ABSTRACT
Since 2003 (US) and 2012 (Europe) the live attenuated influenza vaccine (LAIV) has been used as an alternative to the traditional inactivated influenza vaccines (IIV). The immune responses elicted by LAIV mimic natural infection and have been found to provide broader clinical protection in children compared to the IIVs. However, our knowledge of the detailed immunological mechanisims induced by LAIV remain to be fully elucidated, and despite 14 years on the global market, there exists no correlate of protection. Recently, matters are further complicated by differing efficacy data from the US and Europe which are not understood. Better understanding of the immune responses after LAIV may aid in achieving the ultimate goal of a future “universal influenza vaccine”. In this review we aim to cover the current understanding of the immune responses induced after LAIV.
Introduction
Influenza viruses are a major cause of severe respiratory illness, and annually causes global fatality rates of 250,000–500,000 people, with an estimated 3–5 million hospitalizations.Citation1-3 These numbers are extrapolated from US estimates into the global population of influenza serious airway respiratory infection (SARI), and are probably a major underestimate of the burden of disease. In the US alone, the annual economic costs of influenza are estimated to $8 billion.Citation4 Pandemics cause a large burden on society and healthcare systems, often with higher morbidity and mortality rates in younger individuals, inducing a short-term global health emergency. However, seasonal influenza is an annual challenge with a far greater public heath impact than pandemics over time.
Complications of influenza are commonly pulmonary including: bronchitis, viral pneumonitis, secondary bacterial pneumonia and acute respiratory distress syndrome (ARDS), with a high risk of fatal outcome,Citation5 particularly during pregnancy.Citation6 In children, otitis media, febrile seizures and rare cases of viral myocarditis and meningoencephalitis can occur.Citation7-9
Despite their shortcomings, vaccines have been the most important and cost-effective counter-measure to combat influenza, since their implementation 70 years ago.Citation10 Influenza viruses have a unique ability to mutate and hence escape immune defense mechanisms, necessitating annual vaccine updates. These vaccines are the inactivated influenza vaccines (IIV) and live attenuated influenza vaccines (LAIV). The current influenza vaccines are well tolerated and considered safe, with more than 140–170 million doses distributed annually in the US during the last 5 years.Citation6 LAIV is administered intranasally and vaccination resembles a natural infection.
Natural influenza infection elicits a broad immune response; involving both the humoral and cellular immune compartments with long-term cellular cross-reactive protection to similar strains achieved. This was evident during the 2009 pandemic when elderly people with prior exposure to the H1N1 virus experienced lower infection rates. The current IIVs provide strain-specific antibody mediated protection, which is shorter-lived. Long-term cellular protective responses are not elicited. The LAIV attempts to mimic a natural infection and has been found to elicit protective antibodies both locally and systemically, as well as induce cellular responses. Knowledge of the early mucosal and long-term immunological responses elicited by seasonal LAIV is limited. Currently, there is a substantial global research effort in further understanding influenza immunology towards the desired goal of a “universal influenza vaccine”, a broadly protective vaccine that does not require annual vaccination. This review aims to cover the latest knowledge regarding human immune responses after LAIV.
Influenza ecology
Influenza is an RNA virus, lacking accurate proof reading mechanisms. This causes point mutations in the viral genome resulting in “antigenic drift”, allowing the virus to escape the host´s acquired immunity. Antigenic drift is responsible for annual epidemics, which necessitates biannual vaccine updates for the northern and southern hemisphere by the World Health Organization (WHO). There are four types of influenza virus (A, B, C and D) where types B and C are predominantly human viruses, although C rarely cause infections. Types A and B are responsible for seasonal epidemics. Influenza A viruses infect >20 different animal species, mainly pigs, birds (poultry and waterfowl) and bats, representing a considerable zoonotic potential, which can cause pandemics if the viruses acquire the ability for human-to-human transmission.Citation11,12 Pigs can be infected by avian, swine and human viruses and may act as “mixing vessels”, creating novel human influenza strains.Citation13 Most influenza A subtypes (combinations of H1-16/N1-9) are found in aquatic birds, resulting in global viral dissemination,Citation12 whilst the remaining subtypes (H17/H18 and N10/N11) occurs in bats.
Seasonal influenza vaccines
The currently used seasonal influenza vaccines (IIV and LAIV) are usually trivalent, consisting of two influenza A subtypes and one B lineage. However, due to the two co-circulating influenza B lineages, B/Yamagata and B/Victoria, quadrivalent vaccines containing both influenza B lineages are available.Citation14 Since 1945 influenza vaccines have been produced in embryonated hen´s eggs, and even today this remains the most important production platform. Approximately 10 LAIV doses are produced from one embryonated egg, compared to a single IIV dose.Citation15 Egg supply and vaccine yield achieved from each egg, are the most important factors limiting influenza vaccine production. Production takes roughly six monthsCitation16 and during this time a new strain can arise, leading to a mismatch between the vaccine and circulating strain. Production of quadrivalent vaccines is an improvement, reducing the risk of mismatch of B strains.Citation17
LAIV vaccine strains are produced by reverse genetics, using the HA and NA genes from the WHO recommended strains with six gene segments from a cold adapted, temperature sensitive and attenuated master donor virus (MDV).Citation18 LAIV viruses are restricted in replication to the lower temperatures of the upper airways (33°C) (), but cannot replicate in the warmer, lower respiratory tract (>33°C).Citation18
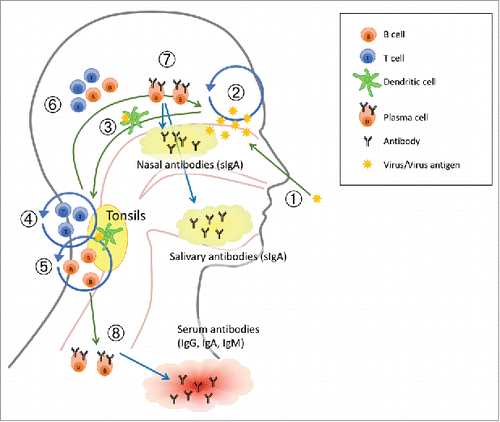
Figure 1. A suggested working model of how live attenuated influenza vaccine (LAIV) elicits immune response. The numbers in the figure refer to 1. adminstration of the LAIV intranasally as a nasal spray; 2. Limited virus replication (IFN-γ) by LAIV; 3. Viral influenza antigen is transported to the tonsils by dendritic cells (DCs); 4. Activation and proliferation of T cells (IL-2); 5. Activation and proliferation of B cells, affinity maturation and isotype switching; 6. Activated B and T cells homes to site of infection (vaccination); 7. Plasma cells secrete specific mucosal antibodies and 8. Plasma cells secrete specific antibodies into circulation. Adapted from.Citation91
Influenza vaccination strategies
Although fatal cases of influenza occur in the elderly, children are the main transmitters of disease in the community, and most often hospitalized during outbreaks; often without influenza as suspected diagnosis.Citation9,19 Seasonal influenza vaccination strategies vary between countries. In the US, influenza vaccination is recommended for all people >6 months, whereas in Europe vaccination is largely recommended for risk groups, and with a few exceptions not children in general.Citation7,20-23
A European consensus report from 2006 recommended children <3 years old to be considered a high-risk population and recommended for vaccination.Citation24 LAIV is recommended for children due to the lower immunogenicity of IIV in this age group and the needle-free administration. The UK introduced seasonal LAIV into their childhood vaccination program (4–11 years old) in 2013 and has estimated large national cost savings by vaccinating children with a reduction in morbidity due to herd immunity.Citation21,25 Finland, Latvia, Slovenia and Germany are the only other European countries to recommend seasonal vaccination of healthy children in this age group.Citation23
Risk groups and recommendations for influenza vaccination
A higher risk of increased morbidity and mortality after influenza virus infection has been consistently observed in young children (<5 years), elderly (>65 years), pregnant women,Citation3,6,26 morbid obesity (Body mass index (BMI)>40)Citation27 and people of all ages with chronic conditions such as: pulmonary or cardiac diseases, immunosuppression (by either medication or disease), diabetes, metabolic, liver, kidney and neurological/neuromuscular disorders.Citation6,28
The WHO has published guidelines for the recommendation of seasonal influenza vaccines to the population at most risk of severe or fatal disease ().Citation6 Each country makes national decisions about prioritisation based upon costs and feasibility of vaccination.
Table 1. A comparison of the immune responses after natural infection, and immunisation with inactivated influenza vaccine (IIV) or live attenuated influenza vaccine (LAIV).
Live attenuated influenza vaccine (LAIV)
The Russians were the first to use a LAIV and have used it for over 40 years.Citation29 LAIV was licensed in the US for healthy people aged 2–49 years old in 2003, and in Europe in 2012, for healthy children 2–17 years old.Citation30 LAIV is administered as a nasal spray with one spray per nostril, and requires replication of the live attenuated virus in the mucosa of the upper airways to induce protection. By mimicking natural infection, but without causing disease or onward transmission, LAIV elicits both humoral and cellular immune responses ().Citation31,32 LAIV induces mucosal IgA antibodies, which provide protection at the site of viral entry against subsequent infection ().Citation33-35 LAIV has shown better immunogenicity than IIV in children,Citation36 and only LAIV has been shown to induce T-cell responses in children, perhaps due to pre-existing immunity limiting infection of LAIV strains in adults.Citation31,37,38
Vaccine safety
Vaccines are given prophylactically to a healthy population including children. The tolerance of side effects is hence very low, and safety is of the utmost importance. LAIV strains are safe, genetically stable, and do not revert to wild type viruses.Citation39 There is the possibility that LAIV can reassort with wild type viruses if simultaneous infection and immunization occurs, however the progeny virus are unlikely to transmit as they maintain the attenuated phenotype of the LAIV.Citation40 LAIV is easy to administer and well tolerated with mostly transient, local side effects observed 2–3 days after vaccination, such as runny or congested nose.Citation32,41,42 An increased hospitalization rate due to wheezing was found in children <2 years old and, LAIV is therefore contraindicated in these children.Citation36 LAIV is also contraindicated in immunocompromised individuals, pregnant women and in children with severe asthma, or who are receiving salicylate therapy (risk of Reye´s syndrome with salicylate and wild-type influenza infection). Several studies have confirmed LAIV to be safe in children with intermittent wheezing and stable asthma, including children 18 months old.Citation32,43 A recent multicentre study of the safety of LAIV in children (2–18 years) with egg allergy and asthma, found that the vaccine was well tolerated with a low risk of systemic allergic reactions.Citation13,44
Measuring protection after LAIV
Vaccine effectiveness and correlates of protection
LAIV has a proven record of efficacy after decades of use (reviewed inCitation45), Cochrane reviews have found approximately 80% efficacy in young children (<6 years old) and 40% in adults to matched strains.Citation46,47 The efficacy varies depending on the outcome measure and the method used, but is higher in children than adults and in vaccinees that experience influenza symptoms and have virological confirmed influenza.Citation48-51 LAIV had superior efficacy compared to IIV in young children (generally 70–90% against strains antigenically similar to the vaccine strains),Citation52-54 and in recipients with a history of respiratory tract infections, asthma and HIV.Citation54,55 Also, LAIV recipients with breakthrough influenza had less severe illness.Citation52,54 A recent review found no evidence of reduced efficacy of LAIV in children who were vaccinated with LAIV for two consecutive seasons.Citation56
The commonly used hemagglutination inhibition (HI) assay measures virus specific antibody and an HI titer of ≥40 is considered protective in adults. Although higher HI titers of 110 or 320 for H3N2 could be more appropriate for protection in children.Citation57 LAIV efficacy trials (clinical trials measuring the number of influenza like illness (ILI) symptoms with a laboratory confirmed influenza in vaccinees) have shown that the HI titer underestimates protection from LAIV.Citation58 This is probably due to the multifaceted immune response elicited by LAIV with induction of local IgA, and T-cell responses, which are not measured by HI. A recent study searching for correlates of protection (COPs) after LAIV could not find a single marker predictive of protection and COPs used for IIV did not correlate with protection after LAIV vaccination and subsequent challenge.Citation59 Non-neutralising antibodies may also be important in limiting influenza through natural killer mediated lysis of infected cells through antibody dependent cell-mediated cytotoxicity (ADCC). Although to date, no increases in ADCC activity after LAIV immunization were found in adults or children.Citation60
LAIV induces cellular IFN-γ responses, which are not yet an established correlate of protection, however they have been used in several studies with different thresholds (range 20–100 spot forming units (SFU)/million peripheral blood mononuclear cells (PBMCs).Citation61-63 A large efficacy trial conducted with >2000 children suggested a COP of T-cellular immunity of 100 IFN-γ SFU/million PBMCs, however the suggested number of 100 is considered arbitrary.Citation38 Background levels of <20 SFU/million PBMCs have been found in a UK child cohort,Citation63 and pre-vaccination levels of >100SFU/million PBMCs were found in a Norwegian cohort,Citation64 probably due to natural infection. LAIV induces both humoral and cellular immune responses and there is an urgent need for new correlates of protection to evaluate the immunogenicity of LAIV in children, which may aid in development of new vaccines.
The early kinetics of the mucosal immune response
Mucosal antibodies are vital in protecting the upper airways, the site of viral entry, while serum antibodies generally protect the lower respiratory tract. LAIV elicits secretory IgA, which plays a major role in influenza protection being the predominant antibody secreted at the mucosal surfaces ().Citation33,65,66 Early work in seronegative (HI≤8) adults found that an important effect of LAIV was the rapid and durable induction of secretory nasal wash IgA persisting for up to 6–12 months, as well as serum IgG.Citation33,67,68 Recent work in children using non-invasive sampling of saliva, found significant increases in influenza specific salivary IgA after only 14 days, lasting up to 6 monthsCitation32 and this could be used as a new possible indicator of vaccine immunogenicity in children.
Nasal IgA has been found to be associated with protection from influenza illness in young children (<3 years old) and adults from experimental influenza challenge.Citation66,69 A placebo-controlled, double blind study in children, found that both serum and nasal IgA antibodies correlated with LAIV induced protection, but that nasal IgA was the stronger correlate.Citation70 Other studies have demonstrated that despite the lack of a robust serum antibody response, the LAIV provides protective immunity.Citation58,71 The induction of mucosal antibodies may hence be the most important effect of LAIV,Citation72,73 and could be a superior indicator of immunogenicity of LAIV compared to serum antibodies.Citation10 The findings of long-term local IgA responses after LAIV indicate that the mucosal immune response is well developed in young children and that the LAIV may provide local protection in the nasal and oral cavities. Furthermore, local, mucosal IgA correlates with HI titers for all vaccine strains.Citation66,74
Several studies have found that the highest humoral responses were directed towards the influenza B strains in LAIV.Citation32,66,72 The LAIV influenza B strain induces both antibodies and T-cells, while the A viruses mostly induced T-cells and to a lesser degree antibodies,Citation66,72 although the reason for this is not clear. It may be due to lack of pre-existing immune responses to the B strain, but it could equally be due to differences in the infectivity of the LAIV strains, or that the B virus may be better adapted to replicate in humans.
Since HI antibodies have proven suboptimal for children or for measuring immunogenicity after LAIV, there is a global interest in finding an early predictor of LAIV immunogenicity. Unlike the HI, there exists no COP for mucosal antibodies,Citation73 due to great variations in quality and quantity and challenges in sampling and assaying of mucosal antibodies.Citation10,66,69 Given the multifaceted response dependent on age, priming status and comorbidities, perhaps a single COP for LAIV will not be feasible, however a partial COP could be of great value.
The longevity of systemic immune response induced by LAIV
LAIV elicits both early and durable humoral and cellular immune responses in children. Increased numbers of Memory B cells (MBCs) as well as T-cellular responses, which were maintained for 6 months in most children, and up to one year in some children, have been found.Citation32,33,65
The effect of priming on the subsequent immune response
It is debated whether LAIV is effective in older age groups, probably as pre-existing immunity derived from previous natural infection limits replication of the LAIV viruses. Children's developing immune system, combined with the impact of previous exposures (priming) to influenza may influence the age-related response to LAIV. Age dependent differences are found in the response, supporting immunization of the youngest children with two doses, although most respond after the first dose.
A paediatric study found that naïve children mounted a significant increase in B-cell responses (ASC and MBC) after LAIV. While primed children (HI titre≥40) had higher numbers, of pre-vaccination MBC which did not boost after LAIV, indicating a possible biological threshold for boosting. Memory B cells responses are rapid, producing more high affinity antibodies than naïve B-cells. Serum IgG levels correlate with resistance to infection.Citation69 This could imply that repeated vaccination with a LAIV could provide a more cross-reactive immune response towards influenza.
The effect of previous priming on the subsequent LAIV immunological response is unclear. The need for two doses of LAIV in young children is based on the belief that priming is essential. This is supported by a study where antibody titers were higher (although not significantly boosted) post-LAIV in children with pre-existing antibodies, indicating that LAIV perhaps assisted maintenance of the response.Citation32 In naïve children, protective HI titers were reached as early as 14 days post-vaccination indicating a rapid induction of protective antibodies.Citation35 The extended duration of the antibody secreting cell (ASC) response observed after LAIV than IIV, could be due to the local application and virus replication in the mucosa providing a longer stimulation period.Citation75
In 2014–15, the US experienced surprisingly and unexpected low efficacy of the H1N1 strain after LAIV but not IIV,Citation76,77 which led the USA advisory committee on immunization practices (ACIP) to withdraw its earlier preferential recommendation of the LAIV to children.Citation78,79 The manufacturer stated that the H1N1 strain had a temperature sensitive mutation rendering it heat instable, possibly explaining the lack of protection observed.Citation79 The manufacturer has updated the H1N1 strain used in LAIV vaccines from 2016, and future efficacy studies will indicate if this resolves the problem. There is also currently a disparity between LAIV vaccine effectiveness data in the US and Europe. In Europe LAIV has been found to provide moderate protection against H1N1pdm09 in the UK (41.5%) and Finland (47.9%), but remained lower than IIV.Citation80-82 Similarly, a study from Senegal found that LAIV failed to protect against H1N1pdm09 in young children,Citation83 whereas protection was found in a similar study in Bangladesh.Citation84 The reason for these differences is currently unknown, but could be related to the vaccine, the viruses or the population´s exposure history.Citation85,86 The main difference between the USA and Europe is the vaccine recommendations, with the USA recommending influenza vaccination for everyone > 6 months.
Tonsil responses after LAIV
Tonsils are secondary lymphoid tissue, located at the site of entry of the upper respiratory tract, draining the oral and nasal cavitiesCitation87 and are an important induction site and reservoir for B-and T-cells.Citation88-90 Since LAIV is administered in the nasal cavity, tonsils may play an important role in inducing immunological responses after LAIV. This is supported by expression of B-and T-cell activation markers in tonsils from children after LAIV.Citation91 A paediatric study found that LAIV induced early (7–14 days post-vaccination) B-cellular responses (ASC and MBC) in the tonsils.Citation35 MBC levels increased significantly post-vaccination in blood and tonsils in naïve children, while primed children maintained their high levels for up to one year in blood. Primed children had higher local MBCs in the tonsils pre-vaccination, which did not boost after LAIV. Furthermore, MBC levels correlated with systemic antibodies (HI), indicating that responses in the tonsils are reflected in the peripheral blood, which has earlier been observed for ASC numbers after IIV.Citation75 Further studies of induction of cell-mediated immunity after LAIV in tonsils are warranted. A recent study found that the nasal-associated lymphoid tissues (NALTs) act as an induction site for the recall and expansion of memory CD8+ T-cells after LAIV but did not activate naïve CD8+ T-cells, and this raises the question of whether LAIV can generate CD8 T-cells in the tonsils.Citation92
T-cell immune responses after LAIV vaccination
T-cells are critical for the control of viral infections, and may protect from severe illness or fatal outcome. Important human studies have shown that CD4+ and CD8+ T-cells are important in limiting disease and may confer heterosubtypic immunity.Citation61-63,93 In a human challenge study, pre-existing CD4+ T-cells resulted in less influenza symptoms.Citation61 During the 2009 pandemic, the presence of CD8+ T-cells was associated with less severe influenza illness.Citation62 Studies comparing the immune response after LAIV and IIV in children and adults, found that only children mounted a T-cellular response after LAIV.Citation31,37 In children, a robust T-cell response was found lasting six months and above the proposed protective level of 100 SFU/million PBMCs.Citation32,38,64 Interestingly, this study found that the few (n = 5) children, who did not respond serologically to the H1N1 strain, had significant increases in virus specific T-cell responses after vaccination.Citation32,64 This indicates that although these children were non-responders in the humoral compartment, they responded in T-cells, which could provide clinical protection.
The possibility of heterosubtypic protection
Natural infection provides the basis for cross-reactive T-cells, and historical studies provide evidence of hetero-variant protection after influenza infection.Citation61,94,95 Activation of T-cells by a viral infection is a dynamic and complex immune reaction where T-cells migrate between the blood and tissues.Citation96 Current research towards the desired universal influenza vaccine includes focus on T-cells and their potential for cross-protection across different IAVs. As opposed to IIV, the LAIV vaccine mimics natural infection inducing broad T-cellular responses.Citation31,32,97 Studies have shown that the LAIV provides protection in animals challenged with heterosubtypic influenza strains,Citation98-100 perhaps through induction of tissue-resident memory T cells (TRM) in the lungs.Citation101 More importantly, human studies have found that children were protected against a drifted H3N2 variant virus, which occurred naturally during clinical studies and was not contained in the vaccine.Citation31,97 Furthermore, the LAIV has shown the potential to confer broader protection than the IIVs in children when used in school settings.Citation50,63 The benefits of herd immunity have also been observed after LAIV vaccination of the child population in the UK, and T-cellular immune responses are considered to provide this observed effect.Citation25 A recent study found that LAIV reduced the risk of hospitalization due to influenza illness in young children,Citation85 perhaps due to boosting pre-existing T-cells to heterovariant strains not included in the vaccine.Citation64 In contrast to IIV, LAIV induces cross-protective CD8+ T-cells in young children, and when a novel pandemic arises, these CD8 T-cells may provide valuable protection.Citation102 In adults these influenza specific CD8+ T-cells have been shown to be long-lived, and are promising for the development of future influenza vaccines.Citation64,103 It has been suggested that using the LAIV in children could be an important step in the protection against a new pandemic.Citation104
Future perspectives
Although advancements have been made, the precise immunological events that ultimately produce long lasting and neutralizing antibodies or cross-reacting T-cells remains unclear. To design better vaccines, it is essential to better understand these complex immune responses induced by natural infection. Future research studying the immune responses after infection and LAIV vaccination will help answer some of these questions. Studying the responses in naïve children may perhaps illustrate an immunological scenario in adults where the population lacks protective antibodies. Studying the responses in primed children could aid in development of improved future seasonal vaccines. The recent licensure of a quadrivalent LAIV is an improvement, protecting from both influenza B lineages. However, some speculation about “competition” between the four LAIV viruses could explain the reported reduced effectiveness against the H1N1pdm09 strain.
The need for annual strain updates, time constraints of production as well as the late arrival of the 2009 pandemic vaccine has further motivated research into developing a “universal influenza vaccine”. Such a vaccine, would ideally afford protection against all influenza strains, a “one shot fix all” approach. The key to such a success lies in identifying conserved epitopes that exist in multiple influenza viruses (including highly conserved HA stalk,Citation105 or T-cell epitopesCitation106) followed by developing a vaccine, which elicits a durable effective immune response.Citation107 Vaccines capable of inducing cross-reactive T-cellular responses would be a major improvement, and such clinical trials are in focus.Citation10,108 However considerable efforts are needed before any replacement of today's influenza vaccines will occur.Citation109 In contrast to an earlier study,Citation96 we have recently shown that LAIV boosts cross-reactive CD8+ T-cells responses.Citation64 If LAIV provides protection across influenza A subtypes, this would have large public health implications. Larger studies will be needed to confirm the level of protection, and studies into the differences between US and European LAIV efficacy data are warranted as well.
There are no vaccines today, which are licensed on the basis of limiting severe disease. In the future, such a vaccine could prove valuable in reducing severe illness and the burden on healthcare systems from a novel virus and buy time before a specific vaccine is available.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We wish to thank Dr. Karl A Brokstad for valuable scientific input and for providing the illustration in .
Additional information
Funding
References
- WHO. Fact sheet about seasonal influenza. 2017. http://www.who.int/mediacentre/factsheets/fs211/en/
- Stohr K. Influenza–WHO cares. Lancet Infect Dis. 2002;2:517. doi:10.1016/S1473-3099(02)00366-3. PMID:12206966.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333-40. doi:10.1001/jama.292.11.1333. PMID:15367555.
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine. 2007;25:5086-96. doi:10.1016/j.vaccine.2007.03.046. PMID:17544181.
- WHO. Vaccines against influenza WHo position paper – November 2012. Weekly Epidemiological Record. 2012;87(47):461-476. PMID:23210147.
- CDC. People at high risk of developing Flu–Related complications. 2016. https://www.cdc.gov/flu/about/disease/high_risk.htm
- Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258-64. doi:10.1016/j.amjmed.2007.10.040. PMID:18374680.
- Gaglani MJ, Piedra PA, Riggs M, Herschler G, Fewlass C, Glezen WP. Safety of the intranasal, trivalent, live attenuated influenza vaccine (LAIV) in children with intermittent wheezing in an open-label field trial. Pediatr Infect Dis J. 2008;27:444-52. doi:10.1097/INF.0b013e3181660c2e. PMID:18401289.
- Miller MA, Viboud C, Olson DR, Grais RF, Rabaa MA, Simonsen L. Prioritization of influenza pandemic vaccination to minimize years of life lost. J Infect Dis. 2008;198:305-11. doi:10.1086/589716. PMID:18558871.
- Sridhar S, Brokstad KA, Cox RJ. Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines (Basel). 2015;3:373-89. doi:10.3390/vaccines3020373. PMID:26343192.
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152-79. PMID:1579108.
- CDC. Transmission of avian influenza a viruses between animals and people. 2015. https://www.cdc.gov/flu/avianflu/virus-transmission.htm
- Turner PJ, Southern J, Andrews NJ, Miller E, Erlewyn-Lajeunesse M, Investigators SS. Safety of live attenuated influenza vaccine in atopic children with egg allergy. J Allergy Clin Immunol. 2015;136:376-81. doi:10.1016/j.jaci.2014.12.1925. PMID:25684279.
- Toback SL, Levin MJ, Block SL, Belshe RB, Ambrose CS, Falloon J. Quadrivalent Ann Arbor strain live-attenuated influenza vaccine. Expert Rev Vaccines. 2012;11:1293-303. doi:10.1586/erv.12.108. PMID:23151111.
- Milian E, Kamen AA. Current and emerging cell culture manufacturing technologies for influenza vaccines. Biomed Res Int. 2015;2015:504831. doi:10.1155/2015/504831. PMID:25815321.
- Russell CA, Jones TC, Barr IG, et al. Influenza vaccine strain selection and recent studies on the global migration of seasonal influenza viruses. Vaccine. 2008;26 Suppl 4:D31-4. doi:10.1016/j.vaccine.2008.07.078. PMID:19230156.
- WHO. WHO recommendations on pandemic (H1N1) 2009 vaccines. 2009
- Bridges CB, Kuehnert MJ, Hall CB. Transmission of influenza: Implications for control in health care settings. Clin Infect Dis. 2003;37:1094-101. doi:10.1086/378292. PMID:14523774.
- Simonsen L, Clarke MJ, Schonberger LB, Arden NH, Cox NJ, Fukuda K. Pandemic versus epidemic influenza mortality: A pattern of changing age distribution. J Infect Dis. 1998;178:53-60. doi:10.1086/515616. PMID:9652423.
- Jefferson T, Rivetti A, Di Pietrantonj C, Demicheli V, Ferroni E. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2012;8:CD004879
- Pebody R, Green H, Andrews N, et al. Uptake and impact of a new live attenuated influenza vaccine programme in England: Early results of a pilot in primary school-age children, 2013/14 influenza season. Euro Surveill. 2014;19 (22). http://www.eurosurveillance.org/content/10.2807/ese.19.22.20823-en.ES2014.19.22.20823
- Folkehelseinstituttet. Fakta om influensa. 2016. https://www.fhi.no/en/id/influensa/seasonal-influenza/influenza---fact-sheet-about-season/
- Allen JE, Gardner SN, Vitalis EA, Slezak TR. Conserved amino acid markers from past influenza pandemic strains. BMC Microbiol. 2009;9:77. doi:10.1186/1471-2180-9-77. PMID:19386124.
- Salo H, Kilpi T, Sintonen H, Linna M, Peltola V, Heikkinen T. Cost-effectiveness of influenza vaccination of healthy children. Vaccine. 2006;24:4934-41. doi:10.1016/j.vaccine.2006.03.057. PMID:16678945.
- Dobson J, Whitley RJ, Pocock S, Monto AS. Oseltamivir treatment for influenza in adults: A meta-analysis of randomised controlled trials. Lancet. 2015;385:1729-37. doi:10.1016/S0140-6736(14)62449-1. PMID:25640810.
- Ampofo K, Gesteland PH, Bender J, et al. Epidemiology, complications, and cost of hospitalization in children with laboratory-confirmed influenza infection. Pediatrics. 2006;118:2409-17. doi:10.1542/peds.2006-1475. PMID:17142526.
- Morgan OW, Bramley A, Fowlkes A, et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS One. 2010;5:e9694. doi:10.1371/journal.pone.0009694. PMID:20300571.
- Lee N, Ison MG. Editorial commentary. “Late” treatment with neuraminidase inhibitors for severely ill patients with influenza: Better late than never?. Clin Infect Dis. 2012;55:1205-8. doi:10.1093/cid/cis642. PMID:22843780.
- Slepushkin AN, Obrosova-Serova NP, Burtseva EI, et al. Comparison of live attenuated and inactivated influenza vaccines in schoolchildren in Russia: I. Safety and efficacy in two Moscow schools, 1987/88. Vaccine. 1993;11:323-8. doi:10.1016/0264-410X(93)90194-3. PMID:8447161.
- Committee for Medicinal Products for Human Use. Guideline on Influenza Vaccines. Non-clinical and Clinical Module. 2016. EMA/CHMP/VWP/457259/2014. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/07/WC500211324.pdf
- Hoft DF, Babusis E, Worku S, et al. Live and inactivated influenza vaccines induce similar humoral responses, but only live vaccines induce diverse T-cell responses in young children. J Infect Dis. 2011;204:845-53. doi:10.1093/infdis/jir436. PMID:21846636.
- Mohn KG, Bredholt G, Brokstad KA, et al. Longevity of B-Cell and T-Cell responses after live attenuated influenza vaccination in children. J Infect Dis. 2015;211(10):1541–9. PMID:25425696.
- Clements ML, Murphy BR. Development and persistence of local and systemic antibody responses in adults given live attenuated or inactivated influenza A virus vaccine. J Clin Microbiol. 1986;23:66-72. PMID:3700610.
- Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667-71. doi:10.1038/nature06890. PMID:18449194.
- Mohn KG, Brokstad KA, Pathirana RD, et al. Live attenuated influenza vaccination in children induces B-cell responses in tonsils. J Infect Dis. 2016;214(5):722–731. doi:10.1093/infdis/jiw230. PMID:27247344.
- Belshe RB, Edwards KM, Vesikari T, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685-96. doi:10.1056/NEJMoa065368. PMID:17301299.
- He XS, Holmes TH, Zhang C, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756-66. doi:10.1128/JVI.01460-06. PMID:16971435.
- Forrest BD, Pride MW, Dunning AJ, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008;15:1042-53. doi:10.1128/CVI.00397-07. PMID:18448618.
- Cha TA, Kao K, Zhao J, Fast PE, Mendelman PM, Arvin A. Genotypic stability of cold-adapted influenza virus vaccine in an efficacy clinical trial. J Clin Microbiol. 2000;38:839-45. PMID:10655394.
- Kiseleva I, Dubrovina I, Bazhenova E, Fedorova E, Larionova N, Rudenko L. Possible outcomes of reassortment in vivo between wild type and live attenuated influenza vaccine strains. Vaccine. 2012;30:7395-9. doi:10.1016/j.vaccine.2012.09.076. PMID:23063833.
- Stiehm ER, Chin TW, Haas A, Peerless AG. Infectious complications of the primary immunodeficiencies. Clin Immunol Immunopathol. 1986;40:69-86. doi:10.1016/0090-1229(86)90070-X. PMID:3521971.
- Block SL, Yogev R, Hayden FG, Ambrose CS, Zeng W, Walker RE. Shedding and immunogenicity of live attenuated influenza vaccine virus in subjects 5–49 years of age. Vaccine. 2008;26:4940-6. doi:10.1016/j.vaccine.2008.07.013. PMID:18662737.
- Ambrose CS, Dubovsky F, Yi T, Belshe RB, Ashkenazi S. The safety and efficacy of live attenuated influenza vaccine in young children with asthma or prior wheezing. Eur J Clin Microbiol Infect Dis. 2012;31:2549-57. doi:10.1007/s10096-012-1595-9. PMID:22410646.
- Ison MG, Hui DS, Clezy K, et al. A clinical trial of intravenous peramivir compared with oral oseltamivir for the treatment of seasonal influenza in hospitalized adults. Antivir Ther. 2013;18:651-61. doi:10.3851/IMP2442. PMID:23111657.
- Caspard H, Heikkinen T, Belshe RB, Ambrose CS. A systematic review of the efficacy of live attenuated influenza vaccine upon revaccination of children. Hum Vaccin Immunother. 2016;12(7):1721–7
- Jefferson T, Rivetti A, Harnden A, Di Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database Syst Rev. 2008;16(2):CD004879. PMID:18425905.
- Jefferson T, Di Pietrantonj C, Rivetti A, Bawazeer GA, Al-Ansary LA, Ferroni E. Vaccines for preventing influenza in healthy adults. Cochrane Database Syst Rev. 2010:CD001269 PMID:20614424.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36-44. doi:10.1016/S1473-3099(11)70295-X. PMID:22032844.
- Coelingh K, Olajide IR, MacDonald P, Yogev R. Efficacy and effectiveness of live attenuated influenza vaccine in school-age children. Expert Rev Vaccines. 2015;14(10):1331–46
- Ambrose CS, Levin MJ, Belshe RB. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir Viruses. 2011;5:67-75. doi:10.1111/j.1750-2659.2010.00183.x. PMID:21306569.
- Tam JS, Capeding MR, Lum LC, et al. Efficacy and safety of a live attenuated, cold-adapted influenza vaccine, trivalent against culture-confirmed influenza in young children in Asia. Pediatr Infect Dis J. 2007;26:619-28. doi:10.1097/INF.0b013e31806166f8. PMID:17596805.
- Belshe RB, Toback SL, Yi T, Ambrose CS. Efficacy of live attenuated influenza vaccine in children 6 months to 17 years of age. Influenza Other Respir Viruses. 2010;4:141-5. doi:10.1111/j.1750-2659.2009.00124.x. PMID:20409210.
- Rhorer J, Ambrose CS, Dickinson S, et al. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine. 2009;27:1101-10. doi:10.1016/j.vaccine.2008.11.093. PMID:19095024.
- Ashkenazi S, Vertruyen A, Aristegui J, et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr Infect Dis J. 2006;25:870-9. doi:10.1097/01.inf.0000237829.66310.85. PMID:17006279.
- Fleming DM, Crovari P, Wahn U, et al. Comparison of the efficacy and safety of live attenuated cold-adapted influenza vaccine, trivalent, with trivalent inactivated influenza virus vaccine in children and adolescents with asthma. Pediatr Infect Dis J. 2006;25:860-9. doi:10.1097/01.inf.0000237797.14283.cf. PMID:17006278.
- Caspard H, Heikkinen T, Belshe RB, Ambrose CS. A systematic review of the efficacy of live attenuated influenza vaccine upon revaccination of children. Hum Vaccin Immunother. 2016;12:1721-7. PMID:26751513.
- Black S, Nicolay U, Vesikari T, et al. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatr Infect Dis J. 2011;30:1081-5. doi:10.1097/INF.0b013e3182367662. PMID:21983214.
- Bandell A, Woo J, Coelingh K. Protective efficacy of live-attenuated influenza vaccine (multivalent, Ann Arbor strain): A literature review addressing interference. Expert Rev Vaccines. 2011;10:1131-41. doi:10.1586/erv.11.73. PMID:21854309.
- Wright PF, Hoen AG, Ilyushina NA, et al. Correlates of immunity to influenza as determined by challenge of children with live, Attenuated influenza vaccine. Open Forum Infect Dis. 2016;3:ofw108. doi:10.1093/ofid/ofw108. PMID:27419180.
- Jegaskanda S, Luke C, Hickman HD, et al. Generation and protective ability of influenza virus-specific antibody-dependent cellular cytotoxicity in humans elicited by vaccination, Natural infection, and Experimental challenge. J Infect Dis. 2016;214:945-52. doi:10.1093/infdis/jiw262. PMID:27354365.
- Wilkinson TM, Li CK, Chui CS, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18:274-80. doi:10.1038/nm.2612. PMID:22286307.
- Sridhar S, Begom S, Bermingham A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305-12. doi:10.1038/nm.3350. PMID:24056771.
- Hayward AC, Wang L, Goonetilleke N, et al. Natural T cell mediated protection against seasonal and pandemic influenza: Results of the flu watch cohort study. Am J Respir Crit Care Med. 2015;191(12):1422–31. doi:10.1164/rccm.201411-1988OC.
- van de Sandt CE, Hillaire ML, Geelhoed-Mieras MM, Osterhaus AD, Fouchier RA, Rimmelzwaan GF. Human influenza a virus-specific CD8+ T-Cell response is long-lived. J Infect Dis. 2015;212:81-5. doi:10.1093/infdis/jiv018. PMID:25583167.
- Ambrose CS, Yi T, Walker RE, Connor EM. Duration of protection provided by live attenuated influenza vaccine in children. Pediatr Infect Dis J. 2008;27:744-8. doi:10.1097/INF.0b013e318174e0f8. PMID:18600188.
- Ambrose CS, Wu X, Jones T, Mallory RM. The role of nasal IgA in children vaccinated with live attenuated influenza vaccine. Vaccine. 2012;30:6794-801. doi:10.1016/j.vaccine.2012.09.018. PMID:23000125.
- Beyer WE, Palache AM, de Jong JC, Osterhaus AD. Cold-adapted live influenza vaccine versus inactivated vaccine: Systemic vaccine reactions, local and systemic antibody response, and vaccine efficacy. A meta-analysis. Vaccine. 2002;20:1340-53. doi:10.1016/S0264-410X(01)00471-6. PMID:11818152.
- Fry AM. Effectiveness of neuraminidase inhibitors for severe influenza. Lancet Respir Med. 2014;2:346-8. doi:10.1016/S2213-2600(14)70068-2. PMID:24815800.
- CDC. Prevention and control of influenza with vaccines: Recommendations of the advisory committee on immunization practices, United States, 2015–16 influenza season. Morbidity and Mortality Weekly Report (MMWR). 2015;64(30):818–825
- Belshe RB, Gruber WC, Mendelman PM, et al. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181:1133-7. doi:10.1086/315323. PMID:10720541.
- Treanor JJ, Kotloff K, Betts RF, et al. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999;18:899-906. doi:10.1016/S0264-410X(99)00334-5. PMID:10580204.
- Park AW, Daly JM, Lewis NS, Smith DJ, Wood JL, Grenfell BT. Quantifying the impact of immune escape on transmission dynamics of influenza. Science. 2009;326:726-8. doi:10.1126/science.1175980. PMID:19900931.
- Couch RB, Atmar RL, Franco LM, et al. Prior infections with seasonal influenza A/H1N1 virus reduced the illness severity and epidemic intensity of pandemic H1N1 influenza in healthy adults. Clin Infect Dis. 2012;54:311-7. doi:10.1093/cid/cir809. PMID:22075792.
- Mohn KG, Brokstad KA, Pathirana RD, et al. Live attenuated influenza vaccine in children induces B-Cell responses in tonsils. J Infect Dis. 2016;214:722-31. doi:10.1093/infdis/jiw230. PMID:27247344.
- Brokstad KA, Cox RJ, Olofsson J, Jonsson R, Haaheim LR. Parenteral influenza vaccination induces a rapid systemic and local immune response. J Infect Dis. 1995;171:198-203. doi:10.1093/infdis/171.1.198. PMID:7798664.
- CDC. Interim Estimates of 2013–14 seasonal influenza vaccine Effectiveness — United States, February 2014. MMWR Morb Mortal Wkly Rep. 2014;63(7):137–142
- Dowdle WR. Influenza A virus recycling revisited. Bull World Health Organ. 1999;77:820-8. PMID:10593030.
- CDC newsroom. Advisory Committee on Immunization . Practices (ACIP) recommends a preference for using the nasal spray flu vaccine. 2014. https://www.cdc.gov/media/releases/2014/s0625-acip.html
- CDC newsroom. ACIP votes down use of LAIV for 2016-2017 flu season. 2016. https://www.cdc.gov/media/releases/2016/s0622-laiv-flu.html
- Nohynek H, Baum U, Syrjanen R, Ikonen N, Sundman J, Jokinen J. Effectiveness of the live attenuated and the inactivated influenza vaccine in two-year-olds – a nationwide cohort study Finland, influenza season 2015/16. Euro Surveill. 2016;21 (38):30346. http://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.38.30346. PMID:27684447.
- Pebody R, Warburton F, Ellis J, et al. Effectiveness of seasonal influenza vaccine for adults and children in preventing laboratory-confirmed influenza in primary care in the United Kingdom: 2015/16 End-of-season results. Euro Surveill. 2016;21(38):30348. http://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.38.30348
- Zimmerman RK, Nowalk MP, Chung J, et al. 2014–2015 influenza vaccine effectiveness in the United States by vaccine type. Clin Infect Dis. 2016;63:1564-1573. doi:10.1093/cid/ciw635. PMID:27702768.
- Victor JC, Lewis KD, Diallo A, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among children in Senegal: A randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2016;4:e955-e965. doi:10.1016/S2214-109X(16)30201-7. PMID:27746224.
- Brooks WA, Zaman K, Lewis KD, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among young children in Bangladesh: A randomised, double-blind, placebo-controlled trial. Lancet Glob Health. 2016;4:e946-e954. doi:10.1016/S2214-109X(16)30200-5. PMID:27746226.
- Pebody R, Sile B, Warburton F, et al. Live attenuated influenza vaccine effectiveness against hospitalisation due to laboratory-confirmed influenza in children two to six years of age in England in the 2015/16 season. Euro Surveill. 2017;22. http://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2017.22.4.30450
- Chung JR, Flannery B, Thompson MG, et al. Seasonal Effectiveness of Live Attenuated and Inactivated Influenza Vaccine. Pediatrics. 2016;137(2):e20153279. doi:10.1542/peds.2015-3279.
- Dressel H, Nowak D. [Influenza vaccination for health care workers: Should we strengthen individual responsibility or should it be mandatory?]. Dtsch Med Wochenschr. 2009;134:1649. doi:10.1055/s-0029-1233995. PMID:19650029.
- Brandtzaeg P. Immunology of tonsils and adenoids: Everything the ENT surgeon needs to know. Int J Pediatr Otorhinolaryngol. 2003;67 Suppl 1:S69-76. doi:10.1016/j.ijporl.2003.08.018. PMID:14662171.
- Sada-Ovalle I, Talayero A, Chavez-Galan L, et al. Functionality of CD4+ and CD8+ T cells from tonsillar tissue. Clin Exp Immunol. 2012;168:200-6. doi:10.1111/j.1365-2249.2012.04573.x. PMID:22471281.
- Quiding-Jarbrink M, Granstrom G, Nordstrom I, Holmgren J, Czerkinsky C. Induction of compartmentalized B-cell responses in human tonsils. Infect Immun. 1995;63:853-7. PMID:7868256.
- Panapasa JA, Cox RJ, Mohn KG, Aqrawi LA, Brokstad KA. The expression of B & T cell activation markers in children's tonsils following live attenuated influenza vaccine. Hum Vaccin Immunother. 2015;11:1663-72. doi:10.1080/21645515.2015.1032486. PMID:26148331.
- Pizzolla A, Wang Z, Groom JR, et al. Nasal-associated lymphoid tissues (NALTs) support the recall but not priming of influenza virus-specific cytotoxic T cells. Proc Natl Acad Sci U S A. 2017;114:5225-5230. doi:10.1073/pnas.1620194114. PMID:28461487.
- Kucharski AJ, Gog JR. The role of social contacts and original antigenic sin in shaping the age pattern of immunity to seasonal influenza. PLoS Comput Biol. 2012;8:e1002741. doi:10.1371/journal.pcbi.1002741. PMID:23133346.
- McMichael AJ, Gotch FM, Noble GR and Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13-7. doi:10.1056/NEJM198307073090103. PMID:6602294.
- Francis T. The doctrine of original antigenic sin. Proceedings of the American Philosophical Society 104, 6 (Dec. 15, 1960), pp. 572-578 1960;104:572-578
- He XS, Holmes TH, Mahmood K, et al. Phenotypic changes in influenza-specific CD8+ T cells after immunization of children and adults with influenza vaccines. J Infect Dis. 2008;197:803-11. doi:10.1086/528804. PMID:18279048.
- Belshe RB, Gruber WC, Mendelman PM, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168-75. doi:10.1016/S0022-3476(00)70097-7. PMID:10657821.
- Cheng X, Zengel JR, Suguitan AL, Jr., et al. Evaluation of the humoral and cellular immune responses elicited by the live attenuated and inactivated influenza vaccines and their roles in heterologous protection in ferrets. J Infect Dis. 2013;208:594-602. doi:10.1093/infdis/jit207. PMID:23656978.
- Rekstin A, Isakova-Sivak I, Petukhova G, et al. Immunogenicity and cross protection in mice afforded by Pandemic H1N1 live attenuated influenza vaccine containing wild-type nucleoprotein. Biomed Res Int. 2017;2017:9359276. doi:10.1155/2017/9359276. PMID:28210631.
- Jang YH, Seong BL. Cross-protective immune responses elicited by live attenuated influenza vaccines. Yonsei Med J. 2013;54:271-82. doi:10.3349/ymj.2013.54.2.271. PMID:23364956.
- Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI Insight. 2016;1(10):e85832. doi:10.1172/jci.insight.85832. PMID:27468427.
- Bodewes R, Kreijtz JH, Rimmelzwaan GF. Yearly influenza vaccinations: A double-edged sword?. Lancet Infect Dis. 2009;9:784-8. doi:10.1016/S1473-3099(09)70263-4. PMID:19879807.
- Soema PC, van Riet E, Kersten G, Amorij JP. Development of cross-protective influenza a vaccines based on cellular responses. Front Immunol. 2015;6:237. doi:10.3389/fimmu.2015.00237. PMID:26029218.
- Dropulic LK, Cohen JI. Severe viral infections and primary immunodeficiencies. Clin Infect Dis. 2011;53:897-909. doi:10.1093/cid/cir610. PMID:21960712.
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167-82. doi:10.1038/nrd4529. PMID:25722244.
- Quinones-Parra S, Grant E, Loh L, et al. Preexisting CD8+ T-cell immunity to the H7N9 influenza A virus varies across ethnicities. Proc Natl Acad Sci U S A. 2014;111:1049-54. doi:10.1073/pnas.1322229111. PMID:24395804.
- Krammer F. Emerging influenza viruses and the prospect of a universal influenza virus vaccine. Biotechnol J. 2015;10:690-701. doi:10.1002/biot.201400393. PMID:25728134.
- Soema PC, Kompier R, Amorij JP, Kersten GF. Current and next generation influenza vaccines: Formulation and production strategies. Eur J Pharm Biopharm. 2015;94:251-63. doi:10.1016/j.ejpb.2015.05.023.
- Wang TT, Tan GS, Hai R, et al. Broadly protective monoclonal antibodies against H3 influenza viruses following sequential immunization with different hemagglutinins. PLoS Pathog. 2010;6:e1000796. doi:10.1371/journal.ppat.1000796.
