ABSTRACT
Background: Leprosy is an infectious disease caused by the bacterium Mycobacterium leprae. Influenza vaccine is an important influenza prevention strategy and the preparations used display good safety and tolerability profiles. But the safety of applying influenza vaccine on the clinical cured leprosy patients is unclear.
Methods: We conducted an observational clinical study, in Wuhan between November 15, 2016 and March 1, 2017. Two groups of participants ≥50 years of age received a 0.5 ml dose of the inactivated split-virion trivalent influenza vaccine and a follow-up 28 days observation of any solicited and unsolicited adverse events.
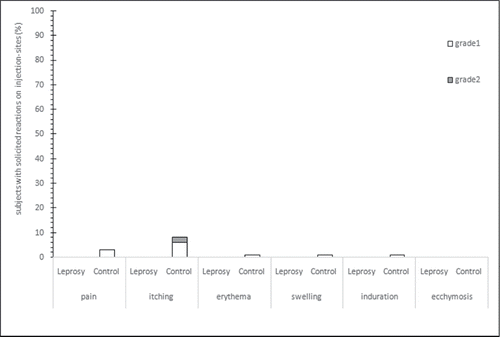
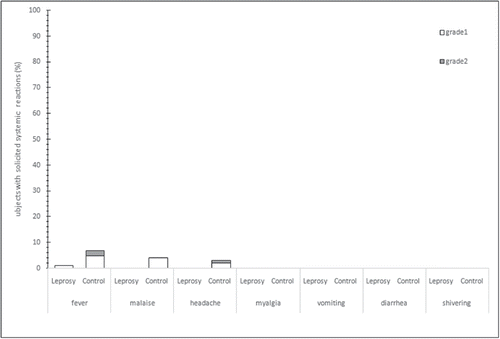
Results: A total of 134 subjects were included in the study. The total rate of reactogenicity was 5.4% [2/37] in leprosy group and 15.5% [15/ 97] in control group, the difference of reactogenicity between two groups was not significant (p = 0.1522). For solicited injection-sites adverse events (AEs), 12.4% [12/ 97] participants in the control group reported of itching, pain, erythema, swelling or induration, and no participants in leprosy group reported of any solicited injection-sites AEs. For solicited systemic AEs, 7.2% [7 / 97] participants in the control group reported of fever, malaise or headache, and 2.7% [1 / 37] participants in the leprosy group reported of fever, statistic result showed that the difference was not significant (p = 0.4438). Unsolicited AEs was reported by one male aged 76, 4 hours after vaccination administration, his plantar ulcer area began bleeding. All AEs were grade 1 or grade 2, and no recurrence of lepra reaction, AEs leading to early withdrawal from the study, or deaths were reported in this study.
Conclusions: To our knowledge, the present study is the first clinical study to evaluate the safety of influenza vaccine in clinically cured leprosy patients. We concluded that clinically cured leprosy patients are relatively safe for influenza vaccine. More importantly, our study make a positive and scientific efforts to eradicate discrimination on leprosy. In our study, we described a patient with plantar ulcer undergoing bleeding for 4 hours after vaccine administration. Based on evidence we have, we interpret that this adverse event may probably associated with vaccine, and patients with ulcer and leprosy need intensive attention after vaccines administration.
Introduction
Leprosy, or Hansen's disease, is an infectious disease caused by the bacterium Mycobacterium leprae. Transmission of leprosy is believed to occur through close contact with an infected person, but the route of its transmission remains largely unknown. Leprosy is diagnosed based on clinical symptoms and treated with multidrug therapy (MDT). Leprosy control depends on early diagnosis and treatment of this disease, which are thought to prevent both transmission and progression to leprosy-related disabilities.Citation1 More than 200,000 new leprosy cases worldwide are reported annually from 121 countries.Citation2 Together, India, Brazil and Indonesia account for 81% of all new cases, and only more than 1000 new cases were reported in 13 countries in 2014. Leprosy sustained low popularity in China in recent years, but it is still keeping significant morbidity throughout the tropical zone. Approximately 30% of patients with leprosy develop nerve damage. Trophic, or neuropathic, ulcer is a common complication of an anesthetic foot. The term plantar, trophic, or perforating ulcer was introduced in 1959. It was defined as a chronic ulceration of the anesthetic foot, situated in well-defined areas overlying bony prominences, resistant to local and/or systemic therapy, and characterized by a marked tendency to recur which accounts for the most cases of morbidity associated with leprosy. China has been established hundreds of leprosariums, where leprosy patients are cured of this disease and receive the life healthcare, and most of them are ≥50 years of age.
Influenza is a highly infectious airborne disease causing the high medical costs and societal burden.Citation3 Influenza vaccine is an important influenza prevention strategy because of its safety and tolerability. But many survivors from leprosarium are disqualified from vaccination for a long time. In China, thousands of clinically cured patients from leprosarium had never received any vaccine, including influenza vaccine, partly due to the safety concern of vaccination. There are few articles focusing on the safety of vaccine after immunization on these groups. Besides, clinically cured leprosy patients have special physical conditions, such as ulcer, nerve system damage and terminal microcirculation disturbance, which may potentially induce safety concern after immunization. In this study, we conducted a clinical observation and evaluated the safety of influenza vaccine in clinically cured leprosy patients in leprosaria in China. The medical status of these patients was monitored by clinical residents from local clinics.
Results
Participants
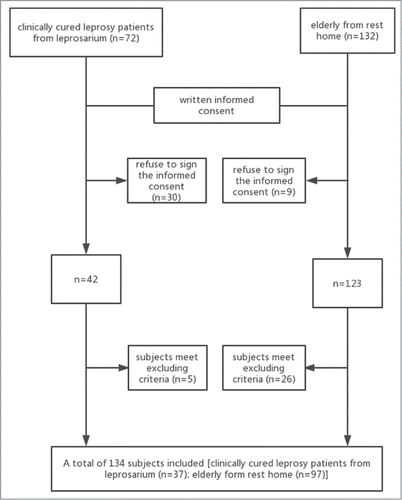
As shown in , between November 15, 2016 and March 1, 2017, a total of 204 patients were screened for the eligibility, 134 subjects were included in the study. All participants received a 0.5 ml dose vaccination and a 28-day follow-up. The leprosy group and control group have similar age, ethnicity, and basic medical history. Leprosy group had a higher proportion of male than control group. Participants aged from 52 to 86 years of the leprosy group and from 51 to 97 years old of the control group.
Safety and tolerability
The total rate of reactogenicity was 5.4% [2/37] in leprosy group and 15.5% [15/97] in control group (). Statistic result indicated that the difference of reactogenicity between two groups not significant (p = 0.1522). For male participants, the total rate of reactogenicity was 6.7% [2/30] in leprosy group and 14.6% [7/48] in control group. Result of Fisher exact test also showed no significant difference of reactogenicity between two groups (p = 0.4695).For solicited injection-sites AEs, 12.4% [12/97] of the participants in the control group reported the itching, pain, erythema, swelling or induration, but no participant in leprosy group reported any solicited injection-sites AEs (). For solicited systemic AEs, 7.2% [7/97] participants in the control group reported fever, malaise or headache, and 2.7% [1/37] participants in the leprosy group reported fever, statistic result showed that the difference was not significant (p = 0.4438)(). In leprosy group, only 1 male reported fever (grade 1, 37.1°∼37.5°) as systemic reaction. In control group, the most common injection-sites reaction was itching and the most common solicited systemic reactions were fever, malaise, and headache. Most solicited reactions were at the grade 1 level, and occurred within the first 3 days after vaccination, and resolved within 7 days after the vaccination.
Unsolicited AEs was reported by one male aged 76, who had a median sized ulcer on the right plantar. Four hours after vaccination, the ulcer area began bleeding, and considered as grade 2 AE, and it was probably related to vaccine. One day after treatment, the ulcer area stopped bleeding. There is no recurrence of lepra reaction, immediate unsolicited SAEs, and AEs leading to early withdrawal from the study, or deaths in this study.
Subgroup analysis by leprosy characteristics
We divided leprosy patients into two subgroups according to the leprosy type (). Turberculoid leprosy and borderline Tuberculoid leprosy were considered as Turberculoid type, and Lepromatous leprosy, borderline Lepromatous leprosy and midborderline leprosy were considered as Lepromatous type. Statistical analysis detected no significant difference of AEs between two subgroups (p = 1.0000). We further divided leprosy patients into subgroups base on eye-hand-foot (EHF) score ().1 patient with EHF sore 0 reported of solicited systematic events, 1 patients with EHF sore 2 reported of unsolicited adverse events, statistical analysis indicated that leprosy patients with lower EHF score had higher rate of AEs (p = 0.0180).
In our study, 40.5% [15/37] of leprosy patients were suffering from ulcer, and most of them were plantar ulcers (). Their wound remained stable before vaccination and the volume of ulcer were all less than 2 ml (). After vaccination, adverse events were reported in 2 of the 11 plantar ulcer patients, including one unsolicited AEs of ulcer bleeding. Other 7 ulcer patients with palm or gluteal or ankle had no any AEs reported.
Discussion
Intramuscular administration of Inactivated influenza vaccines (IIVs) ly usually provides short-term and strain specific humoral immunity, while the irreversible nerve damage and deformities of leprosy patient is associated with poor cellular immunity caused by bacterium Mycobacterium leprae.Citation4,5 The present clinical observation evaluated the safety of influenza vaccine in clinically cured leprosy patients over than 50 years of age. The leprosy patients have been living in leprosarium for decades, isolated from outside society and never received any vaccine, including influenza vaccine. Although the vaccination is free, over 40% of our leprosy patient refuse to receive it, compared to those less than 10% in control group. After informed consent and exclusion, a total of 134 subjects were enrolled in the study. In our study, rate of reactogenicity was lower in leprosy patients than that in the residents from local rest home (5.4% [2/37] vs. 15.5% [15/97]), but fisher's exact test showed that the difference of reactogenicity rate was not significantly different. The difference of rates of AEs was also not significant for solicited systemic AEs. Local solicited adverse reactions were about 0.0% [0/37] in leprosy patients and 12.4% [12/97] in residents from rest home. Most solicited reactions were grade1, and no SAEs or deaths were reported in this study.
In our study, we found that influenza vaccine had a relatively good tolerability in clinically cured leprosy patients. Based on the evidence above, we suggest that clinically cured leprosy patients are relatively safe for influenza vaccine. We also suggested that the clinicians would be more proactive in monitoring the safety while vaccination in leprosy patients. The result aroused our interesting that leprosy patients had not report any solicited injection site reactions. We initially suspected that host immune response may play a role in local solicited AEs of the two groups. Host response to Micobacterium leprae (M. leprae) lead to the presentation with varied clinicopathological disease. The results showed a negative correlation between leprosy humoral immunity and cellular immunity in each type of Leprosy. lepromatous patients have high levels of antibodies, while tuberculoid patients are very difficult to detect the antibody.Citation6 Clones of CD4+cells from tuberculoid patients produce high levels of interferon-gamma (IFN-γ), interleukin-2 (IL-2), and TNF-α. Clones of CD8+cells from lepromatous patients produce high levels of suppressor cytokines on macrophage activity, such as interleukin4 (IL-4), interleukin-5 (IL-5), and IL-10, as well as low levels of IFN-γ.81 These cell clones contribute to the stimulation of Blymphocytes, with increased humoral immune response and production of antibodies, making the individual susceptible to disease development.Citation7 The different level of humoral immune response in lepromatousor tuberculoid patients may affect their immune response to influenza vaccine. But in our study, the difference is not significant in the subgroup analysis of leprosy patients divided by leprosy type. Another explanation of the difference of AEs between two groups is that, leprosy patients have tissue damage and suffer sensory loss caused by Mycobacterium, therefore perceive less uncomfortable sense of injection site. The damage to the peripheral nerve system (PNS) is a marker of M. leprae infection that develops as a result of the M. leprae invasion to the Schwann cells.Citation8,9 Skin-deep and stimulating electromyography revealed the subclinical manifestation of neuromuscular system injuries in clinically normal muscles.Citation10 This damage may result in the less reports on self-conscious symptoms, such as pain, swelling and itching. Our subgroup analysis of nerve function impairment showed that leprosy patient with less impairment (EHF sore = 0) were more likely to report the AEs. Cured leprosy patients suffering from nerve system damage and terminal microcirculation disturbance, so AEs after vaccination may not be easily sensed. So, we arranged the routine AE report by both self-report and active observation. Meanwhile the conventional reported mechanism is only self-report, but not active observation. Actually, the unsolicited AE of ulcer bleeding was reported by our clinician's visitation. Based on this, we believe that the influenza vaccine is relatively safe in leprosy patients, and we suggested that clinicians need to be more proactive in safety monitoring.
Foot affection due to neuropathy is common in leprosy patients, about 30% of the leprosy patients have or had plantar ulcer. It occurs due to breakdown of tissue from within, on account of intrinsic muscle paralysis or trauma from outside or fissure foot due to autonomic imbalances.Citation11,12 These ulcers should be prevented and treated promptly to avoid serious problems occur, such as secondary infections, sepsis, carcinomatous degeneration and amputations. The sensory loss and affected shape of the foot make the foot liable to trauma and pressure, and subsequently cause more blisters and ulcers. Foot ulcers are usually liable to secondary infection as cellulitis or osteomyelitis, and may result in amputations. Foot ulcers are responsible for the most morbidity associated with leprosy. In our study, 43% (16/37) of the leprosy patient have ulcer. In our study we noticed that a 76 years old male patient of tuberculoid leprosy (TT) reported an immediately wound bleeding of ulcer area. He had been living in leprosarium for more than 30 years, and had survived with plantar ulcer for 8 years. The ulcer area was about 2*2.5 cm2 before vaccination. Four hours after immunization, he had a 50 ml volume small artery bleeding of ulcer area. After treated by wound cleaning and bind up, and injected 2 units of HemocoagulaseBothropsAtrox, his wound area stanched bleeding within 24 hours. To our knowledge, the association between ulcer and influenza vaccine has been little reported. We speculate that the unsolicited AE is probably related to vaccine. We tried to explain the ulcer bleeding after vaccination by immune response mechanisms. It is widely accepted that leprosy is an infectious disease associated with the immune function of the organism. In the process of leprosy immunity, many physiological effects of cytokines are mutual influenced and restricted forming a complex network.Citation13–15 It is important to maintain the network balance, in other words, the body's immune homeostasis. Any intervention factors break this balance (such as immunization) is likely to lead to the body's immune disorder, thereby cause the development of diseases.Citation16 Studies also confirm that higher reactivity of lymphocyte transformation test with Dharmendara's antigen (DL-LTT) was found in the patients with plantar ulcer than those without.Citation17 The discretion of the lymphocyte conversion rate can reflect the body's cellular immune level, therefore, as one of the indicators of the determination of the body's immune function. Polarized (Th1/Th2 paradigm) T cell response to M.leprae is considered as an important factor for the pathogenesis of leprosy in different clinical forms. The tuberculoid type (BT/TT) leprosy patients show good recall of cell-mediated immune with prevalence of CD4+ T cells, Th1 cytokines in the lesions and restricted growth of M.leprae.Citation6 After influenza vaccination, CD4+ and CD8+ T cells divide rapidly. T cells aggregated, infiltrated in ulcer tissues and secreted IFN-γ that may turn into an open sore.Citation18–20 Additionally, regulatory T cells modulating the immune responses in inflammation have been identified, and showed higher frequencies in ulcers such as peptic.Citation21 As for other patients with plantar ulcer, observation of flap size showed that no changes occurred after vaccination. Base on the evidence above, we suggest that leprosy patients with ulcer need intensive attention after influenza vaccine administration.
Leprosy has been recognized as one of the neglected tropical diseases (NTDs), but it keeps significant level of morbidity throughout the tropical zone.Citation22 Medical Care for disabilities and reduction of the discrimination against persons affected by the disease are still the challenge in most countries. The proportion of new grade-2 disabilities cases is around 6.7% globally (14059 cases).Citation23 Leprosy affected persons are often experiencing stigma and discrimination. Stigma and discrimination negatively impacts the patients accessing to diagnosis, treatment or care, as well as affects their societal characters. Our study established an effective effort to promote health and reduce burden for persons affected by leprosy. Furthermore, our study provides a powerful evidence to support that persons affected by leprosy should be treated equally in medical care, thus reduce stigma and discrimination against persons affected by leprosy and promote social inclusion.
The preliminary study has some limitation that need to be considered. First, cytokine bioactivity methods were not conducted in the study, which help further elaborate how biological mechanisms relate to AEs after immunization. And the immunogenicity analysis was not performed. Second, our conclusions may have bias limited by a relatively small number of subject, only 37 leprosy patients were included in present study. The relatively small sample size may lead to premature estimation. Further studies are needed with larger sample size. At last, the disparity between the leprosy and control groups in respect of gender may lead to potential bias.
To our knowledge, the present study is the first clinical study to evaluate the safety of influenza vaccine in clinically cured leprosy patients. We concluded that clinically cured leprosy patients are relatively safe for influenza vaccine. Our safety research results will hopefully serve as useful information for similar studies of vaccination on leprosy patients. We suggested that clinicians need to be more proactive in monitoring medical status while vaccination in leprosy patients. More importantly, our study made a positive and scientific effort to eradicate discrimination on leprosy. In our study, we described a patient with plantar ulcer bleeding within 4 hours after vaccination. Based on evidence we have, we interpret that this adverse event may probably associated with vaccine, and ulcer patients need extensive attention while vaccination. Further clinical studies on the association between ulcer and influenza vaccine are needed. In the following studies we will focus on larger group of subjects, laboratory testing of titer and seroprotection, and cytokine bioactivity methods, so that the safety of vaccination can be monitored in a larger sample size, and the immunogenicity of vaccination can be analyzed.
Methods
Study design and ethics
This was an observational clinical study, conducted at two centers in Wuhan between November 15, 2016 and March 1, 2017. The objective of the study was to describe the safety of influenza vaccination. The protocol of this study was approved by the Institutional Review Board of Wuhan Institution of Dermatology and Venerology, and written informed consent was obtained from all participants, and all participants were adults.
Participants
Medically stable adults from Wuhan rest home (control group) or clinically cured leprosy patients from leprosarium (leprosy group) who were above or equal to 50 years old were considered for enrollment. Participants were excluded if they had fever, or severe allergic history for vaccination, or thrombocytopenia or other disturbance of blood coagulation which would lead to muscle injection taboo, or serious cardiovascular disease, or receipt of vaccines within two weeks, or receipt of aspirin because of chronic diseases, or any other conditions that that clinicians thought that they should be excluded.
Vaccine
Subjects received intramuscular injections (deltoid muscle) of a single 0.5 ml dose of the inactivated split-virion trivalent influenza vaccine (“Vaxigrip”, Sanofi Pasteur). The vaccine was provided in prefilled syringes of 0.5 ml (containing 15 μg hemagglutinin per strain) of A/California/7/2009 (H1N1)pdm09, A/Texas/50/2012(H3N2), and B/Massachusetts/2/2012, in compliance with World Health Organization recommendations.
Safety monitoring
Side effects were observed for 30 min after vaccination for both groups. At initial follow-up, a telephone visit was conducted at 24 h, 48 h, 72 h, 14 d and 28 d post-immunization, to record any adverse reactions. Before the injection of influenza, a face-to-face survey was also performed to collect the demographic and clinical information during the period between the 2 visits. Safety information were collected including the occurrence, nature, duration, intensity, action taken, and relationship to vaccination of any solicited adverse event (AE), unsolicited AE, or serious adverse events (SAE). AEs and SAEs were recorded according to National Institute of Allergy and Infectious Diseases (NIAID)Citation24 and “preventive vaccine clinical trials, adverse events grading guidelines” issued by the China Food and Drug Administration (CFDA). Solicited systemic reactions (fever, malaise, headache, myalgia, vomiting, diarrhea and shivering) and solicited injection site reactions (pain, itching, erythema, swelling, induration, and ecchymosis) were recorded by participants on diary cards for 7 days after vaccination. Other non-serious unsolicited AEs were recorded by patients for 28 days after vaccination. The intensity of solicited reactions was graded as mild (1), moderate (2), or severe (3) according to the China Food and Drug Administration scale (Supplement ).
Table 1. Population Demographics.
Table 2. number of subjects experiencing adverse reactions within 28 days of vaccination.
Table 3. Characteristics of leprosy type and nerve function impairment.
Table 4. Characteristics of the Ulcers.
SAEs were recorded by investigators for 28 days after vaccination. AEs of special interest were reported and analyzed as SAEs. After being informed of SAEs, the investigator should immediately report to the person in charge of the site and report to the sponsor and the principal investigator by telephone, fax or E-mail within 24 hours. All SAEs were reported to the Ethical Review Committee and the drug adverse reaction monitoring system. The investigator categorized all AEs and SAEs as probably related, possibly related or not related to vaccine, according to the WHO standard.Citation25
Statistical analysis
All calculations were performed using SAS version 9.1 (SAS Institute, Cary, NC), figures were drawn by Prism 5 software (GraphPad). Safety endpoints were assessed in the safety analysis set. The difference of frequency rates between two groups were compared by Chi-square test or Fisher Exact test or Cochran-Mantel-Haenszel test.
Ethical clearance
The study was conducted in accordance with the ethical principles originated from the Declaration of Helsinki and in compliance with ICH-GCP, ISO 14155–1 and -2, and the applicable laws and regulations of the participating country.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Britton WJ., Lockwood DN. Leprosy. Lancet. 2004;363:1209–19. doi:10.1016/S0140-6736(04)15952-7. PMID:15081655.
- WHO. Global leprosy update, 2013. Reducing disease burden. Wkly Epidemiol Rec. 2014;89(36):389–400
- Quandelacy TM., Viboud C., Charu V., Lipsitch M., Goldstein E. Age- and sex-related risk factors for influenza-associated mortality in the united states between 1997–2007. Am J Epidemiol. 2014;179:156–67. doi:10.1093/aje/kwt235. PMID:24190951.
- Tamura S., Tanimoto T., Kurata T. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn J Infect Dis. 2005;58:195–207. PMID:16116250.
- Bobosha K., Wilson L., van Meijgaarden KE., Bekele Y., Zewdie M., van der Ploeg-van Schip JJ., Abebe M., Hussein J., Khadge S., Neupane KD, et al. T-cell regulation in lepromatous leprosy. PLoS Negl Trop Dis 2014;8:e2773. doi:10.1371/journal.pntd.0002773. PMID:24722473.
- Modlin RL. The innate immune response in leprosy. Curr Opin Immunol. 2010;22:48–54. doi:10.1016/j.coi.2009.12.001. PMID:20060279.
- Lastória JC., Abreu MA. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects - part 1. An Bras Dermatol. 2014;89:205. doi:10.1590/abd1806-4841.20142450. PMID:24770495.
- Belopasov VV., Androsiuk Iu G., Diachina MN. [Leprous neuropathies]. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova. 2004;104:19–24.
- Lockwood DN., Saunderson PR. Nerve damage in leprosy: a continuing challenge to scientists, clinicians and service providers. Int Health. 2012;4:77–85. doi:10.1016/j.inhe.2011.09.006. PMID:24029146.
- Scollard DM., Truman RW., Ebenezer GJ. Mechanisms of nerve injury in leprosy. Clin Dermatol. 2015;33:46–54. doi:10.1016/j.clindermatol.2014.07.008. PMID:25432810.
- Ismail HEA. Sural artery perforator flap with posterior tibial neurovascular decompression for recurrent foot ulcer in leprosy patients. GMS Interdiscip Plast Reconstr Surg DGPW. 2017;6:Doc01. PMID:28194322.
- Brandsma JW., Macdonald MR., Warren AG., Cross H., Schwarz RJ., Solomon S., Kazen R., Gravem PE., Shrinivasan H. Report from the workshop on the Neurologically Impaired foot: 5–9 June 2000, Green Pastures Hospital, Pokhara, Nepal. Assessment and examination of the neurologically impaired foot. Lepr Rev. 2001;72:254. PMID:11715271.
- Fukutomi Y., Matsuoka M., Minagawa F., Toratani S., McCormick G., Krahenbuhl J. IL-10 treatment of macrophages bolsters intracellular survival of Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 2004;72:16–26. PMID:15217319.
- Mangan PR., Harrington LE., O'Quinn DB, Helms WS., Bullard DC., Elson CO., Hatton RD., Wahl SM., Schoeb TR., Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231. PMID:16648837.
- Korn T., Bettelli E., Gao W, Awasthi A., Jäger A., Strom TB., Oukka M., Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484. PMID:17581588.
- Cheng WU., Xiao Hong ZHOU., Cai Xia LI. The Expression and Significance of Th1, Th2 and Th17 Cells Related Cytokines in Peripheral Blood of Leprosy patients. Chin J Derm Venereol. 2014:998–1000.
- Minagawa F., Yoshino Y., Abe M., Saito N., Ohsawa Y., Ozawa T., Saikawa K. [Epidemiological immunological studies on leprosy in Okinawa. 6. Lepromin, lymphocyte transformation, FLA-ABS and leproagglutination tests in the in-and out-patients with leprosy and the relationships among these tests and personal or family history or clinical findings of the patients]. Nihon Rai Gakkai zasshi. 1989;58:92–111. PMID:2697714.
- Surls J., Nazarov-Stoica C., Kehl M., Casares S., Brumeanu TD. Differential effect of CD4+Foxp3+ T-regulatory cells on the B and T helper cell responses to influenza virus vaccination. Vaccine. 2010;28:7319–30. PMID:20832492.
- Brown DM., Román E., Swain SL. CD4 T cell responses to influenza infection. Semin Immunol. 2004;16:171. PMID:15130501.
- Roy-Ghanta S., Van dMR, Li P., Vaughn DW. Responses to A(H1N1)pdm09 Influenza Vaccines in Participants Previously Vaccinated With Seasonal Influenza Vaccine: A Randomized, Observer-Blind, Controlled Study. J Infect Dis. 2014;210:1419–30. doi:10.1093/infdis/jiu284. PMID:24864125.
- Mesali H., Ajami A., Hussein-Nattaj H, Rafiei A., Rajabian Z., Asgarian-Omran H., Hosseini V., Taghvaei T., Tehrani M. Regulatory T Cells and Myeloid-Derived Suppressor Cells in Patients with Peptic Ulcer and Gastric Cancer. Iran J Immunol. 2016;13:167. PMID:27671508.
- Hollingsworth TD., Adams ER., Anderson RM., Atkins K., Bartsch S., Basáñez MG., Behrend M., Blok DJ., Chapman LA., Coffeng L, et al. Quantitative analyses and modelling to support achievement of the 2020 goals for nine neglected tropical diseases. Parasit Vectors. 2015;8:630. doi:10.1186/s13071-015-1235-1. PMID:26652272.
- Global leprosy update, 2015. time for action, accountability and inclusion. Releve Epidemiologique Hebdomadaire. 2015;91:405. PMID:27592500.
- Surhone LM., Timpledon MT., Marseken SF. National Institute of Allergy and Infectious Diseases. Science. 2011;50(1):22–22.
- WHO/HIS/EMP/QSS Causality Assessment of an Adverse Event Following Immunization [AEFI]; 2013. ISBN 978924150533d.