried influenza vaccine. Int J Pharm. 2016;511(2):1098–111. doi:10.1016/j.ijpharm.2016.08.022. PMID:27523619
- Zhu C , Shoji Y , McCray S , Burke M , Hartman CE , Chichester JA , Breit J , Yusibov V , Chen D , Lal M . Stabilization of HAC1 influenza vaccine by spray drying: Formulation development and process scale-up. Pharm Res. 2014;31:3006–18. doi:10.1007/s11095-014-1394-3. PMID:24858396
- Roy I , Gupta MN . Freeze-drying of proteins: Some emerging concerns. Biotechnol Appl Biochem. 2004;39(Pt 2):165–77. doi:10.1042/BA20030133. PMID:15032737
- Katja S . Spray drying of protein precipitates and evaluation of the nano spray dryer B-90 [Dissertation]. LMU Munich; 2011.
- Vehring R . Pharmaceutical particle engineering via spray drying. Pharm Res. 2008;25(5):999–1022. doi:10.1007/s11095-007-9475-1. PMID:18040761
- Masters K . Spray drying handbook. 5th ed. Burnt Mill, Harlow, Essex, England New York: Longman Scientific & Technical; Wiley; 1991. xiv, 725.
- Gala RP , Popescu C , Knipp GT , McCain RR , Ubale RV , Addo R , Bhowmik T, Kulczar CD, D'Souza MJ. Physicochemical and preclinical evaluation of a novel buccal measles vaccine. AAPS PharmSciTech. 2016;18(2):283–292. doi:10.1016/j.ijpharm.2015.04.053. PMID:27357420
- Wanning S , Suverkrup R , Lamprecht A . Pharmaceutical spray freeze drying. Int J Pharm. 2015;488(1–2):136–53. PMID:25900097
- Maa YF , Zhao L , Payne LG , Chen D . Stabilization of alum-adjuvanted vaccine dry powder formulations: Mechanism and application. J Pharm Sci. 2003;92(2):319–32. doi:10.1002/jps.10294. PMID:12532382
- Dean HJ , Chen D . Epidermal powder immunization against influenza. Vaccine. 2004;23(5):681–6. doi:10.1016/j.vaccine.2004.06.041. PMID:15542190
- Saluja V , Amorij JP , Kapteyn JC , de Boer AH , Frijlink HW , Hinrichs WL . A comparison between spray drying and spray freeze drying to produce an influenza subunit vaccine powder for inhalation. J Control Release. 2010;144(2):127–33. doi:10.1016/j.jconrel.2010.02.025. PMID:20219608
- Amorij JP , Saluja V , Petersen AH , Hinrichs WL , Huckriede A , Frijlink HW . Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine. 2007;25(52):8707–17. doi:10.1016/j.vaccine.2007.10.035. PMID:17996993
- Maa YF , Shu C , Ameri M , Zuleger C , Che J , Osorio JE , Payne LG , Chen D . Optimization of an alum-adsorbed vaccine powder formulation for epidermal powder immunization. Pharm Res. 2003;20(7):969–77. doi:10.1023/A:1024493719236. PMID:12880281
- Tonnis WF , Amorij JP , Vreeman MA , Frijlink HW , Kersten GF , Hinrichs WL . Improved storage stability and immunogenicity of hepatitis B vaccine after spray-freeze drying in presence of sugars. Eur J Pharm Sci. 2014;55:36–45. doi:10.1016/j.ejps.2014.01.005. PMID:24468629
- Mikszta JA , Sullivan VJ , Dean C , Waterston AM , Alarcon JB , Dekker JP 3rd , Brittingham JM , Huang J , Hwang CR , Ferriter M , et al. Protective immunization against inhalational anthrax: A comparison of minimally invasive delivery platforms. J Infect Dis. 2005;191(2):278–88. doi:10.1086/426865. PMID:15609239
- Wang SH , Kirwan SM , Abraham SN , Staats HF , Hickey AJ . Stable dry powder formulation for nasal delivery of anthrax vaccine. J Pharm Sci. 2012;101(1):31–47. doi:10.1002/jps.22742. PMID:21905034
- Huang J , D'Souza AJ , Alarcon JB , Mikszta JA , Ford BM , Ferriter MS , Evans M , Stewart T , Amemiya K , Ulrich RG , et al. Protective immunity in mice achieved with dry powder formulation and alternative delivery of plague F1-V vaccine. Clin Vaccine Immunol. 2009;16(5):719–25. doi:10.1128/CVI.00447-08. PMID:19261773
- Jovanovic N , Bouchard A , Hofland GW , Witkamp GJ , Crommelin DJ , Jiskoot W . Stabilization of proteins in dry powder formulations using supercritical fluid technology. Pharm Res. 2004;21(11):1955–69. doi:10.1023/B:PHAM.0000048185.09483.e7. PMID:15587916
- Sievers RE , Karst U . Inventor Methods for fine particle formation 1997 June 17. Patent US5639441 A.
- Sievers RESPS , Carpenter JF . inventorSupercritical fluid-assisted nebulization and bubble drying 2003 October 7. Patent US 6630121 B1.
- Cape SP , Villa JA , Huang ET , Yang TH , Carpenter JF , Sievers RE . Preparation of active proteins, vaccines and pharmaceuticals as fine powders using supercritical or near-critical fluids. Pharm Res. 2008;25(9):1967–90. doi:10.1007/s11095-008-9575-6. PMID:18581212
- Sellers SP , Clark GS , Sievers RE , Carpenter JF . Dry powders of stable protein formulations from aqueous solutions prepared using supercritical CO(2)-assisted aerosolization. J Pharm Sci. 2001;90(6):785–97. doi:10.1002/jps.1032. PMID:11357179
- Burger JL , Cape SP , Braun CS , McAdams DH , Best JA , Bhagwat P , Pathak P , Rebits LG , Sievers RE . Stabilizing formulations for inhalable powders of live-attenuated measles virus vaccine. J Aerosol Med Pulm drug deliv. 2008;21(1):25–34. doi:10.1089/jamp.2007.0658. PMID:18518829
- Kissmann J , Ausar SF , Rudolph A , Braun C , Cape SP , Sievers RE , Federspiel MJ , Joshi SB , Middaugh CR . Stabilization of measles virus for vaccine formulation. Hum Vaccin. 2008;4(5):350–9. doi:10.4161/hv.4.5.5863. PMID:18382143
- LeClair DA , Cranston ED , Xing Z , Thompson MR . Optimization of spray drying conditions for yield, particle size and biological activity of thermally stable viral vectors. Pharm Res. 2016;33(11):2763–76. doi:10.1007/s11095-016-2003-4. PMID:27450412
- Grasmeijer N . Improving protein stabilization by spray drying [PhD thesis]. University of Groningen; 2016.
- Maa YF , Prestrelski SJ . Biopharmaceutical powders: Particle formation and formulation considerations. Curr Pharm Biotechnol. 2000;1(3):283–302. doi:10.2174/1389201003378898. PMID:11469385
- Webb SD , Golledge SL , Cleland JL , Carpenter JF , Randolph TW . Surface adsorption of recombinant human interferon-gamma in lyophilized and spray-lyophilized formulations. J Pharm Sci. 2002;91(6):1474–87. doi:10.1002/jps.10135. PMID:12115847
- Mumenthaler M , Hsu CC , Pearlman R . Feasibility study on spray-drying protein pharmaceuticals: Recombinant human growth hormone and tissue-type plasminogen activator. Pharm Res. 1994;11(1):12–20. doi:10.1023/A:1018929224005. PMID:8140042
- Ohtake S , Martin RA , Yee L , Chen D , Kristensen DD , Lechuga-Ballesteros D , Truong-Le V . Heat-stable measles vaccine produced by spray drying. Vaccine. 2010;28(5):1275–84. doi:10.1016/j.vaccine.2009.11.024. PMID:19944152
- Stahl K , Claesson M , Lilliehorn P , Linden H , Backstrom K . The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int J Pharm. 2002;233(1–2):227–37. doi:10.1016/S0378-5173(01)00945-0. PMID:11897427
- Maltesen MJ , Bjerregaard S , Hovgaard L , Havelund S , van de Weert M . Quality by design - Spray drying of insulin intended for inhalation. Eur J Pharm Biopharm. 2008;70(3):828–38. doi:10.1016/j.ejpb.2008.07.015. PMID:18755270
- Rabbani NR , Seville PC . The influence of formulation components on the aerosolisation properties of spray-dried powders. J Control Release. 2005;110(1):130–40. doi:10.1016/j.jconrel.2005.09.004. PMID:16226334
- Wong YL , Sampson S , Germishuizen WA , Goonesekera S , Caponetti G , Sadoff J , Bloom BR , Edwards D . Drying a tuberculosis vaccine without freezing. Proc Natl Acad Sci U S A. 2007;104(8):2591–5. doi:10.1073/pnas.0611430104. PMID:17299039
- Lin WH , Griffin DE , Rota PA , Papania M , Cape SP , Bennett D , Quinn B , Sievers RE , Shermer C , Powell K , et al. Successful respiratory immunization with dry powder live-attenuated measles virus vaccine in rhesus macaques. Proc Natl Acad Sci U S A. 2011;108(7):2987–92. doi:10.1073/pnas.1017334108. PMID:21282608
- Lovalenti PM , Anderl J , Yee L , Nguyen V , Ghavami B , Ohtake S , Saxena A , Voss T , Truong-Le V . Stabilization of live attenuated influenza vaccines by freeze drying, spray drying, and foam drying. Pharm Res. 2016;33:1144–60. doi:10.1007/s11095-016-1860-1. PMID:26818839
- Sou T , Morton DA , Williamson M , Meeusen EN , Kaminskas LM , McIntosh MP . Spray-Dried influenza antigen with trehalose and leucine produces an aerosolizable powder vaccine formulation that induces strong systemic and mucosal immunity after pulmonary administration. J Aerosol Med Pulm Drug Deliv. 2015;28:361–71. doi:10.1089/jamp.2014.1176. PMID:25714115
- Garcia-Contreras L , Wong YL , Muttil P , Padilla D , Sadoff J , Derousse J , Germishuizen WA , Goonesekera S , Elbert K , Bloom BR , et al. Immunization by a bacterial aerosol. Proc Natl Acad Sci U S A. 2008;105(12):4656–60. doi:10.1073/pnas.0800043105. PMID:18344320
- Jin TH , Tsao E , Goudsmit J , Dheenadhayalan V , Sadoff J . Stabilizing formulations for inhalable powders of an adenovirus 35-vectored tuberculosis (TB) vaccine (AERAS-402). Vaccine. 2010;28(27):4369–75. doi:10.1016/j.vaccine.2010.04.059. PMID:20444437
- Tyne AS , Chan JG , Shanahan ER , Atmosukarto I , Chan HK , Britton WJ , West NP . TLR2-targeted secreted proteins from Mycobacterium tuberculosis are protective as powdered pulmonary vaccines. Vaccine. 2013;31(40):4322–9. doi:10.1016/j.vaccine.2013.07.022. PMID:23880366
- Lu D , Garcia-Contreras L , Xu D , Kurtz SL , Liu J , Braunstein M , McMurray DN , Hickey AJ . Poly (lactide-co-glycolide) microspheres in respirable sizes enhance an in vitro T cell response to recombinant Mycobacterium tuberculosis antigen 85B. Pharm Res. 2007;24(10):1834–43. doi:10.1007/s11095-007-9302-8. PMID:17657598
- Lu D , Garcia-Contreras L , Muttil P , Padilla D , Xu D , Liu J , Braunstein M , McMurray DN , Hickey AJ . Pulmonary immunization using antigen 85-B polymeric microparticles to boost tuberculosis immunity. AAPS J. 2010;12(3):338–47. doi:10.1208/s12248-010-9193-1. PMID:20422340
- Muttil P , Prego C , Garcia-Contreras L , Pulliam B , Fallon JK , Wang C , Hickey AJ , Edwards D . Immunization of guinea pigs with novel hepatitis B antigen as nanoparticle aggregate powders administered by the pulmonary route. AAPS J. 2010;12(3):330–7. doi:10.1208/s12248-010-9192-2. PMID:20419360
- Tawde SA , Chablani L , Akalkotkar A , D'Souza MJ . Evaluation of microparticulate ovarian cancer vaccine via transdermal route of delivery. J Control Release. 2016;235:147–54. doi:10.1016/j.jconrel.2016.05.058. PMID:27238440
- Saboo S , Tumban E , Peabody J , Wafula D , Peabody DS , Chackerian B , Muttil P . Optimized formulation of a thermostable spray-dried virus-like particle vaccine against human papillomavirus. Mol Pharm. 2016;13(5):1646–55. doi:10.1021/acs.molpharmaceut.6b00072. PMID:27019231
- Peabodya J , Muttil P , Chackeriana B , Tumban E . Characterization of a spray-dried candidate HPV L2-VLP vaccine stored for multiple years at room temperature. Papillomavirus Res. 2017;10(3):116–20. doi:10.1016/j.pvr.2017.03.004
- LeClair DA , Cranston ED , Xing Z , Thompson MR . Evaluation of excipients for enhanced thermal stabilization of a human type 5 adenoviral vector through spray drying. Int J Pharm. 2016;506(1–2):289–301. doi:10.1016/j.ijpharm.2016.04.067. PMID:27130366
- Afkhami S , LeClair DA , Haddadi S , Lai R , Toniolo SP , Ertl HC , Cranston ED , Thompson MR , Xing Z . Spray dried human and chimpanzee adenoviral-vectored vaccines are thermally stable and immunogenic in vivo. Vaccine. 2017;35:2916–24. doi:10.1016/j.vaccine.2017.04.026. PMID:28438408
- Pastor M , Esquisabel A , Marquinez I , Talavera A , Pedraz JL . Cellulose acetate phthalate microparticles containing Vibrio cholerae: Steps toward an oral cholera vaccine. J Drug Target. 2014;22(6):478–87. doi:10.3109/1061186X.2014.888071. PMID:24731056
- Ano G , Esquisabel A , Pastor M , Talavera A , Cedre B , Fernandez S , Sifontes S , Aranguren Y , Falero G , García L , et al. A new oral vaccine candidate based on the microencapsulation by spray-drying of inactivated Vibrio cholerae. Vaccine. 2011;29(34):5758–64. doi:10.1016/j.vaccine.2011.05.098. PMID:21683110
- Muttil P , Pulliam B , Garcia-Contreras L , Fallon JK , Wang C , Hickey AJ , Edwards DA . Pulmonary immunization of guinea pigs with diphtheria CRM-197 antigen as nanoparticle aggregate dry powders enhance local and systemic immune responses. AAPS J. 2010;12(4):699–707. doi:10.1208/s12248-010-9229-6. PMID:20878294
- Jones RM , Burke M , Dubose D , Chichester JA , Manceva S , Horsey A , Streatfield SJ , Breit J , Yusibov V . Stability and pre-formulation development of a plant-produced anthrax vaccine candidate. Vaccine. 2017;35(41):5463–5470. doi:10.1016/j.vaccine.2016.12.009
- Chen D , Kapre S , Goel A , Suresh K , Beri S , Hickling J , Jensen J , Lal M , Preaud JM , Laforce M , et al. Thermostable formulations of a hepatitis B vaccine and a meningitis A polysaccharide conjugate vaccine produced by a spray drying method. Vaccine. 2010;28(31):5093–9. doi:10.1016/j.vaccine.2010.04.112. PMID:20478345
- Anish C , Upadhyay AK , Sehgal D , Panda AK . Influences of process and formulation parameters on powder flow properties and immunogenicity of spray dried polymer particles entrapping recombinant pneumococcal surface protein A. Int J Pharm. 2014;466(1–2):198–210. doi:10.1016/j.ijpharm.2014.03.025. PMID:24631054
- Kunda NK , Alfagih IM , Miyaji EN , Figueiredo DB , Goncalves VM , Ferreira DM , Dennison SR , Somavarapu S , Hutcheon GA , Saleem IY . Pulmonary dry powder vaccine of pneumococcal antigen loaded nanoparticles. Int J Pharm. 2015;495(2):903–12. doi:10.1016/j.ijpharm.2015.09.034. PMID:26387622
- Grasmeijer N , Stankovic M , de Waard H , Frijlink HW , Hinrichs WL . Unraveling protein stabilization mechanisms: Vitrification and water replacement in a glass transition temperature controlled system. Biochim Biophys Acta. 2013;1834(4):763–9. doi:10.1016/j.bbapap.2013.01.020. PMID:23360765
- Crowe JH , Crowe LM , Chapman D . Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science. 1984;223(4637):701–3. doi:10.1126/science.223.4637.701. PMID:17841031
- Schule S , Schulz-Fademrecht T , Garidel P , Bechtold-Peters K , Frieb W . Stabilization of IgG1 in spray-dried powders for inhalation. Eur J Pharm Biopharm. 2008;69(3):793–807. doi:10.1016/j.ejpb.2008.02.010. PMID:18477504
- Aschenbrenner M , Grammueller E , Kulozik U , Foerst P . The contribution of the inherent restricted mobility of glassy sugar matrices to the overall stability of freeze-dried bacteria determined by low-resolution solid-state 1H-NMR. Food Bioproc Tech. 2014;7(4):1012–24. doi:10.1007/s11947-013-1095-7
- Carpenter JF , Crowe JH . An infrared spectroscopic study of the interactions of carbohydrates with dried proteins. Biochemistry. 1989;28(9):3916–22. doi:10.1021/bi00435a044. PMID:2526652
- Kunda NK , Wafula D , Tram M , Wu TH , Muttil P . A stable live bacterial vaccine. Eur J Pharm Biopharm.2016;103:109–17. doi:10.1016/j.ejpb.2016.03.027. PMID:27020530
- Tonnis WF , Mensink MA , de Jager A , van der Voort Maarschalk K , Frijlink HW , Hinrichs WL . Size and molecular flexibility of sugars determine the storage stability of freeze-dried proteins. Mol Pharm. 2015;12(3):684–94. doi:10.1021/mp500423z. PMID:25581526
- Bam NB , Cleland JL , Yang J , Manning MC , Carpenter JF , Kelley RF , Randolph TW . Tween protects recombinant human growth hormone against agitation-induced damage via hydrophobic interactions. J Pharm Sci. 1998;87(12):1554–9. doi:10.1021/js980175v. PMID:10189266
- Jones LS , Cipolla D , Liu J , Shire SJ , Randolph TW . Investigation of protein-surfactant interactions by analytical ultracentrifugation and electron paramagnetic resonance: The use of recombinant human tissue factor as an example. Pharm Res. 1999;16(6):808–12. doi:10.1023/A:1018809632395. PMID:10397598
- Kreilgaard L , Frokjaer S , Flink JM , Randolph TW , Carpenter JF . Effects of additives on the stability of recombinant human factor XIII during freeze-drying and storage in the dried solid. Arch Biochem Biophys. 1998;360(1):121–34. doi:10.1006/abbi.1998.0948. PMID:9826437
- Prince AM , Stephan W , Kotitschke R , Brotman B . Inactivation of hepatitis B and non-A, non-B viruses by combined use of Tween 80, beta-propiolactone, and ultraviolet irradiation. Thromb Haemost. 1983;50(2):534–6. doi:10.1006/abbi.1997.0111. PMID:6415846
- Cherepova N , Veljanov D . Effect of sorbitan monolaurate polyoxyalkylene (Tween 20) on the ultrastructure of some bacteria. Cytobios. 1994;80(322):179–85. PMID:7539732
- Newman JF , Tirrell S , Ullman C , Piatti PG , Brown F . Stabilising oral poliovaccine at high ambient temperatures. Dev Biol Stand. 1996;87:103–11. PMID:8854007
- Chen CH , Wu R , Roth LG , Guillot S , Crainic R . Elucidating mechanisms of thermostabilization of poliovirus by D2O and MgCl2. Arch Biochem Biophys. 1997;342(1):108–16. PMID:9185619
- Erk I , Huet JC , Duarte M , Duquerroy S , Rey F , Cohen J , Lepault J . A zinc ion controls assembly and stability of the major capsid protein of rotavirus. J Virol. 2003;77(6):3595–601. doi:10.1128/JVI.77.6.3595-3601.2003. PMID:12610135
- Shirley JA , Beards GM , Thouless ME , Flewett TH . The influence of divalent cations on the stability of human rotavirus. Arch Virol. 1981;67(1):1–9. doi:10.1007/BF01314596. PMID:6263223
- Turnbull AE , Skulimowski A , Smythe JA , Alexander IE . Adeno-associated virus vectors show variable dependence on divalent cations for thermostability: Implications for purification and handling. Hum Gene Ther. 2000;11(4):629–35. doi:10.1089/10430340050015815. PMID:10724041
- Duddu SP , Dal Monte PR . Effect of glass transition temperature on the stability of lyophilized formulations containing a chimeric therapeutic monoclonal antibody. Pharm Res. 1997;14(5):591–5. doi:10.1023/A:1012144810067. PMID:9165528
- Sarciaux JM , Hageman MJ . Effects of bovine somatotropin (rbSt) concentration at different moisture levels on the physical stability of sucrose in freeze-dried rbSt/sucrose mixtures. J Pharm Sci. 1997;86(3):365–71. doi:10.1021/js960217k. PMID:9050807
- Walters RH , Bhatnagar B , Tchessalov S , Izutsu K , Tsumoto K , Ohtake S . Next generation drying technologies for pharmaceutical applications. J Pharm Sci. 2014;103(9):2673–95. doi:10.1002/jps.23998. PMID:24916125
- Calcott P . Implementation of quality by design in vaccine development: BioProcess international; 2013 [ cited 2013 February]. Web Article.
- Administration. UFaD . Guidance for industry: Q10 pharmaceutical cGMP regulations, US Department of Health and Human Service. Rockville (MD): FDA; September, 2006. FDA Guideline.
- Rathore AS . Roadmap for implementation of quality by design (QbD) for biotechnology products. Trends Biotechnol. 2009;27(9):546–53. doi:10.1016/j.tibtech.2009.06.006. PMID:19647883
- Rathore AS , Winkle H . Quality by design for biopharmaceuticals. Nat Biotechnol. 2009;27(1):26–34. doi:10.1038/nbt0109-26. PMID:19131992
- Haas J , Franklin A , Houser M , Maraldo D , Mikola M , Ortiz R , Sullivan E , Otero JM . Implementation of QbD for the development of a vaccine candidate. Vaccine. 2014;32(24):2927–30. doi:10.1016/j.vaccine.2014.02.028. PMID:24598725
- group Ma , Cape S , Chaudhari A , Vaidya V , Mulay R , Agarkhedkar S , Shermer C , Collins M , Anderson R , Agarkhedkar S , et al. Safety and immunogenicity of dry powder measles vaccine administered by inhalation: A randomized controlled Phase I clinical trial. Vaccine. 2014;32(50):6791–7. doi:10.1016/j.vaccine.2014.09.071. PMID:25446830
- Weniger BG , Papania MJ . 61 - Alternative vaccine delivery methods A2 - Plotkin, Stanley A. In: Orenstein WA , Offit PA , editors. Vaccines (Sixth Edition). London: W.B. Saunders; 2013. p. 1200–31.
- Satti I , Meyer J , Harris SA , Manjaly Thomas ZR , Griffiths K , Antrobus RD , Rowland R , Ramon RL , Smith M , Sheehan S , et al. Safety and immunogenicity of a candidate tuberculosis vaccine MVA85A delivered by aerosol in BCG-vaccinated healthy adults: A phase 1, double-blind, randomised controlled trial. Lancet Infect Dis. 2014;14(10):939–46. doi:10.1016/S1473-3099(14)70845-X. PMID:25151225
- Pastor M , Esquisabel A , Talavera A , Ano G , Fernandez S , Cedre B , Infante JF , Callicó A , Pedraz JL . An approach to a cold chain free oral cholera vaccine: in vitro and in vivo characterization of Vibrio cholerae gastro-resistant microparticles. Int J Pharm. 2013;448(1):247–58. doi:10.1016/j.ijpharm.2013.02.057. PMID:23518363
- Minne A , Louahed J , Mehauden S , Baras B , Renauld JC , Vanbever R . The delivery site of a monovalent influenza vaccine within the respiratory tract impacts on the immune response. Immunology. 2007;122(3):316–25. doi:10.1111/j.1365-2567.2007.02641.x. PMID:17521369
- Holmgren J , Czerkinsky C . Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45–53. doi:10.1038/nm1213. PMID:15812489
- Thomas C , Gupta V , Ahsan F . Particle size influences the immune response produced by hepatitis B vaccine formulated in inhalable particles. Pharm Res. 2010;27(5):905–19. doi:10.1007/s11095-010-0094-x. PMID:20232117
- Lu D , Hickey AJ . Pulmonary vaccine delivery. Expert Rev Vaccines. 2007;6(2):213–26. doi:10.1586/14760584.6.2.213. PMID:17408371
- Sou T , Meeusen EN , de Veer M , Morton DA , Kaminskas LM , McIntosh MP . New developments in dry powder pulmonary vaccine delivery. Trends Biotechnol. 2011;29(4):191–8. doi:10.1016/j.tibtech.2010.12.009. PMID:21255854
- Frijlink HW , De Boer AH . Dry powder inhalers for pulmonary drug delivery. Expert Opin Drug Deliv. 2004;1(1):67–86. doi:10.1517/17425247.1.1.67. PMID:16296721
- Amorij JP , Hinrichs W , Frijlink HW , Wilschut JC , Huckriede A . Needle-free influenza vaccination. Lancet Infect Dis. 2010;10(10):699–711. doi:10.1016/S1473-3099(10)70157-2. PMID:20883966
- de Boer AH , Hagedoorn P , Westerman EM , Le Brun PP , Heijerman HG , Frijlink HW . Design and in vitro performance testing of multiple air classifier technology in a new disposable inhaler concept (Twincer) for high powder doses. Eur J Pharm. 2006;28(3):171–8. doi:10.1016/j.ejps.2005.11.013. PMID:16650739
- Hoppentocht M , Akkerman OW , Hagedoorn P , Alffenaar JW , van der Werf TS , Kerstjens HA , Frijlink HW , de Boer AH . Tolerability and pharmacokinetic evaluation of inhaled dry powder tobramycin free base in non-cystic fibrosis bronchiectasis patients. PloS One. 2016;11(3):e0149768. doi:10.1371/journal.pone.0149768. PMID:26959239
- Jabbal-Gill I . Nasal vaccine innovation. J Drug Target. 2010;18(10):771–86. doi:10.3109/1061186X.2010.523790. PMID:21047271
- Mutsch M , Zhou W , Rhodes P , Bopp M , Chen RT , Linder T , Spyr C , Steffen R . Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. New Engl J Med. 2004;350(9):896–903. doi:10.1056/NEJMoa030595. PMID:14985487
- Tafaghodi M , Abolghasem Sajadi Tabassi S , Jaafari MR , Zakavi SR , Momen-Nejad M . Evaluation of the clearance characteristics of various microspheres in the human nose by gamma-scintigraphy. Int J Pharm. 2004;280(1–2):125–35. doi:10.1016/j.ijpharm.2004.05.009. PMID:15265553
- Vidgren MT , Kublik H . Nasal delivery systems and their effect on deposition and absorption. Adv Drug Deliv Rev. 1998;29(1–2):157–77. doi:10.1016/S0169-409X(97)00067-7. PMID:10837586
- Newman SP , Pitcairn GR , Dalby RN . Drug delivery to the nasal cavity: In vitro and in vivo assessment. Crit Rev Ther Drug Carrier Syst. 2004;21(1):21–66. doi:10.1615/CritRevTherDrugCarrierSyst.v21.i1.20. PMID:15099184
- Ugwoke MI , Agu RU , Verbeke N , Kinget R . Nasal mucoadhesive drug delivery: Background, applications, trends and future perspectives. Adv Drug Deliv Rev. 2005;57(11):1640–65. doi:10.1016/j.addr.2005.07.009. PMID:16182408
- Huang J , Mikszta JA , Ferriter MS , Jiang G , Harvey NG , Dyas B , Roy CJ , Ulrich RG , Sullivan VJ . Intranasal administration of dry powder anthrax vaccine provides protection against lethal aerosol spore challenge. Hum Vaccin. 2007;3(3):90–3. doi:10.4161/hv.3.3.4011. PMID:17375001
- Huang J , Garmise RJ , Crowder TM , Mar K , Hwang CR , Hickey AJ , Mikszta JA , Sullivan VJ . A novel dry powder influenza vaccine and intranasal delivery technology: Induction of systemic and mucosal immune responses in rats. Vaccine. 2004;23(6):794–801. doi:10.1016/j.vaccine.2004.06.049. PMID:15542204
- Garmise RJ , Staats HF , Hickey AJ . Novel dry powder preparations of whole inactivated influenza virus for nasal vaccination. AAPS PharmSciTech. 2007;8(4):E81. doi:10.1208/pt0804081. PMID:18181542
- Papania MJJJB , Bagley MC , Friets EM , Knaus DA . inventor; The United States Of America, As Represented By The Secretary, Department Of Health And Human Services, Creare Llc,, assignee. Nasal aerosol delivery system 2011.
- Patel VF , Liu F , Brown MB . Advances in oral transmucosal drug delivery. J Control Release. 2011;153(2):106–16. doi:10.1016/j.jconrel.2011.01.027. PMID:21300115
- Kraan H , Vrieling H , Czerkinsky C , Jiskoot W , Kersten G , Amorij JP . Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580–92. doi:10.1016/j.jconrel.2014.05.060. PMID:24911355
- Kraan H , van der Stel W , Kersten G , Amorij JP . Alternative administration routes and delivery technologies for polio vaccines. Expert Rev Vaccines. 2016;15(8):1029–40. doi:10.1586/14760584.2016.1158650. PMID:26912100
- Ubale RV , D'Souza MJ , Infield DT , McCarty NA , Zughaier SM . Formulation of meningococcal capsular polysaccharide vaccine-loaded microparticles with robust innate immune recognition. J Microencapsul. 2013;30(1):28–41. doi:10.3109/02652048.2012.692402. PMID:22657751
- Mitragotri S . Immunization without needles. Nat Rev Immunol. 2005;5(12):905–16. doi:10.1038/nri1728. PMID:16239901
- Chen D , Burger M , Chu Q , Endres R , Zuleger C , Dean H , Payne LG . Epidermal powder immunization: Cellular and molecular mechanisms for enhancing vaccine immunogenicity. Virus Res. 2004;103(1–2):147–53. doi:10.1016/j.virusres.2004.02.027. PMID:15163503
- Dean HJ . Epidermal delivery of protein and DNA vaccines. Expert Opin Drug Deliv. 2005;2(2):227–36. doi:10.1517/17425247.2.2.227. PMID:16296750
- Chen D , Endres R , Maa YF , Kensil CR , Whitaker-Dowling P , Trichel A , Youngner JS , Payne LG . Epidermal powder immunization of mice and monkeys with an influenza vaccine. Vaccine. 2003;21(21–22):2830–6. doi:10.1016/S0264-410X(03)00175-0. PMID:12798624
- Lycke N . Recent progress in mucosal vaccine development: Potential and limitations. Nat Rev Immunol. 2012;12(8):592–605. doi:10.1038/nri3251. PMID:22828912
- Akande J , Yeboah KG , Addo RT , Siddig A , Oettinger CW , D'Souza MJ . Targeted delivery of antigens to the gut-associated lymphoid tissues: 2. Ex vivo evaluation of lectin-labelled albumin microspheres for targeted delivery of antigens to the M-cells of the Peyer's patches. J Microencapsul. 2010;27(4):325–36. doi:10.3109/02652040903191834. PMID:20055749
- Wilkhu J , McNeil SE , Kirby DJ , Perrie Y . Formulation design considerations for oral vaccines. Ther Deliv. 2011;2(9):1141–64. doi:10.4155/tde.11.82. PMID:22833911
- Xiang SD , Scholzen A , Minigo G , David C , Apostolopoulos V , Mottram PL , Plebanski M . Pathogen recognition and development of particulate vaccines: Does size matter? Methods. 2006;40(1):1–9. doi:10.1016/j.ymeth.2006.05.016. PMID:16997708
- Hori M , Onishi H , Machida Y . Evaluation of Eudragit-coated chitosan microparticles as an oral immune delivery system. Int J Pharm. 2005;297(1–2):223–34. doi:10.1016/j.ijpharm.2005.04.008. PMID:15885938
- Chablani L , Tawde SA , D'Souza MJ . Spray-dried microparticles: A potential vehicle for oral delivery of vaccines. J Microencapsul. 2012;29:388–97. doi:10.3109/02652048.2011.651503. PMID:22283700
- Shastri PN , Kim MC , Quan FS , D'Souza MJ , Kang SM . Immunogenicity and protection of oral influenza vaccines formulated into microparticles. J Pharm Sci. 2012;101(10):3623–35. doi:10.1002/jps.23220. PMID:22711602
- Chablani L , Tawde SA , Akalkotkar A , D'Souza C , Selvaraj P , D'Souza MJ . Formulation and evaluation of a particulate oral breast cancer vaccine. J Pharm Sci. 2012;101(10):3661–71. doi:10.1002/jps.23275. PMID:22828873
- Scherliess R , Ajmera A , Dennis M , Carroll MW , Altrichter J , Silman NJ , Scholz M , Kemter K , Marriott AC . Induction of protective immunity against H1N1 influenza A(H1N1)pdm09 with spray-dried and electron-beam sterilised vaccines in non-human primates. Vaccine. 2014;32(19):2231–40. doi:10.1016/j.vaccine.2014.01.077. PMID:24631078
- Siew A . Exploring the use of aseptic spray drying in the manufacture of biopharmaceutical injectables. Pharmaceutical Technology. 2016;40(7):24 -27. http://www.pharmtech.com/exploring-use-aseptic-spray-drying-manufacture-biopharmaceutical-injectables
