ABSTRACT
Hepatitis B vaccines are highly effective in preventing hepatitis B virus infection and have been included in the national immunization program of Japan since 2016. Heptavax®-II is one of two hepatitis B vaccine products licensed in Japan, and its manufacturing process is being modified to reduce variability of manufacturing and optimize immunogenicity. In this study (NCT01463683), the immunogenicity and safety of a modified-process hepatitis B vaccine (mpHBV) were compared to those of the licensed Heptavax®-II. Overall, 722 Japanese adults aged 20-to-35 years old were randomized in a 3:3:1 ratio to either the mpHBV subcutaneous (SC) injection group (mpHBV SC), the Heptavax®-II SC injection group (Heptavax®-II SC), or the mpHBV intramuscular (IM) injection group (mpHBV IM). All participants received a 3-dose series of either mpHBV or Heptavax®-II at Day 1, Month 1, and Month 6. Serum antibody to hepatitis B virus surface antigen (anti-HBs) was assayed on Day 1 prior to the first vaccination and Month 7 (1 month Postdose 3). Seroprotection rates in mpHBV SC were non-inferior to that in Heptavax®-II SC and anti-HBs geometric mean titers were numerically higher in mpHBV SC as compared to Heptavax®-II SC. The incidences of injection-site and systemic adverse events (AEs) observed in mpHBV SC were comparable to those in Heptavax®-II SC, except for erythema which was higher in mpHBV SC than in Heptavax®-II SC. Most injection-site and systemic AEs were mild-to-moderate in intensity and there were no reports of vaccine-related serious AEs in any group. IM administration of mpHBV was well-tolerated and more immunogenic compared to SC administration. In conclusion, mpHBV and Heptavax®-II were well-tolerated and elicited satisfactory immune responses for the prevention against hepatitis B virus-associated diseases.
Introduction
Disease caused by hepatitis B virus has a worldwide distribution. It is estimated that more than 2 billion people worldwide had evidence of past or present hepatitis B virus infection in 1995.Citation1 In 2015, the global prevalence of hepatitis B virus infection in the general population was estimated at 3.5%, with about 257 million persons living with chronic hepatitis B virus infection.Citation2 Persons with chronic hepatitis B infection are at risk for serious illness and death, and more than 880,000 persons are estimated to die annually as a result of hepatitis B virus-associated acute and chronic liver disease.Citation3
Hepatitis B virus is transmitted through contact with the infected blood or other specific body fluids. Particularly, transmission can occur perinatally from mother to child.Citation4 Hepatitis B virus infection is a vaccine-preventable disease; therefore, hepatitis B vaccination is recommended as part of any national immunization program (NIP) by the World Health Organization.Citation5 In Japan, hepatitis B vaccination has been incorporated in the NIP for infants as a 3-dose vaccination schedule since October 2016.
A recombinant hepatitis B vaccine (RECOMBIVAX HB® in the United States; Heptavax®-II in Japan: Merck & Co., Inc., Kenilworth, NJ, USA) is indicated for the prevention of hepatitis B virus infection and has been used worldwide since its initial licensure in the United States in 1986; subsequently, Heptavax®-II was licensed in Japan in 1988. In the United States, the incident of acute hepatitis B has decreased since 1991 when universal vaccination for infants was introduced.Citation6
To reduce variability of manufacturing and optimize immunogenicity, a modified process hepatitis B vaccine (mpHBV) was developed. The antigen component of the mpHBV is consistent with that of the licensed Heptavax®-II, with only the composition of amorphous aluminum hydroxyphosphate sulfate adjuvant having been modified by utilizing a higher phosphate content (relative to the current product, Heptavax®-II) during the manufacturing process.
In this study, the immunogenicity, safety, and tolerability of the subcutaneous administration of mpHBV (mpHBV SC) were evaluated comparing to those of the subcutaneous administration of the licensed Heptavax®-II (Heptavax™-II SC) in Japanese health young adults. Subcutaneous administration of vaccines are generally recommended in Japan, however there are few studies that examine directly the immunogenicity and safety in subcutaneous compared to intramuscular administration. Therefore, mpHBV given by the intramuscular route (mpHBV IM) was included as an additional control group.
Results
Participant accounting and demographics
A total 722 Japanese adults aged 20-to-35 years old were randomized to this study; 70 participants at one of the sites were excluded from all analyses set due to the potential non-compliance, and one participant randomized to the Heptavax®-II SC group did not receive study vaccine. Therefore, a total of 651 participants received the first vaccination of the 3-dose series and are included in the safety analysis population (). Overall, 264 participants in the mpHBV SC group, 250 participants in Heptavax®-II SC group, and 84 participants in the mpHBV IM group completed the study; 54 participants (15, 29 and 10; mpHBV SC, Heptavax®-II SC, and mpHBV IM participants, respectively) discontinued from the study. The most common reason for discontinuation was lost to follow-up (mpHBV SC: 3.2%; Heptavax®-II SC: 2.9%; and mpHBV IM: 7.4%) and withdrawal of consent (mpHBV SC: 1.4%; Heptavax®-II SC: 5.7%; and mpHBV IM: 1.1%). Of the participants in mpHBV SC group, one participant discontinued due to a vaccine-related adverse event (AE). Overall, 23 participants (8 in mpHBV SC, 11 in Heptavax®-II SC and 4 in mpHBV IM, respectively) were anti-HBs seropositive (anti-HBs titer: ≥5 mIU/mL) and 18 participants (9 in mpHBV SC, 7 in Heptavax®-II SC and 2 in mpHBV IM, respectively) were seropositive to antibody to hepatitis B virus core antigen (anti-HBc) at baseline prior to receiving study vaccination. Seropositive participants were excluded from the per-protocol immunogenicity population. Therefore, 564 participants (249 in mpHBV SC, 236 in Heptavax®-II SC and 79 in mpHBV IM, respectively) were included in the per-protocol population for immunogenicity analysis. A slightly higher number of females were enrolled in the mpHBV SC group (56.6%) than in the Heptavax®-II SC group and mpHBV IM group (45.3% and 44.7%, respectively) (). The mean age was comparable among the three groups.
Figure 1. Participant disposition, † Potential non-compliance, ‡ Reason discontinued mpHBV SC = 15 (5.4%), Adverse event = 1 (0.4%), Lost to follow-up = 9 (3.2%), Pregnancy = 1 (0.4%), Withdrew consent = 4 (1.4%), § Reason discontinued Heptavax®-II SC = 29 (10.4%), Lost to follow-up = 8 (2.9%), Physician decision = 3 (1.1%), Pregnancy = 2 (0.7%), Withdrew consent = 16 (5.7%), || Reason discontinued mpHBV IM = 10 (10.6%), Lost to follow-up = 7 (7.4%), Physician decision = 1 (1.1%), Pregnancy = 1 (1.1%), Withdrew consent = 1 (1.1%).
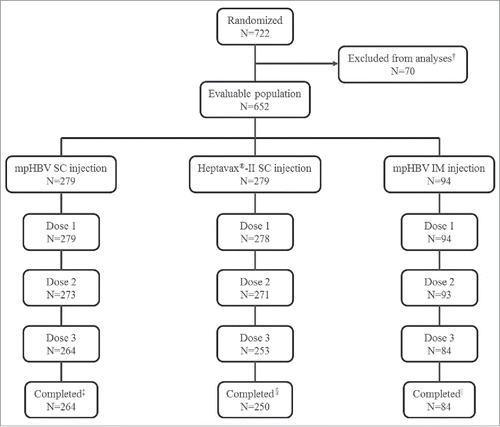
Table 1. Demographics of participants at baseline.
No participant received any vaccines other than mpHBV or Heptavax®-II within 28 days before the first study vaccination. The percentage of participants given concomitant medications within 15 days after each study vaccination was similar across the groups. The most frequently used concomitant medications were anti-inflammatory/anti-rheumatic drugs (mpHBV SC: 3.2%; Heptavax®-II SC: 5.0%; and mpHBV IM: 8.5%) and analgesics (mpHBV SC: 3.2%; Heptavax®-II SC: 2.9%; and mpHBV IM: 4.3%).
Immunogenicity
The seroprotection rates (SPRs), which is defined as the percentage of participants with anti-HBs titer ≥10 mIU/mL in the per-protocol population, among the three groups are presented in . The SPRs in the mpHBV SC group and Heptavax®-II SC group were 91.6% (228/249) and 82.6% (195/236), respectively. The lower bound of the two-side 95% confidence interval (CI) for the between-treatment difference in SPR (mpHBV SC group minus Heptavax®-II SC group) was 3.0%, exceeding the predefined criterion of −10%, demonstrating that mpHBV SC is not inferior to Heptavax®-II SC. The geometric mean titer (GMT) of anti-HBs at Month 7 following vaccination was higher in the mpHBV SC group than in the Heptavax®-II SC group (231.4 mIU/mL and 91.2 mIU/mL, respectively).
Table 2. Summary of anti-HBs responses at Month 7 (1 month Postdose 3).
The SPR (95% CI) in the mpHBV IM group was 98.7% (95% CI: 93.1%, 100%) and was higher than those in both the mpHBV SC and Heptavax®-II SC groups. Also, the GMT of anti-HBs at Month 7 following vaccination in the mpHBV IM group was 1064 mIU/mL, which was higher than the GMTs in both the mpHBV SC and Heptavax®-II SC groups (231.4 mIU/mL and 91.2 mIU/mL, respectively).
Safety
AEs occurring within 15 days following any study vaccination in each group are summarized in . The incidences of vaccine-related AEs were generally higher in the mpHBV SC group compared to the Heptavax®-II SC group. The incidences of solicited injection-site AEs were also generally higher in the mpHBV SC group compared to the Heptavax®-II SC group (pain [69.5% and 62.2%], erythema [57.0% and 48.2%] and swelling [54.1% and 47.5%], respectively).; the 95% CI of the differences (%) for all solicited injection-site AEs crossed zero, except for erythema (8.8 [95% CI: 0.5, 17.0]). The incidences of vaccine-related systemic AEs in mpHBV SC injection group were similar to that those in Heptavax®-II SC injection group. The most common vaccine-related systemic AEs were headache (mpHBV SC: 3.6%; Heptavax®-II SC: 3.6%), fever (mpHBV SC: 2.5%; Heptavax®-II SC: 2.5%), and malaise (mpHBV SC: 1.1%; Heptavax®-II SC: 2.2%). Only one participant in the mpHBV SC group had an oral temperature ≥39°C (on Day 5 after the first vaccination), but decreased to normal within a day without any medications. One participant in mpHBV SC group discontinued from the study due to vaccine-related systemic AEs (diarrhea, fatigue, headache, and fever) on the Day 1 Postdose 2 and injection-site AEs (erythema and swelling) on the Day 2 Postdose 2; these resolved within 1 day and 4 days, respectively. There were no reports of serious vaccine-related AEs (including death) throughout the duration of study.
Table 3. Summary of adverse events from Day 1 to 15 days following any vaccination visits.
The incidences of solicited injection-site AEs in mpHBV IM group were lower than those elicited by SC administration: pain 57.4%, erythema 22.3%, and swelling 23.4%.
Discussion
The vaccine-induced IgG antibodies to hepatitis B virus surface antigen (anti-HBs) are efficacious in the protection against hepatitis B virus infection. The anti-HBs titer of ≥10 mIU/mL measured after completion of vaccination series is considered a reliable serological marker of protection against hepatitis B virus infection.Citation7 Therefore, it is crucial to evaluate the proportion of immune responses achieving anti-HBs titer of ≥10 mIU/mL (SPR) after the completion of the standard vaccination schedule.
The immunogenicity results from the present study demonstrated that SPR at 1 month after a 3-dose series of mpHBV SC was 91.6%, and not inferior to the licensed Heptavax®-II SC. Moreover, mpHBV SC as a 3-dose series was shown to be highly immunogenic and anti-HBs GMTs at 1 month after 3-dose series tended to be higher than Heptavax®-II SC. These data indicate that both mpHBV SC and Heptavax®-II SC induce satisfactory immune responses to achieve the seroprotection level of ≥ 10 mIU/mL.
The mpHBV SC was generally well-tolerated and there were no clinically meaningful differences between mpHBV SC and Heptavax®-II SC with respect to vaccine-related AEs, including solicited injection-site AEs, systemic AEs, serious vaccine-related AE, and discontinuation. Most injection-site AEs were mild-to-moderate in intensity. One participant who received mpHBV SC reported severe injection-site pain and nodule, but this resolved without discontinuation from the study.
The route of administration is an important factor to evaluate immunogenicity and safety of a vaccine. Intramuscular administration is generally recommended for adjuvant-containing vaccines worldwide because reactogenicity is increased when subcutaneous administration is employed.Citation8,Citation9 However, most pediatric vaccines containing adjuvants are generally administered subcutaneously in Japan. Questions have remained on the route of administration of vaccines including adjuvants regarding the immunogenicity and tolerability in the Japanese. We therefore investigated the immunogenicity and tolerability of mpHBV IM in comparison to mpHBV SC. The present study showed that mpHBV IM in young Japanese adults was highly immunogenic and generally well-tolerated. Although this study was not design to statistically compare mpHBV IM to mpHBV SC and/or Heptavax®-II SC, SPR and GMT in the mpHBV IM given as a 3-dose series were generally higher than those of subcutaneous administration and are consistent with the results seen with intramuscular administration of RECOMBIVAX HB® manufactured by a modified process in healthy young adults.Citation10 As a result, these findings may encourage the intramuscular route of administration in Japan.
Potential limitations of these results included the small sample size for the mpHBV IM group and the lack of a Heptavax®-II intramuscular administration arm in this study. Though 70 participants were excluded from the analyses for safety and immunogenicity, there was not a significant impact on the interpretation of study results in the primary objectives for safety and immunogenicity which was predefined in the study protocol.
Conclusion
The results of the present study demonstrated that SC administration of recombinant hepatitis B vaccine manufactured by a modified process (mpHBV) was well-tolerated, was not inferior to SC administration of the licensed Heptavax®-II and elicited robust immune responses. Furthermore, IM administration of mpHBV showed better safety and immunogenicity profiles compared to SC administration of mpHBV. These results indicate that IM administration may be effective route for the delivery of mpHBV in young adults.
Materials and methods
Study design
This is a partially double-blind (both mpHBV SC and Heptavax®-II SC in double-blind manner with regards to vaccine administered, and mpHBV IM in an unblinded manner), randomized, multicenter trial conducted at 10 sites in Japan from December 2011 through November 2012. This study evaluated the immunogenicity, safety, and tolerability of mpHBV compared to Heptavax®-II in healthy Japanese young adults. Participants aged 20–35 years old were randomly assigned to one of three groups in a 3:3:1 ratio (mpHBV SC: Heptavax®-II SC: mpHBV IM). The route of administration was unblinded. Investigators, study coordinator, participants, study personnel monitoring the study data, and laboratory testing personnel remained blinded to treatment group throughout the study. Participants received a 3-dose series of either mpHBV SC, Heptavax®-II SC, or mpHBV IM at Day 1 (study entry), Month 1, and Month 6. Blood samples for antibody testing against hepatitis B surface antigen (anti-HBs) were to be collected from all participants at Day 1 (study entry) prior to the first vaccination and at Month 7 (1 month Postdose 3).
Study objectives
The primary immunogenicity objective of this study was to demonstrate that in young Japanese adults immunogenicity of mpHBV SC was non-inferior to Heptavax®-II SC, as measured by SPR (defined as the percent of participants with anti-HBs titer ≥ 10 mIU/mL) at Month 7 (1 month Postdose 3). The safety objective was to evaluate the safety and tolerability of mpHBV SC. An exploratory objective was to describe the anti-HBs GMTs at Month 7 (1 month Postdos 3) of mpHBV SC and Heptavax®-II SC. Another exploratory objective was to summarize the immunogenicity and safety of mpHBV IM.
Study population
Healthy Japanese males and females between 20 and 35 of age were eligible for the study. Female participants had to have a negative urine pregnancy test. Participants were excluded if they: had a history of previous hepatitis B infection; had a history of vaccination with any hepatitis B vaccine; had a known or suspected hypersensitivity to any component of study vaccine; had recent receipt of immune globulin and/or blood products; were undergoing immunosuppressive therapy; received a live vaccine within 28 or an inactivated vaccine within 14 days of enrollment; were pregnant or nursing; or had a coagulation disorder contraindicating intramuscular injection. The study was conducted in accordance with principles of Good Clinical Practice, approved by the Institutional Review Board of each participating site, and written informed consent was obtained from each participant prior to study entry. This study is registered with ClinicalTrials.gov, number NCT01463683.
Vaccine descriptions
Study vaccines (mpHBV, lot WL00042309; Heptavax®-II, lot WL00042807, Merck & Co., Inc., Kenilworth, NJ, USA) is a liquid product containing hepatitis B virus surface antigen (HBsAg), prepared from recombinant yeast cells. Both vaccines contained 10μg of HBsAg. The mpHBV was produced by modifying the manufacturing process by adjusting the amount of the adjuvant to optimize the phosphate-to-aluminum ratio of the adjuvant in the final product. Clinical materials were provided as a single-dose glass vials containing of 0.5 mL of products and stored at 2 to 8°C.
Measures
Immunogenicity
Blood samples were to be obtained on Day 1 prior to the first vaccination and Month 7 (1 month Postdose 3). Anti-HBs titer was determined by using the VITROS anti-HBs assay and performed at PPD Vaccines and Biologics Laboratory (Wayne, PA, USA). For the primary objective, the endpoint was the percentage of participants who achieved anti-HBs titer threshold levels of ≥10 mIU/mL at 1 month Postdose 3.
Safety
All participants were followed for safety for 15 days after each scheduled study visit. Each participant was instructed to record oral temperatures from Day 1 to Day 5 and all AEs (including solicited injection-site AEs: pain, erythema and swelling) from Day 1 through Day 15 on a vaccination report card following each study visit. A maximum oral temperature of ≥37.8°C (100.0 °F) was defined as fever in this study.
Statistical analysis
Immunogenicity
The primary immunogenicity analyses were based on the per–protocol population, defined as participants who received all 3 scheduled doses of study vaccines, were anti-HBs seronegative at baseline (anti-HBs titer prior to the first vaccination was <5 mIU/mL), were anti-HBc seronegative at baseline, and adhered to the study procedures. For the primary analysis of SPR with subcutaneous injection at Month 7, the two-sided 95% CI for the between-treatment difference was calculated using Miettinen and Nurminen method.Citation11 For the primary hypothesis, mpHBV SC would be considered non-inferior to Heptavax®-II SC if the lower bound of the two-sided 95% CI of the between-treatment difference in SPR (mpHBV SC minus Heptavax®-II SC) at Month 7 is not lower than −10 percentage points (non-inferiority margin).
Safety
All randomized participants who received at least 1 dose of study vaccine and had safety follow-up were included in the safety analysis (All Participants as Vaccinated). The frequencies and percentages of AEs were summarized. Regarding the injection-site AEs (including erythema, swelling and pain) and the systemic AE of fever (defined as oral temperature ≥ 37.8°C [≥ 100.0°F]) between Day 1 through Day 15 after each vaccination and 95% confidence intervals for between-treatment differences in the percentage of participants with events were calculated using the Miettinen and Nurminen method.Citation11
Sample size
This study randomized 300 participants into both SC groups and had 91% power to establish that mpHBV SC would be non-inferior to Heptavax®-II SC with regards to SPR at Month 7 at an overall one-sided, 2.5% alpha-level, if the underlying treatment difference is 0. The power and sample size were based on the following assumptions: 1) an approximately 11% dropout and/or protocol violation rate including 1.6% seropositive rate at baseline, 2) a non-inferiority margin of 10% (mpHBV SC minus HeptavaxTM-II SC), and 3) an underlying response rate of 86% for both subcutaneous groups based on data from clinical study conducted in ex-Japan and Japan. The minimum criterion for success was that the lower bound of 95% CI of difference > −10%.
Disclosure of potential conflicts of interest
In conjunction with the external investigators, this study was designed, executed, and analyzed by the sponsor. The sponsor formally reviewed a penultimate draft. All co-authors approved the final version of the manuscript. All authors are employees of Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA and may hold stock and/or stock options in the company.
Acknowledgments
The authors would like to thank all the participants for participating in this trial. Also, the authors greatly appreciate the contributions to this study provided the investigators: Hikaru Ishii, MD (Shin-nihonbashi Ishii Clinic), Masahiro Endo, MD (Osaki Hospital Tokyo Heart Center), Masayoshi Sone, MD (Sone Clinic), Chihiro Morita, MD (Nakameguro Atlas Clinic), Suehiro Shimotsuura, MD (Komaba Clinic), Michitaka Tsukioka, MD (Tsukioka Clinic), Tsuyoshi Yamaguchi, MD (Yamaguchi Clinic), Kenichi Furihata, MD (P-One Clinic) and Makoto Sanomura, MD (Hokusetsu General Hospital). The authors were deeply grateful to Jon E. Stek (Merck & Co., Inc., Kenilworth, NJ, USA) for the editorial support.
Additional information
Funding
References
- Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13(Suppl 1):S47–9. doi:10.1016/0264-410X(95)80050-N. PMID:7571830
- World Health Organization. Global hepatitis Report, 2017 [accessed 2017 Sep 15] http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf?ua=1.
- WHO global health estimated for 2015 published in 2016 (Global Health Estimates 2015: deaths by cause, age, sex, by country and region, 2000–2015. http://www.who.int/entity/healthinfo/global_burden_disease/GHE2015_Deaths_Global_2000_2015.xls?ua=1.
- Bodihar NP. Hepatitis B infection in pregnancy. Hepatitis B. Annual. 2004;1:199–209.
- World Health Organization. Hepatitis B vaccines: WHO position paper—July 2017. Weekly epidemiological record. 2017;92:369–92. PMID:28685564
- Centers for Disease Control and Prevention. Surveillance for Acute Viral Hepatitis —United States, 2007. MMWR. 2009;58(SS-3).
- Jack AD, Hall AJ, Maine N, Mendy M, Whittle HC. What level of hepatitis B antibody is protective? J Infect Dis. 1999;179(2):489–92. doi:10.1086/314578. PMID:9878036
- Petousis-Harris H. Vaccine injection technique and reactogenicity-Evidence for practice. Vaccine. 2008;26(50):6299–304. doi:10.1016/j.vaccine.2008.08.052. PMID:18804137
- Cook IF. Evidence based route of administration of vaccines. Hum Vaccin. 2008;4(1):67–73. doi:10.4161/hv.4.1.4747. PMID:17881890
- Van Damme P, Minervini G, Liss CL, McCarson B, Vesikari T, Boslego JW, Bhuyan PK. Safety, tolerability and immunogenicity of a recombinant hepatitis B vaccine manufactured by a modified process in healthy young adults. Hum Vaccin. 2009;5(2):92–7. doi:10.4161/hv.5.2.6587. PMID:18690015
- Miettinen O, Nurminen M. Comparative analysis of two rates. Stat Med. 1985;4:213–26. doi:10.1002/sim.4780040211. PMID:4023479
