ABSTRACT
Background: Bacillus Calmette-Guérin (BCG) vaccination may have beneficial non-specific effects on child survival, the effects being stronger for children developing a scar. In a prospective cohort study, we examined determinants for not developing a BCG scar within 6 months of vaccination.
Methods: Bandim Health Project (BHP) runs a Health and Demographic Surveillance System site in rural Guinea-Bissau. BHP provides BCG at monthly visits. We studied determinants for not developing a BCG scar using binomial regression models to obtain relative risks (RR).
Results: From May 2012 until October 2014, BHP nurses vaccinated 2415 infants with BCG. We assessed BCG scar between 6 and 12 months of age for 2156 (89%) of these children and 2115 (98%) had developed a scar. In comparison, among 785 children BCG vaccinated elsewhere, 622 (79%) had a scar, the RR of not having a scar being 10.91 (7.52-15.85) compared with children vaccinated by BHP.
Among children vaccinated by BHP, those receiving the Russian BCG strain were more likely not to develop a scar (RR = 2.98 (1.52–5.81)) compared with children receiving Danish BCG strain. Children with no post-injection wheal or a wheal <3 mm were more likely to not develop a scar (RR = 9.05 (3.69–22.20) and RR = 4.74 (1.96–11.45), respectively). Nutritional status and socioeconomic status were not associated with scarification.
Conclusion: Vaccination technique and vaccine strain were associated with BCG scar development while nutritional status and socioeconomic status were not. Scarring rate may therefore be a better indicator of vaccination programme performance than coverage.
Background
Bacillus Calmette Guérin (BCG) vaccine is recommended at birth in low-income countries to prevent tuberculosis. Observational studies have indicated that the vaccine has broader effects: In addition to protection against tuberculosis, the vaccine seems to have beneficial non-specific effects on child mortality.Citation1-6 In a combined analysis of three recent randomised trials among low-birth-weight infants in Guinea-Bissau, BCG vaccination at birth compared with the usual delayed BCG was associated with 38% (17%-54%) lower neonatal mortality.Citation7-9 A recent WHO review concluded that BCG vaccine is associated with nearly a halving of all-cause mortality, which was not fully explained by prevention of tuberculosis.Citation10
Correct intradermal administration of BCG, usually causes scarification at the vaccination site. Development of a BCG scar is associated with improved survival.Citation11-15 The survival advantage is unlikely to be explained by underlying health status: among cohorts resembling the general population of children in Guinea-Bissau, scarring rates in different cohorts have varied from 52% to 92%.Citation11,Citation15 Regardless of the scarring frequency, having a scar is associated with a 45–55% lower mortality during the first 1½ year of life.Citation11-15
Vaccination technique is important for scarring after BCG vaccinationCitation16 and an intradermal vaccine is difficult to administer; BCG vaccine is administered subcutaneously in 5% of the cases, even in closely monitored settings.Citation16 Vaccination technique may explain the difference in scarring rates in the cohorts: In a setting with trained staff in urban Guinea-Bissau, more than 90% developed a BCG scarCitation17 whereas in the rural areas, where less specialised health centre staff administered the vaccine, only 52% of BCG-vaccinated children had developed a scar.Citation15
Since BCG scarification is associated with lower mortality, it is important to investigate factors related to scar development after BCG. If these factors can be modified, there is potential for further enhancing the survival benefits of BCG vaccination. In the present study, we assessed determinants for BCG scarification 6 months after vaccination.
Results
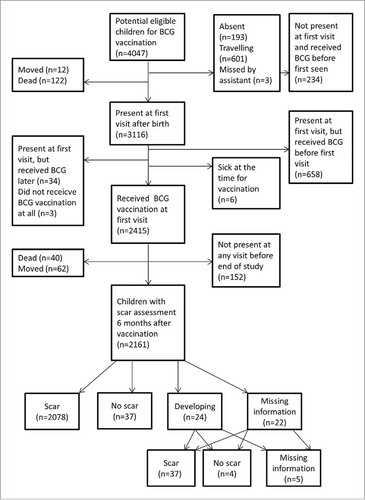
Between 28 May 2012 and 1 October 2014, 4047 children registered during pregnancy were born in the study villages and were therefore eligible for the study (). Of these, 3116 (77%) were present at first visit after birth and 2415 (78%) of these children received BCG by nurses from Bandim Health Project (BHP). Of the 701 children who did not receive BCG, 658 (94%) had already received BCG at government health centres or hospitals before being seen by the BHP assistant. Six children (1%) were sick at the time of vaccination and 37 children (5%) did not receive BCG vaccination for unspecified reasons.
Scar prevalence and determinants among children vaccinated by BHP
Among the 2415 children vaccinated by the BHP, 2161 children (89%) had their scar status assessed at a visit at least 6 months after vaccination. The median time between BCG vaccination and scar assessment was 201 days (inter-quartile range: 190–219 days). Scars were registered for 2115 (98%) children and no scars were registered for 41 (2%) children. Scar information was missing for 5 (0.2%) children ().
Determinants for scar development
Children vaccinated with the Russian strain had a higher risk of being scar-negative (Relative Risk (RR) 2.98 (1.52-5.81)) compared with children vaccinated with the Danish strain (). Children with no post-injection wheal or with a post-injection wheal of less than 3 mm had a higher risk of being scar-negative than children with a post-injection wheal above 3 mm (RR 9.05 (3.69–22.20) and RR 4.74 (1.96–11.45), respectively). Changing the cut-off for the post-injection wheal size to 2, 2.5, 3.5, 4 or 5 mm revealed the same pattern, and they were all statistically significant.
Table 1. Determinants of scar prevalence 6 months after vaccination among children vaccinated by the Bandim Health Project nurses. Guinea-Bissau, 2012–2014.
Children vaccinated during the first 3 months of the study when the nurses were still inexperienced tended to have a higher risk of being scar-negative (). Children from the Biombo region, where we first started the monthly visits and the nurses were thus more inexperienced, had a higher risk of being scar-negative (RR 2.61 (1.29–5.28)).
Maternal schooling was associated with being scar-negative; however, there was no consistent pattern as mothers with some schooling had higher risk of being scar-negative than mothers without schooling, whereas the opposite tendency was seen for women with most schooling (). There was an increased risk of being scar-negative for children who did not have a toilet in their household (RR 1.91 (1.21–3.04)), but we did not see a difference for any of the other socioeconomic factors.
Children of women who did not attend antenatal consultations had a higher risk of not developing a scar (RR 2.33 (1.27–4.27)). Mid-upper-arm-circumference (MUAC), age of the mother, and whether or not the mother had a scar (BCG, smallpox or no scar) were not associated with the risk of being scar-negative. Weight-for-age of the child, MUAC of the child and age at scar assessment were not associated with scarring.
Scar prevalence among children not vaccinated by the BHP A total of 892 children were vaccinated at government health centres or hospitals and 792 (89%) children had their scar assessed 6 months after vaccination. Among these, 622 (79%) were registered as scar-positive and 163 (21%) were registered as scar-negative. Scar information was missing for 7 children (0.9%). Children vaccinated at government health centres or hospitals had a significantly higher risk of being scar-negative compared with children vaccinated by the BHP nurses (RR 10.91 (7.52–15.85)).
Children vaccinated elsewhere were younger at the time of BCG vaccination (P<0.0001), and more likely not to receive and oral polio vaccine (OPV) at the time of BCG vaccination (Supplementary Table 1). Among those vaccinated elsewhere, more mothers had attended antenatal consultations, and the mothers had higher levels of education (P<0.001). We saw a consistent pattern that those vaccinated elsewhere had higher socioeconomic status compared with those vaccinated by BHP (Supplementary Table 1).
Among children vaccinated at government health centres or hospitals, children vaccinated in the dry season had a higher risk of being scar-negative, as did children who had OPV co-administered with BCG (). Socioeconomic status and maternal health seeking behaviour was not associated with scaring.
Table 2. Determinants of scar prevalence 6 months after BCG vaccination among children vaccinated at health centres and hospitals. Guinea-Bissau, 2012–2014.
Children lost to follow-up before age 6 months The MUAC and weight-for-age (z-score) at the time of vaccination were lower for children who died or migrated before 6 months after vaccination compared with children who remained in the study, otherwise there were no associations between background factors and death or migration before 6 months after vaccination (Supplementary Table 2).
Discussion
Main results
We found an almost 11-fold higher risk of being scar-negative for children who received BCG vaccination at government health centres or hospitals compared with children vaccinated by the BHP. Among children vaccinated by the trained BHP nurses, children vaccinated with the Russian strain had an almost 3-fold higher risk of being scar-negative compared with children vaccinated with the Danish strain. Child nutritional and socioeconomic factors were not associated with the risk of not developing a scar.
Strengths and weaknesses
The data originate from a Health and Demographic Surveillance System (HDSS) and were collected by experienced field workers at monthly visits. For each child a detailed questionnaire was completed at the time of BCG vaccination, which gave us the possibility to study the vaccination-related determinants. Contrary to our expectations, based on a study from rural Guinea-Bissau in 2009–201115, very few children were classified as scar-negative, which lowered the power of the study. Scar classification was subject to the judgement of our field assistants and not detecting small scars may have caused some children to be misclassified. We sought to minimize classification errors through frequent supervision during the study period, and it is therefore not likely that the high scar prevalence is due to overestimation of scarring frequency. It is more likely due to intensive training of the nurses in administration of BCG, an interpretation, which is supported by the that the scar frequency became higher after the first 3 months.
Another weakness is that the nurses classified the post-injection wheal themselves. They were aware that a large and visible wheal reflects good vaccination technique. However, this type of misclassification would tend to conceal an association between post-injection wheal size and BCG scars. Hence, this misclassification cannot explain the observed association between wheal size and BCG scar. Our classification of underlying health status is crude, and we cannot rule out that underlying health status, which was not reflected in weight- or MUAC-for-age of the child may have played a role in the formation of a scar.
A BCG vaccine should be stored between 2°C and 8°C.Citation18 At the BHP, the vaccines are stored in temperature-monitored refrigerators. The cold chain in the national vaccination programme is also monitored. However, we cannot rule out that some vaccines may have been exposed to temperatures outside the recommended range, which could lead to lower viabilityCitation19 and possibly a lower scarring rate among children vaccinated at health centres/hospitals.
Consistency with previous studies
An accumulating volume of both animal and human studies indicates that the virulence and immunologic response differ by BCG strain.Citation20,Citation21 The finding that BCG strain is also associated with scar development is consistent with two observational studies where different strains were used for sequential cohorts: An observational study in urban Guinea-Bissau found that the Danish strain was associated with higher rates of scar development (99%) than BCG Merieux and BCG Connaught with rates around 90%.Citation16 A prospective cohort study from Uganda found a significant association between the Danish strain and higher prevalence of scar (93%) at 12 months of age compared with the Russian (52%) or the Bulgarian (64%) BCG strain.Citation22 In a recent study from urban Guinea-Bissau with different strains in use at the same time, we also found that the Danish strain was associated with higher scarring rates (97%) than the Russian strain (87%).Citation23
Children with small or no post-injection wheal developed fewer scars in our study. Results from prior studies were in line with this with larger post-injection wheals being associated with BCG scar development.Citation16,Citation23,Citation24
Only 2% of the children vaccinated by the BHP were classified as scar-negative. Previous studies conducted in urban Guinea-Bissau have found that 10–15% of the children vaccinated by trained nurses did not develop a scar,Citation14,Citation23 while in rural Guinea-Bissau 48% of children vaccinated with BCG Russia at government health centres or hospitals between 2004–2011 were scar-negative.Citation15 The higher prevalence of scar-failure among children vaccinated at government health centres or hospitals was also seen in our data, albeit somewhat weaker with 21% not developing a scar.
Interpretation
The marked difference in scar prevalence among children vaccinated by the BHP and children vaccinated at government health centres or hospitals cannot be explained by the assessed background factors. The slightly higher socioeconomic status of children vaccinated elsewhere does not explain the difference, since socioeconomic status was not associated with scar prevalence neither in the cohort of children vaccinated by BHP nurses nor in the cohort of children vaccinated elsewhere. It is noteworthy that weight-for-age and MUAC of the child was not associated with scar development, nor was nutritional status of the mother during pregnancy. Thus, none of the underlying health markers investigated predicted which children would develop a scar.
Instead, development of a BCG scar is likely a result of both vaccination technique and BCG strain for several reasons: First, scarring frequencies improved over the duration of the study as the nurses gained experience. Second, post-injection wheal size was associated with scar development, the children with no post-injection wheal or a post-injection wheal below 3 mm all had a higher risk of not developing a scar. Third, among children vaccinated by BHP nurses, the children who had received the BCG Russia had lower rates of scarring and the national programme only used BCG Russia.
Implications
As scar development after BCG vaccination is associated with better child survival, strategies to optimise scarring rates should be considered. The present study shows that strain and vaccination technique is associated with scar development. These are factors that can be targeted and scar development could easily be improved by better training of nurses. Our findings support, that using BCG scar rate as a marker for a good vaccination programme should be considered. Further studies should assess whether re-vaccination of scar-negative children should be recommended and randomised studies of BCG strain are warranted.
Conclusion
BCG scarring rates depend on vaccination technique and vaccine strain and are not associated with the underlying health and socioeconomic status indicators. High scarring frequencies may therefore better reflect a well-performing BCG vaccination programme than BCG coverage.
Participants and methods
Setting
The study was conducted in the rural study area of the BHP in Guinea-Bissau where BHP established an HDSS in 1990. Women of fertile age and their children below the age of 5 years are followed through home visits by mobile data-collection teams. Since 2012, monthly visits have been conducted in 75 village clusters in three regions (Biombo, Cacheu and Oio). The present study was conducted in these regions.
As part of the BHP routine all women are registered with information on their age, past obstetric history, ethnicity, scar status (smallpox vaccination scar yes/no, BCG scar yes/no, or no scar) and whether they have attended school. When a pregnancy is registered, the woman's nutritional status is assessed by measurement of the MUAC and socio-economic factors (type of roof, type of toilet, possession of a mobile phone, radio and generator) are registered. Information on antenatal care is collected prior to giving birth, and at the first visit after delivery. After delivery, the place of delivery (home, health facility) and who assisted the birth are also registered.
The mobile data-collection teams are accompanied by a nurse, who administers routine vaccinations according to the Guinean vaccination schedule: BCG and OPV at birth, pentavalent (diphtheria-tetanus-pertussis, Haemophilus-influenza type B and Hepatitis B) vaccine and OPV at 6, 10 and 14 weeks of age, and measles and yellow fever vaccines at 9 months of age.
Study population
Children who were registered by our HDSS before birth and born between 28 May 2012 and 1 October 2014 in the three rural regions were eligible for the present study. The monthly visits started at 28 May 2012 and 1 October 2014 was chosen as cut off, to ensure that data were complete within the project period of the first author (KMF).
BCG vaccination procedure and assessment of outcomes
The nurse accompanying the mobile team provides routine vaccinations at the monthly visits.
Before the initiation of the monthly visits in May 2012, all the BHP nurses received intensive training in correct administration of the intradermal BCG vaccine. The monthly visits were first implemented in Biombo region, and subsequently in Oio and Cacheu.
During the present study, all children were offered BCG vaccination by BHP nurses on the first day the village was visited after the birth of the child. BCG was given as a 0.05 mL intradermal injection in the left, upper deltoid area. Two different BCG strains were used by the BHP; the Danish BCG strain from Statens Serum Institut, Denmark (batch numbers: 112032A, 111005A, 113010B, 110050B, 111013B, 110016B, 111023B, 113042B, 113033C) and the Russian BCG strain from the Serum Institute of India (batch numbers: 004M2138, 034G2047, 034G2074, 037G1072, 037G1119, 037G1145, 037G2074). The Russian strain vaccines were supplied by the national vaccination programme. According to the national vaccination practice, BCG vaccine is only administered if there are more than 10 children present for BCG vaccination.Citation25 However, BHP nurses would administer BCG vaccines to all unvaccinated infants regardless of the number of children present. Hence, the supply had to be supplemented with BCG vaccines purchased from Statens Serum Institut, Denmark by BHP.
At the time of BCG vaccination by the BHP, information on child weight, temperature, symptoms and use of medicine on the day of vaccination, prior vaccinations, BCG strain, time since reconstitution of the vaccine, type of syringe and post-injection wheal size were registered. At all visits during the first year of life the upper arms of the child were inspected for the presence of a BCG scar; if a BCG scar was found, two perpendicular diameters were measured. Scar prevalence increased over the first 6 months after vaccination (Supplementary ). To ensure that we did not classify children who would later develop a scar as scar-negative, we used a time interval of 6 months between vaccination and scar reading.
Statistical analyses
The main analysis focused on determinants for BCG scarring among children vaccinated by the BHP. We studied determinants for being classified as BCG scar-negative 6 months after vaccination among children BCG-vaccinated by the BHP at the first visit after birth. If there were signs of infection at the vaccination site at the first visit after 6 months where the child was present (scar still developing (N = 24; 1%)), we used the scar assessment from the subsequent visit. This was also done if information on scar status was missing (N = 22; 1%).
Vaccination-related determinants and underlying determinants of BCG scarification were studied in univariate binominal regression models to obtain RR of being BCG scar-negative.
We considered the following potential vaccination-related determinants; monitored injection of BCG vaccine, post-injection wheal size, time since reconstitution of the vaccine, BCG strain and who vaccinated the child. We considered the following underlying determinants: sex, age at vaccination, weight at vaccination transformed to Z-score for weight-for-age using the WHO growth reference (version 3.2.2, January 2011),Citation26 season of birth, season of vaccination, region and socioeconomic factors (type of roof, possession of a toilet, telephone, radio or generator).
We also compared scar prevalence among children, who had received BCG at a health centre or hospital, with the scar prevalence among children vaccinated by the BHP nurses and assessed determinants for scar development among children vaccinated elsewhere. Furthermore, to assess whether those having their scar assessed was a selected group, we analysed determinants for leaving the study (migration or death) within 6 months after vaccination.
To examine whether an association between strain and scar prevalence was confounded by the other factors assessed, we included them one-by-one as explaining factors in the regression model with scar and strain. None of the factors changed the RR estimate by more than 10% and adjusted estimates are therefore not presented.
Ethics approval and consent to participate
The health and demographic surveillance of BHP has been in place in rural Guinea-Bissau since 1990 and is conducted on request from the Guinean Ministry of Health. Women of fertile age provide oral consent for themselves and their children at the time of registration. No written consent was sought. The observational study of BCG was approved by the Guinean Ethics committee (Ref. 044/CNES/INASA/2012). No separate consent was sought for the present study.
Disclosure of potential conflicts of interest
The authors report no conflicts of interest.
Author contribution
SMT and ABF: designed the study with inputs from PA and CB. KMF, SMT, AR, CLM and ABF: supervised data collection, data entry and/or data cleaning; KMF, SMT and ABF: analysed the data. KMF wrote the first manuscript draft with inputs from SMT and ABF; ABF has primary responsibility for its final content. All authors contributed to and approved the final manuscript.
KHVI_A_1421879_Supplemental.docx
Download MS Word (26.4 KB)Acknowledgments
We thank the team at the BHP office in Guinea-Bissau and the field teams including nurses at Equipa Movel in Guinea-Bissau for their contributions to this paper.
Additional information
Funding
References
- Roth A, Garly ML, Jensen H, Nielsen J, Aaby P. Bacillus calmette-guerin vaccination and infant mortality. Expert Review of Vaccines. 2006;5:277–93. doi:10.1586/14760584.5.2.277. PMID:16608427
- Roth A, Jensen H, Garly M-L, Djana Q, Martins CL, Sodemann M, et al. Low birth weight infants and calmette-guérin bacillus vaccination at birth. Pediatr Infect Dis J. 2004;23:544–50. doi:10.1097/01.inf.0000129693.81082.a0. PMID:15194836
- Velema JP, Alihonou EM, Gandaho T, Hounye FH. Childhood mortality among users and non-users of primary health care in a rural west african community. Int J Epidemiol. 1991;20:474–9. doi:10.1093/ije/20.2.474. PMID:1917252
- Niobey FM, Duchiade MP, Vasconcelos AG, de Carvalho ML, Leal Mdo C, Valente JG. [Risk factors for death caused by pneumonia in children younger than 1 year old in a metropolitan region of southeastern Brazil. A case- control study]. Rev Saude Publica. 1992;26:229–38. doi:10.1590/S0034-89101992000400004. PMID:1342506
- Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in guinea-bissau, West Africa. BMJ. 2000;321:1435–8. doi:10.1136/bmj.321.7274.1435. PMID:11110734
- Aaby P, Vessari H, Nielsen J, Maleta K, Benn CS, Jensen H, et al. Sex differential effects of routine immunizations and childhood survival in rural Malawi. Pediatr Infect Dis J. 2006;25:721–7. doi:10.1097/01.inf.0000227829.64686.ae. PMID:16874172
- Biering-Sorensen S, Aaby P, Napirna BM, Roth A, Ravn H, Rodrigues A, et al. Small randomized trial among low-birth-weight children receiving bacillus calmette-gueerin vaccination at first health center contact. Pediatr Infect Dis J. 2012;31:306–8. doi:10.1097/INF.0b013e3182458289. PMID:22189537
- Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis. 2011;204:245–52. doi:10.1093/infdis/jir240. PMID:21673035
- Biering-Sørensen S, Aaby P, Lund N, Monteiro I, Jensen KJ, Eriksen HB, et al. Early BCG-denmark and neonatal mortality among infants weighing <2500 g: a randomized controlled trial. Clin Infect Dis. 2017;65:1183–90. doi:10.1093/cid/cix525.
- Higgins JP, Soares-Weiser K, Lopez-Lopez JA, Kakourou A, Chaplin K, Christensen H, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ. 2016;355:i5170. doi:10.1136/bmj.i5170. PMID:27737834
- Garly ML, Martins CL, Bale C, Balde MA, Hedegaard KL, Gustafson P, et al. BCG scar and positive tuberculin reaction associated with reduced child mortality in West Africa. A non-specific beneficial effect of BCG? Vaccine. 2003;21:2782–90. doi:10.1016/S0264-410X(03)00181-6. PMID:12798618
- Roth A, Sodemann M, Jensen H, Poulsen A, Gustafson P, Weise C, et al. Tuberculin reaction, BCG scar, and lower female mortality. Epidemiology. 2006;17:562–8. doi:10.1097/01.ede.0000231546.14749.ab. PMID:16878042
- Roth A, Gustafson P, Nhaga A, Djana Q, Poulsen A, Garly ML, et al. BCG vaccination scar associated with better childhood survival in Guinea-Bissau. Int J Epidemiol. 2005;34:540–7. doi:10.1093/ije/dyh392. PMID:15659474
- Timmermann CA, Biering-Sorensen S, Aaby P, Fisker AB, Monteiro I, Rodrigues A, et al. Tuberculin reaction and BCG scar: association with infant mortality. Trop Med Int Health. 2015;20:1733–44. doi:10.1111/tmi.12614. PMID:26426863
- Storgaard L, Rodrigues A, Martins C, Nielsen BU, Ravn H, Benn CS, et al. Development of BCG scar and subsequent morbidity and mortality in rural Guinea-Bissau. Clin Infect Dis: an official publication of the Infectious Diseases Society of America. 2015;61:950–9. doi:10.1093/cid/civ452.
- Roth A, Sodemann M, Jensen H, Poulsen A, Gustafson P, Gomes J, et al. Vaccination technique, PPD reaction and BCG scarring in a cohort of children born in Guinea-Bissau 2000–2002. Vaccine. 2005;23:3991–8. doi:10.1016/j.vaccine.2004.10.022. PMID:15899539
- Sartono E, Lisse IM, Terveer EM, van de Sande PJ, Whittle H, Fisker AB, et al. Oral polio vaccine influences the immune response to BCG vaccination. A natural experiment. PloS One. 2010;5:e10328. doi:10.1371/journal.pone.0010328. PMID:20502641
- World Health Organisation. WHO–UNICEF joint statement on effective vaccine store management (WHO/IVB/04.16). 2005.
- World Health Organisation. Thermostability of vaccines. 1988.
- Shann F. Different strains of bacillus calmette-guerin vaccine have very different effects on tuberculosis and on unrelated infections. Clin infect dis: an official publication of the Infectious Diseases Society of America. 2015. doi:10.1093/cid/civ454.
- Zhang L, Ru HW, Chen FZ, Jin CY, Sun RF, Fan XY, et al. Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol ther: the journal of the American Society of Gene Therapy. 2016;24:398–405. doi:10.1038/mt.2015.216.
- Anderson EJ, Webb EL, Mawa PA, Kizza M, Lyadda N, Nampijja M, et al. The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine. 2012;30:2083–9. doi:10.1016/j.vaccine.2012.01.053. PMID:22300718
- Frankel H, Byberg S, Bjerregaard-Andersen M, Martins CL, Aaby P, Benn CS, et al. Different effects of BCG strains – A natural experiment evaluating the impact of the danish and the russian BCG strains on morbidity and scar formation in Guinea-Bissau. Vaccine. 2016;34:4586–93. doi:10.1016/j.vaccine.2016.07.022. PMID:27491688
- Birk NM, Nissen TN, Ladekarl M, Zingmark V, Kjaergaard J, Jensen TM, et al. The association between Bacillus Calmette-Guerin vaccination (1331 SSI) skin reaction and subsequent scar development in infants. BMC Infect Dis. 2017;17:540. doi:10.1186/s12879-017-2641-0. PMID:28774269
- Thysen SM, Byberg S, Pedersen M, Rodrigues A, Ravn H, Martins C, et al. BCG coverage and barriers to BCG vaccination in Guinea-Bissau: an observational study. BMC Public Health. 2014;14:1037. doi:10.1186/1471-2458-14-1037. PMID:25282475
- World Health Organisation. WHO Anthro (version 3.2.2, January 2011) and macros. 2011.