ABSTRACT
Meningococcal group B outer membrane vesicle vaccines have been used widely in Cuba, New Zealand, and Brazil. They are immunogenic and initially assessed largely by their ability to induce serum bactericidal activity. Measures of efficacy indicate good protection against homologous strains in older children and adults. Effectiveness appears broader than predicted by immunogenicity and efficacy studies. The recent discovery that meningococcal group B OMVs may protect against the related Neisseria species N.gonorrhoeae suggests more to these interesting antigen collections than meets the eye.
Currently there are two OMV-containing group B vaccines available, the new recombinant protein-based Bexsero® developed by Novartis and VA-MENGOC-BC® developed by the Finlay institute in Cuba. Also, a third group B vaccine based on two recombinant factor H binding proteins (Trumenba®, Pfizer), has recently been licenced but it does not include OMV. This commentary explores the population impact that group B OMV vaccines have had on meningococcal and gonorrhoea diseases. Given the heterologous effect against diverse strains of the meningococcus observed in older children and adults, and recent evidence to suggest moderate protection against gonorrhoea, there may be a role for these vaccines in programmes targeting adolescents and groups high at risk for both meningococcal disease and gonorrhoea.
The Neisseria challenge
Gram negative Neisseria is an important cause of both invasive meningococcal disease and gonorrhoea infections globally. While effective vaccines against meningococcal groups A, C, W and Y have been available for over 50 years, meningococcal group B vaccines have posed challenges.Citation1,2 Despite efforts, little progress has been made toward effective vaccines against the gonococcus, now a multidrug resistant super bug.Citation3,4
The most celebrated vaccine strategies against Neisseria meningitides (Nm) have resided in the conjugation of capsular polysaccharide to an immunogenic carrier protein such as tetanus or diphtheria toxoid.Citation5 However, vaccines against Nm serogroup B required alternative approaches. This is due to poor immunogenicity of the polysaccharide and likely homology of this antigen to fetal neural tissue.Citation6
The solution, in part, to this problem has been in the development of group B strain specific vaccines based on the OMV expressing, in particular, the immunodominant protein Porin A (PorA). OMVs are usually extracted by detergent, purified, and the lipopolysaccharides (LPS) further detoxified by adsorption to aluminium adjuvant.Citation7 Based largely on immunogenicity studiesCitation8 these vaccines have traditionally been considered useful in situations where disease is primarily caused by a clonal outbreak arising from a single strain.Citation9,10
Accurate estimates of the vaccine effectiveness against meningococcal disease are currently hampered by the low number of cases. While devastating, meningococcal disease is a relatively rare disease. Studies that have assessed the effectiveness of OMVs conducted in Cuba, Brazil, NZ, and now England, have wide confidence intervals around the estimates. Assessing effectiveness is further compounded by the rapid reduction in cases once the vaccine is introduced.Citation11
OMV vaccines in older children and adults
Cuba
The history of OMV vaccines began in Cuba and Norway. Cuba had high rates of meningococcal disease, noted as a major problem from 1976. Initially most disease was caused by groups B and C, then in the latter 70s and early 80s 95% of disease became dominated by a single serotype (B4:P1.15). The epidemic peaked from 1983. To address the epidemic Finlay Institute, Havana, Cuba, developed and successfully tested the first effective group B OMV vaccine (VA-MENGOC-BC®).Citation12 This vaccine also included group C polysaccharide and was initially assessed in a cluster randomised trial conducted between 1987 and 1989 among 133,600 10–14 year olds in a two dose schedule. Vaccine efficacy against meningococcal disease was estimated to be 83%.Citation12
In addition to efficacy, immunological studies in healthy adults determined long-lasting bactericidal and specific antibody against several group B strains and a strong anamnestic response after a booster at 18 months. The lead author attributed the cross reactivity to the presence of high molecular weight proteins of which there are six, and noted the importance of these for immunological memory and cross reactivity, in particular those associated with iron metabolism.Citation12
Following the success of the efficacy trials Cuba implemented a mass vaccination campaign during 1989 and 1990, commencing with the 10–14 age group. The estimate of effectiveness was 92%. The vaccine was then administered to 3,572,900 infants, children and adolescents aged from 3-months to 20-yearsCitation13 before being incorporated into the national immunisation schedule for 3-month old infants.
In addition to the epidemic strain, the vaccine appeared to induce long lasting bactericidal activity against all pathogenic group B species with an estimated efficacy of 83–94% in children aged 10—14 years after two doses, depending on geographical region.Citation12 Since implementation of VA-MENGOC-BC® the meningococcal rates in Cuba have remained low, with a population incidence of <1 per 100,000 maintained since 1993 and <0.5 per 100,000 since 2000. The rate for the years 2008 to 2016 was 0.1 per 100,000.Citation14
Further effectiveness data for VA-MENGOC-BC® came from Brazil during a group B epidemic in Sao Paolo, dominated by a strain with the same sub-type as Cuba (B:15:P1.15). Brazil evaluated the effectiveness in a case-control study after 2.4 million children aged under 6-years were vaccinated between 1990 and 1991. Effectiveness was estimated to be 74% (95% CI: 16–92%) in children over 4-years of age, while there was no evidence of effectiveness in the younger children. In this large population, as observed in Cuba, the effectiveness against strain specific disease was the same as the effectiveness against heterologous strains,Citation15 a finding that supports the value of the other OMV antigens present in the vaccine.
Norway
From 1974 to 1990, like Cuba, Norway experienced a meningococcal epidemic dominated by a single subtype (B:15:P1.7,16). An OMV vaccine (MenBVac) was developed in 1983 and tested in efficacy trials, delivering two doses of vaccine or placebo to 171,800 adolescents from 1988 to 1991.Citation16 Efficacy was estimated to be 87% after 10 months of observation, falling to 57% after 29 months. The rapid decline of serum bactericidal activity (SBA) prevented the vaccine being used in a national vaccination campaignCitation16 and effectiveness was not explored until 2011 when MenBVac was used to control an epidemic in France.
Between 2006 and 2009, MenBVac was delivered to 26,014 individuals aged under 20-years living in Normandy, France in response to a group B outbreak dominated by B:14:P1.7,16, the same serosubtype as Norway. Most recipients received a 2+1 schedule at weeks 0, 6 and month 8. Although a formal effectiveness study was not performed, the epidemiology of the dominant type was described. The incidence of B:14:P1.7,16 cases decreased from 31.6 in 2006 to 5.9 per 100,000 in 2009 among the group targeted for vaccination.Citation17
The NZ experience
Rates of meningococcal disease in NZ increased in the early 1990s with most cases caused by B:4:P1.7b. A partnership was formed between the Norwegian Institute of Public Health, Chiron (which later became Novartis), the University of Auckland, the Institute of Environmental Science and Research and the World Health Organization to develop a vaccine strategy. The outcome was MeNZB™, a tailor-made OMV vaccine, based on the Norwegian MenBVac, developed, and tested in phase I and II trials, and then rolled out in a mass immunisation campaign. Between 2004 and 2006 one million individuals aged 6-weeks to 20-years were vaccinated with MeNZB™, which was subsequently withdrawn in 2008 due to low uptake among infants, and lack of data to support the co-administration with pneumococcal vaccine, a priority for the national schedule.Citation18
Using data from 2001 to June 2006, the effectiveness of MeNZB™ was estimated to be 75% (95% CI; 52–85%).Citation19 Two years later the effectiveness against strain-specific disease in people aged 6-months to 19-years was estimated to be 68%. After three doses and adjustments for confounders and a programmatic effect, effectiveness against non-strain specific group B was conservatively estimated to be 56% (95% CI; 17–77%), and against all meningococcal disease 67% (95% CI; 57–76%), demonstrating protection beyond the PorA subtype.Citation20
However, the overall impact of MeNZB™ on the epidemic was moderate. By the time MeNZB™ had been developed, tested, and rolled out, the epidemic had naturally waned.Citation21
Bexsero®
The OMV vaccines in Cuba, Brazil, Norway and NZ performed reasonably well in older children and adults, and cross protection was clearly evidenced. Most data indicated limited application in infants and younger children, and immune responses are highly sero-subtype-specific in this group.Citation7 To address this three group B vaccine candidates were identified using reverse vaccinology and developed into an investigational recombinant group B vaccine (rMenB). Immunogenicity studies compared three doses of rMenB administered alone or with an rMenB formulation that had the MeNZB™ OMV added. The rMenB alone induced bactericidal antibody in more than 80% of participants against three out of seven reference group B strains. However, with the rMenB plus MeNZB™ OMV more than 90% of participants developed a greater than 4-fold hSBA titre for five strains and 70% for a sixth strain.Citation22
The combination of recombinant proteins with the OMV also resulted in improved performance of the OMV in this age group when compared with the immunogenicity of the OMV alone observed in the NZ studies,Citation23 supporting a synergistic effect.
Prior to licensure Bexsero® was used in response to a meningococcal group B outbreak at a university in the United States in 2013. Two doses of vaccine were administered 10 weeks apart to 499 vaccinees. No cases were observed among vaccinated students.Citation24 In May 2014, Canada implemented a brief mass immunisation campaign with Bexsero® in a region in Quebec. Of 59,000 target residents aged under 20-years 82% were vaccinated in the 7 months to 31 December 2014. There were four cases in unvaccinated persons and none among the vaccinated,Citation25 providing early indications of vaccine effectiveness.
The UK is the first country able to estimate the effectiveness of Bexsero® after addition of the vaccine to the infant immunisation programme. Though the predicted effectiveness based on the Meningococcal Antigen Typing System (MATS) was 75%, the effectiveness of pooled sera was 88%. In a cohort study conducted 10-months after implementation, two-doses of vaccine were estimated to be 82.9% (95% CI; 24·1–95·2) against all meningococcal B cases, and 94.2% effective against vaccine specific cases. Within this short period, the cases in eligible infants halved.Citation26
Evidence to date supports that Bexsero® offers broad meningococcal group B protection in all age groups.
Performance of OMV vaccines in younger age groups
With the exception of Bexsero®, the overall performance of OMV vaccines in younger age cohorts is markedly lower than that observed in older age groups. The problem is likely twofold. First, studies evaluating two OMV vaccine doses consistently found low or no evidence for effectiveness in younger age groups. The NZ experience indicated that a three-dose primary regime for younger children and the addition of a fourth/booster dose for infants could overcome this to a certain extent.Citation20 Secondly, younger children are more likely to be un-primed by environmental Neisseria species.
The VA-MENGOC-BC® showed poor immunogenicity among young children in Brazil, where a high proportion of the disease was caused by the same serotype or subtype antigens as the Cuban vaccine strain. Whilst 74% protective in older children, it had no measurable effect in children aged under two years.Citation15 However, specific IgG of lasting duration was observed as was the induction of a T-cell immune memory response, even in infancy, and both boost strongly.Citation27,28
To assess the potential effectiveness of the VA-MENGOC-BC for an outbreak in Chile, immunogenicity studies were conducted in infants, children aged 2–4 years, and young adults, in a three-dose regime given 2-months apart. All age groups responded well to the homologous strain. However, older children and adults also developed SBA to heterologous types but infants did not, limiting but not precluding the use of the vaccine against a heterologous strain.Citation8
Immunogenicity versus impact
While there is little doubt as to the importance of SBA in protection against meningococcal disease, and as a gold standard correlate of protection, not all individuals with less than a 4-fold rise in SBA are susceptible to disease.Citation29 A systematic review evaluating the predicted versus observed effectiveness of group B OMV vaccines found that predicted effectiveness based on SBA tended to be lower than the observed effectiveness. Two possible reasons for this have been posed. One, effectiveness studies may have overestimated the protective effect due to unadjusted residual confounding;Citation20 or two, while SBA is a correlate, it is not necessarily exclusive and other mechanisms also play a role, such as opsonophagocytosis.Citation30 This is supported by several lines of evidence including the lack of a relationship between disease incidence and seroprevalence of SBA;Citation31 and the higher than predicted efficacy measured after SBA titres have declined below 1:4.Citation32 Demonstration of immunogenicity using the SBA assay, may be only an indirect and incomplete estimate of protective immunity, and an absence of SBA does not mean an absence of protection.Citation33
Although the lofty goal of vaccine development are vaccines that induce complete protective immunity with no adverse side effects, there may be reason not to throw the baby out with the water when one falls well short of this ideal. The question is what is adequate enough to warrant the effort and expense? Rather than focusing on measures of vaccine immunogenicity or efficacy it can be helpful to also consider the overall impact of a vaccine on the disease in the population. An important example is that of Cuba. Since the initial mass campaign, followed by implementation of the VE-MENGOC-BC® in 1988 the incidence of all meningococcal disease across all age groups has declined from a pre-vaccine high of 14.3 per 100,000 in 1983Citation34 to a sustained less than 1 per 100,000 since 1993 through to 2006. Between 2008 and 2016 the rates dropped further and have remained at 0.1.Citation14 While this 25-year meningococcal hiatus (<1 per 100,000 population) could reflect a natural decline and current baseline of Nm in Cuba, it could alternatively indicate a significant vaccine impact on community immunity.
Beyond meningococcal disease – gonorrhoea
Epidemiological evidence from Cuba, Brazil, and NZ has demonstrated that Nm OMV vaccines can provide broad protection against meningococcal disease. This led to the hypothesis they may affect a more distantly related bacterium. Graphed surveillance data clearly show a marked decline in the incidence of gonorrhoea in Cuba following implementation of the VA-MENGOC-BC®, in contrast to syphilis and genital warts for which incidences remained the same. A double peak and lag in gonorrhoea cases before the decline coincide with the mass catch-up immunisation campaign and then the age of sexual onset in the birth cohort.Citation13
NZ also saw a decline in reported gonorrhoea cases during and after use of MeNZB™ (). No other sexually transmitted infections (STI)s described in the national surveillance reports declined during this period.Citation35 These ‘eyeball’ observations suggested that these Nm OMV vaccines could possibly offer cross protection against gonorrhoea.
Figure 1. Gonorrhoea rates in selected regions, 1998–2014.Citation35
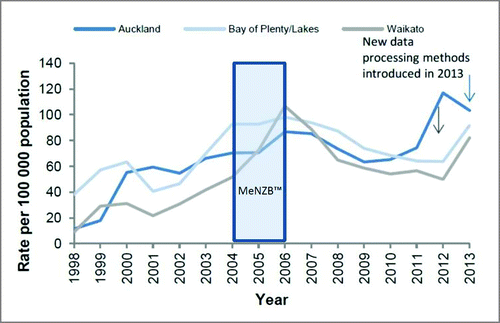
To test this hypothesis a retrospective case-control study of 14,730 sexual health clinic patients aged 15–30 years, who had been eligible to receive the MeNZB™ vaccine, was carried out in NZ. The outcomes of interest were laboratory confirmed gonorrhoea and, as a control, chlamydia. Demographic information and vaccine status were linked to the cases using the NZ unique personal identifier (National Health Index Number). The odds of disease outcomes in vaccinated and unvaccinated participants were compared. Individuals who had received the MeNZB™ vaccine were significantly less likely to be cases than controls with an adjusted OR 0·69 (95% CI 0·61–0·79%); p < 0·0001.Citation36 A subsequent national cohort study found a significant vaccine effectiveness against gonorrhoea-associated hospitalisation.Citation37 While causality is best assessed in a randomised controlled trial these data support the value of undertaking such an assessment. Given the lack of correlates of protection against gonorrhoea infection and progress in vaccine candidates these findings were both surprising and intriguing.
Perhaps some clues reside in the fact that OMV vaccines are notably more qualitatively and quantitatively immunogenic in older age groups, evidenced by the strong heterologous boosting in older ages.Citation38,39 Nm carriage rates can be high,Citation31 as demonstrated in cross sectional studies, and protective immunity appears to ensue. However, the resulting immunity from carriage has not yet been well characterised.Citation31
If carriage results in protective immunity then perhaps we would expect to see patterns of gonorrhoea incidence affected by Nm epidemics. Conversely would we see these patterns affected by meningococcal vaccine programs? Perhaps to make sense of this we will need to step back from SBA and give more consideration to cellular and mucosal immunity, of which SBA may not be directly predictive.Citation40 Some evidence suggests that when superimposed on naturally acquired immunity, an OMV vaccine can selectively re-programme the mucosal compartment,Citation40 a potential mechanistic avenue for the somewhat paradoxical effect of the group B meningococcal vaccines on both heterologous strains as well as gonorrhoea.
Commensal bacteria are noted to be efficient promoters of mucosal lymphoid tissue developmentCitation41 and Neisseria species establish in the mucosa where IgA and transduction of IgG are the primary humoral effectors. Human parotid saliva positive for Nm IgA has cross recognition against Neisseria gonorrhoeae (Ng).Citation13 Boosting individuals positive for Nm IgA with VA-MENGOC-BC® results in an increase in this immunity.Citation13 Also, given this vaccine induces a strong cellular immune response, the presence of SBA may underestimate protection.Citation27
Implications
There are currently two Nm group B vaccines that include OMVs in wide use. Both appear to provide broad protection against their Nm target diseases and both are likely to offer cross protection against gonorrhoea to a greater or lesser extent. While Bexsero® has not yet been formally assessed against Ng it not only contains the antigen that has been (MeNZB™),Citation36 but also two of the three recombinant proteins (fHbp and NHBP) in the formulation are variably expressed in Ng isolates and NHBP induces cross reactive antibodies against the gonoccocus.Citation42 While also not formally assessed, VA-MENGOC-BC® appears ecologically associated with a remarkable decline in Ng disease incidence in Cuba.Citation13 Mathematical modelling suggests that even a moderate efficacy and duration of effect could have considerable public health impact with respect to gonorrhoea.Citation43-45 Provision of a booster dose of one of these vaccines at an age prior to sexual debut, where commensal carriage and possibly priming with an infant dose of OMV vaccine may provide a basis for heterologous boosting, could be worth exploring as a strategy to mitigate the growing burden of gonorrhoea, as well as enhancing population immunity against Nm.
These OMV vaccines may hold clues to inform gonorrhoea vaccine development and existing off-the-shelf vaccines containing Nm OMV may offer us an exciting way forward in the vexatious problem of super-gonorrhoea.
Disclosure of potential conflicts of interest
HPH has been a consultant for GSK, Merck, and Pfizer but has not received honorarium.
References
- Holst J, Feiring B, Naess LM, Norheim G, Kristiansen P, Høiby EA, Bryn K, Oster P, Costantino P, Taha MK, et al. The concept of “tailor-made”, protein-based, outer membrane vesicle vaccines against meningococcal disease. Vaccine. 2005;23:2202-5. doi:10.1016/j.vaccine.2005.01.058. PMID:15755595
- Giuliani MM, Adu-Bobie J, Comanducci M, Aricò B, Savino S, Santini L, Brunelli B, Bambini S, Biolchi A, Capecchi B, et al. A universal vaccine for serogroup B meningococcus. Proc Natl Acad Sci U S A. 2006;103:10834-9. doi:10.1073/pnas.0603940103. PMID:16825336
- Wi T, Lahra MM, Ndowa F, Bala M, Dillon JR, Ramon-Pardo P, Eremin SR, Bolan G, Unemo M. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLoS Med. 2017;14:e1002344. doi:10.1371/journal.pmed.1002344. PMID:28686231
- Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, et al. Sexually transmitted infections: Challenges ahead. Lancet Infect Dis. 2017;17(8):e235-e79. doi:10.1016/S1473-3099(17)30310-9. PMID:28701272
- Zahlanie YC, Hammadi MM, Ghanem ST, Dbaibo GS. Review of meningococcal vaccines with updates on immunization in adults. Hum Vaccin Immunother. 2014;10:995-1007. doi:10.4161/hv.27739. PMID:24500529
- Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis–implications for vaccine development and pathogenesis. Lancet. 1983;2:355-7. doi:10.1016/S0140-6736(83)90340-9. PMID:6135869
- Wang NY, Pollard AJ. The next chapter for group B meningococcal vaccines. Crit Rev Microbiol. 2017:1-17. doi:10.1080/1040841X.2017.1329276. PMID:28557577
- Tappero JW, Lagos R, Maldonado Ballesteros A, Plikaytis B, Williams D, Dykes J, Gheesling LL, Carlone GM, Høiby EA, Holst J, et al. Immunogenicity of 2 serogroup b outer-membrane protein meningococcal vaccines: A randomized controlled trial in chile. JAMA. 1999;281:1520-7. doi:10.1001/jama.281.16.1520. PMID:10227322
- Tauseef I, Ali YM, Bayliss CD. Phase Variation of PorA, a major outer membrane protein, mediates escape of bactericidal antibodies by Neisseria meningitidis. Infect Immun. 2013;81:1374-80. doi:10.1128/IAI.01358-12. PMID:23403557
- Urwin R, Russell JE, Thompson EAL, Holmes EC, Feavers IM, Maiden MCJ. Distribution of surface protein variants among hyperinvasive meningococci: Implications for vaccine design. Infect Immun. 2004;72:5955-62. doi:10.1128/IAI.72.10.5955-5962.2004. PMID:15385499
- Campbell H, Andrews N, Borrow R, Trotter C, Miller E. Updated postlicensure surveillance of the meningococcal C conjugate vaccine in England and Wales: effectiveness, validation of serological correlates of protection, and modelling predictions of the duration of herd immunity. Clin Vaccine Immunol. 2010;17(5):840-7.
- Sierra G, Campa H, Varcacel N, Garcia IL, Izquierdo PL, Sotolongo PF, Casanueva GV, Rico CO, Rodriguez CR, Terry MH. Vaccine against group B Neisseria meningitidis: Protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195-207; discussion 8–10. PMID:1812432
- Pérez O, Campo Jd, Cuello M, Gonalez E, Nunez N, Cabrera O, Llanes R, Acevedo R, Zayas C, Balboa M. Mucosal approaches in Neisseria vaccinology. Vaccimonitor. 2009;18:53-5.
- Ministerio De Salud Publica. Annula Estadistico De Salud 2016. La Habana: Dirección de Registros Médicos y Estadísticas de Salud; 2017.
- de Moraes JC, Perkins BA, Camargo MC, Hidalgo NT, Barbosa HA, Sacchi CT, Landgraf IM, Gattas VL, Vasconcelos Hde G, et al. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074-8. doi:10.1016/0140-6736(92)93086-3. PMID:1357461
- Bjune G, Hoiby EA, Gronnesby JK, Arnesen O, Fredriksen JH, Halstensen A, Holten E, Lindbak AK, Nøkleby H, Rosenqvist E, et al. Effect of outer membrane vesicle vaccine against group B meningococcal disease in Norway. Lancet. 1991;338:1093-6. doi:10.1016/0140-6736(91)91961-S. PMID:1682541
- Caron F, du Châtelet IP, Leroy J-P, Ruckly C, Blanchard M, Bohic N, Massy N, Morer I, Floret D, Delbos V, et al. From tailor-made to ready-to-wear meningococcal B vaccines: Longitudinal study of a clonal meningococcal B outbreak. Lancet Infect Dis. 2011;11:455-63. doi:10.1016/S1473-3099(11)70027-5. PMID:21489881
- Loring BJ, Turner N, Petousis-Harris H. MeNZB™ vaccine and epidemic control: When do you stop vaccinating? Vaccine. 2008;26:5899-904. doi:10.1016/j.vaccine.2008.08.062. PMID:18804134
- Kelly C, Arnold R, Galloway Y, O'Hallahan J. A prospective study of the effectiveness of the New Zealand meningococcal B vaccine. Am J Epidemiol. 2007;166:817-23. doi:10.1093/aje/kwm147. PMID:17615088
- Arnold R, Galloway Y, McNicholas A, O'Hallahan J. Effectiveness of a vaccination programme for an epidemic of meningococcal B in New Zealand. Vaccine. 2011;29:7100-6. doi:10.1016/j.vaccine.2011.06.120. PMID:21803101
- Holst J, Oster P, Arnold R, Tatley MV, Næss LM, Aaberge IS, Galloway Y, McNicholas A, O'Hallahan J, Rosenqvist E, et al. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): Lessons from past programs and implications for the future. Hum Vaccin Immunother. 2013;9:1241-53. doi:10.4161/hv.24129. PMID:23857274
- Snape MD, Dawson T, Oster P, Evans A, John TM, Ohene-Kena B, Findlow J, Yu LM, Borrow R, Ypma E. Immunogenicity of two investigational serogroup B meningococcal vaccines in the first year of life: A randomized comparative trial. Pediatr Infect Dis J. 2010;29:e71. PMID:20844462
- Hosking J, Rasanathan K, Mow FC, Jackson C, Martin D, O'Hallahan J, Oster P, Ypma E, Reid S, Aaberge I, et al. Immunogenicity, reactogenicity, and safety of a P1.7b,4 strain-specific serogroup B meningococcal vaccine given to preteens. Clin Vaccine Immunol. 2007;14:1393-9. doi:10.1128/CVI.00167-07. PMID:17898183
- Basta NE, Mahmoud AAF, Wolfson J, Ploss A, Heller BL, Hanna S, Johnsen P, Izzo R, Grenfell BT, Findlow J, et al. Immunogenicity of a meningococcal B vaccine during a university outbreak. N Engl J Med. 2016;375:220-8. doi:10.1056/NEJMoa1514866. PMID:27468058
- De Wals P, Deceuninck G, Lefebvre B, Tsang R, Law D, De Serres G, Gilca V, Gilca R, Boulianne N. Impact of an immunization campaign to control an increased incidence of serogroup B meningococcal disease in one region of Quebec, Canada. Clin Infect Dis. 2017;64:1263-7. doi:10.1093/cid/cix154. PMID:28207068
- Parikh SR, Andrews NJ, Beebeejaun K, Campbell H, Ribeiro S, Ward C, White JM, Borrow R, Ramsay ME, Ladhani SN. Effectiveness and impact of a reduced infant schedule of 4CMenB vaccine against group B meningococcal disease in England: A national observational cohort study. Lancet. 2016;388:2775-82. doi:10.1016/S0140-6736(16)31921-3. PMID:28100432
- Pérez O, Lastre M, Lapinet J, Pérez A, Díaz M, Zayas M, Batista A, Quintero Y, Aguiar F, Sánchez R. Long-lasting cellular immune response in babies, children, and pre-teenagers vaccinated with a proteoliposome based anti-meningococcal BC vaccine. Inmunología. 2002;20:177-83.
- Camaraza MA, Martínez I, Ochoa R, Arnet A, Sotolongo F, Hernández D, Cuevas I, Pérez AE. Respuesta de anticuerpos inducidos por la vacuna antimeningocócica cubana VA-MENGOC-BC® frente a la cepa de Neisseria meningitidis B: 4: P1. 19, 15 en adolescentes después de 12 años de inmunizados. Vaccimonitor. 2006;15:1-4.
- Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. J Exp Med. 1969;129:1307-26. doi:10.1084/jem.129.6.1307. PMID:4977280
- Granoff DM. Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease. Vaccine. 2009;27:B117-B25. doi:10.1016/j.vaccine.2009.04.066. PMID:19477054
- Trotter C, Findlow J, Balmer P, Holland A, Barchha R, Hamer N, Andrews N, Miller E, Borrow R. Seroprevalence of bactericidal and anti-outer membrane vesicle antibodies to Neisseria meningitidis group B in England. Clin Vaccine Immunol. 2007;14:863-8. doi:10.1128/CVI.00102-07. PMID:17494636
- Harder T, Koch J, Wichmann O, Hellenbrand W. Predicted vs observed effectiveness of outer membrane vesicle (OMV) vaccines against meningococcal serogroup B disease: Systematic review. J Infect. 2017;75:81-94. doi:10.1016/j.jinf.2017.05.001. PMID:28487177
- Welsch JA, Granoff D. Naturally acquired passive protective activity against Neisseria meningitidis group C in the absence of serum bactericidal activity. Infect Immun. 2004;72:5903-9. doi:10.1128/IAI.72.10.5903-5909.2004. PMID:15385492
- Perez AE, Dickinson F, Llanes R. Invasive meningococcal disease. Cuba, 1983–2006. Vaccinmonitor. 2010;19:8-14.
- The Institute of Environmental Science and Research Ltd. Sexually Transmitted Infections in New Zealand: Annual Surveillance Report 2014. Porirua (New Zealand); 2015.
- Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, Black S. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: A retrospective case-control study. Lancet. 2017;390(10102):1603-1610. doi:10.1016/S0140-6736(17)31449-6. PMID:28705462
- Petousis-Harris H, Paynter J, Morgan J, Saxton P, Goodyear-Smith F, McArdle B, Black S. Effectiveness of a group B OMV meningococcal vaccine against gonorrhoea–the New Zealand experience. IV International Congress of Pharmacology of Vaccines (Vaccipharma) 2017; 2017; Varadero Beach, Cuba.
- Rosenqvist E, Høiby EA, Wedege E, Bryn K, Kolberg J, Klem A, Rønnild E, Bjune G, Nøkleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642-52. PMID:7591118
- Ruijne N, Lea RA, O'Hallahan J, Oster P, Martin D. Understanding the immune responses to the meningococcal strain-specific vaccine MeNZB measured in studies of infants. Clin Vaccine Immunol. 2006;13:797-801. doi:10.1128/CVI.00038-06. PMID:16829618
- Davenport V, Groves E, Horton RE, Hobbs CG, Guthrie T, Findlow J, Borrow R, Naess LM, Oster P, Heyderman RS, et al. Mucosal immunity in healthy adults after parenteral vaccination with outer-membrane vesicles from Neisseria meningitidis serogroup B. J Infect Dis. 2008;198:731-40. doi:10.1086/590669. PMID:18636953
- Brandtzaeg P. Induction of secretory immunity and memory at mucosal surfaces. Vaccine. 2007;25:5467-84. doi:10.1016/j.vaccine.2006.12.001. PMID:17227687
- Hadad R, Jacobsson S, Pizza M, Rappuoli R, Fredlund H, Olcén P, Unemo M. Novel meningococcal 4CMenB vaccine antigens–prevalence and polymorphisms of the encoding genes in Neisseria gonorrhoeae. Apmis. 2012;120:750-60. doi:10.1111/j.1600-0463.2012.02903.x. PMID:22882265
- Seib KL. Gonorrhoea vaccines: A step in the right direction. Lancet. 2017. doi:10.1016/S0140-6736(17)31605-7. PMID:28705461
- Craig AP, Gray RT, Edwards JL, Apicella MA, Jennings MP, Wilson DP, Seib KL. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine. 2015;33:4520-5. doi:10.1016/j.vaccine.2015.07.015. PMID:26192351
- Régnier SA, Huels J. Potential impact of vaccination against Neisseria meningitidis on Neisseria gonorrhoeae in the United States: Results from a decision-analysis model. Hum Vaccin Immunother. 2014;10:3737-45. doi:10.4161/hv.36221. PMID:25483706
