ABSTRACT
Sabin-based inactivated poliovirus vaccine(sIPV) is gradually replacing live-attenuated oral polio vaccine(OPV). Sabin-inactivated poliovirus vaccine(sIPV) has played a vital role in reducing economic burden of poliomyelitis and maintaining appropriate antibody levels in the population. However, due to its high cost and limited manufacturing capacity, sIPV cannot reach its full potential for global poliovirus eradication in developing countries. Therefore, to address this situation, we designed this study to evaluate the dose-sparing effects of AS03, CpG oligodeoxynucleotides (CpG-ODN) and polyinosinic:polycytidylic acid (PolyI:C) admixed with sIPV in rats. Our results showed that a combination of 1/4-dose sIPV adjuvanted with AS03 or AS03 with BW006 provides a seroconversion rate similar to that of full-dose sIPV without adjuvant and that, this rate is 5-fold higher than that of 1/4-dose sIPV without adjuvant after the first immunization. The combination of AS03 or AS03 with BW006 as an adjuvant effectively reduced sIPV dose by at least 4-fold and induced both humoral and cellular immune responses. Therefore, our study revealed that the combination of AS03 or AS03 with BW006 is a promising adjuvant for sIPV development.
Introduction
Poliomyelitis is a highly infectious disease that poses a great threat to the children's health. Since the World Health Organization (WHO) established the Global Polio Laboratory Network (GPLN) in 1988, poliomyelitis outbreaks have been largely controlled by extensive use of poliovirus vaccines.Citation1-3 The live-attenuated oral polio vaccine (OPV) and the inactivated polio vaccine (IPV) are the two main poliovirus vaccine used throughout the world since the 1950s. Despite the high effectiveness and low cost of OPV against all 3 serotypes of poliovirus, OPV can also be the source of vaccine-associated paralytic poliomyelitis (VAPP), a disease caused by either circulating vaccine-derived polioviruses (cVDPVs) or immunodeficiency-associated VDPVs (iVDPVs).Citation4,Citation5
Therefore, in 2013, the WHO endorsed the new Polio Eradication and Endgame Strategic Plan 2013–2018, which recommended a new, inactivated poliovirus vaccine (i.e, Sabin-inactivated polio vaccine (sIPV)), bivalent OPV and monovalent OPV for use in developing countries and regions, aiming to completely eradicate wild type poliovirus as well as VAPP and VDPVs.Citation6 Wild-type polio virus type 2 was eliminated in 1999, but the continued use of trivalent OPV contributes to cVDPV2. In 2012, the WHO Strategy Advisory Group of Experts (SAGE) recommended a switch from tOPV to bOPV and noted that although risk of cVDPV emergence following OPV2 cessation is very low, it cannot be ruled out, and the use of IPV would mitigate the consequences of an emergence.Citation7 The aim of the project was to establish immunogenicity against type 2 poliovirus among the entire population before immunization with bivalent OPV.Citation6 Meanwhile, use of sIPV avoids the risk of both VAPP and VDPV infection, which makes it very promising for use in developing countries.
However, the price of sIPV is approximately 5.5 US dollars (USD), this cost has been identified as a primary barrier for universal IPV introduction.Citation8 The high costs attributed to IPV preparation results in lower production capacity, and a large supply of viral antigen, is rarely affordable in low- and middle- income countries. In response to this dilemma, the Global Polio Eradication Initiative (GPEI) has developed a comprehensive strategy to achieve lower priced IPV, wherein intradermal (ID) fractional dose and adjuvant are viable options.Citation9
ID delivery has been proposed as a means to reduce the dose required for vaccination, and it has been shown in several phase 3 trials in infants and adults to be a promising option to dose-spare by 60–80%, despite certain limitations(e.g., dose regimen and age).Citation10-13 For sIPV, our laboratory conducted pioneering ID tests in ratsCitation14 and found out that the dose could be reduced by 80%, thus, further clinical trials are in preparation. In parallel with ID tests for sIPV, we also seek to determine optimal adjuvants for sIPV. Adjuvants for inactivated vaccines(e.g., MF59 for the Influenza vaccine) have been evaluated in several preclinical and clinical studies, which have demonstrated that adjuvant is likely to enhance immunogenicity and reduce the antigen dose and number of immunizations needed.Citation15-19 Only a few investigations have been conducted to evaluate adjuvant for IPV immunogenicity enhancement (e.g., oil-in-water emulsion and chitosan),Citation20,Citation21 and clinical information is limited to aluminum hydroxide.Citation22,Citation23
At present, AS03, CpG oligodeoxynucleotides (CpG-ODN) and polyinosinic:polycytidylic acid (PolyI:C) are the 3 adjuvants that have been comprehensively studied. AS03 is an adjuvant system comprised of α-tocopherol, squalene and polysorbate 80 in an oil-in-water emulsion. Currently, AS03 is included in licensed H5N1 prepandemic and H1N1 pandemic influenza vaccines, and has been shown to activate the innate immune system, increase antigen uptake and be present in draining lymph nodes.Citation15,Citation24 The vertebrate immune system can detect bacterial or viral DNA containing unmethylated CpG dinucleotides as a danger signal through Toll-like receptor 9 (TLR9).Citation25 Synthetic ODNs contain unmethylated CpG motifs that can mimic the activities of natural DNA, activating the innate immune system.Citation26 CpG 7909 has been evaluated by BioThrax(®)(Anthrax Vaccine Adsorbed, AVA) in phase 1 and 2 clinical trials and is registered as Nuthrax (®).Citation27,Citation28 PolyI:C is a synthetic double-strand RNA that can stimulate Toll-like receptor 3 (TLR3); PolyI:C, was one of the first therapeutic agents used to treat HIV and leukemia patients, but abandoned due to its toxicity.Citation29 In the US, PolyI:C has been used in rintatolimod (Ampligen) in several clinical trials (for HIV and chronic fatigue syndrome, among others) but was not approved by the U.S. Food and Drug Administration due to controversial clinical outcomes, and no additional trials were carried out. In China, a rabies vaccine with PolyI:C showed promising safety and efficacy in preclinical studiesCitation30 and phase 1 clinical trials. In addition, several studies have recently shown that Poly I:C can be a safe and effective adjuvant for improving both humoral and cellular immunity in animals.Citation31,Citation32 In this study, we evaluated the immuno-potentiating effects of AS03, CpG-ODN and Poly I:C alone or in combination admixed with sIPV.
Results
Seroconversion analysis
After the first immunization, all of the groups' seroconversion rate for type I poliovirus was higher than for the other two types (2 negative seroconversion groups for type II, 1 negative seroconversion group for type III; ). Within groups, the seroconversion rates for different types of poliovirus were significantly different. The seroversion rate for two of the groups(AS03 and AS03 combined with BW006(AS03/BW006)) were 100% across all three types of virus after the first immunization. For type I and type II poliovirus, the seroconversion rate of the 1/4-dose group without adjuvant was significantly lower than that of the full-dose group and 1/4-dose adjuvanted groups. We compared the 1/4-dose adjuvanted groups to the full-dose sIPV group and the 1/4-dose sIPV group (p1 value for the full-dose sIPV group, p2 values for the 1/4-dose sIPV group; ). The seroconversion rates for type I and type II poliovirus in the adjuvanted groups, except for the 1/4-dose with Poly I:C group, were equivalent to that of the full-dose group and were significantly higher than that of 1/4-dose group(p values are presented in ). The type III seroconversion rates of the AS03 and AS03/BW006 groups were significantly higher than those of the 1/4-dose group and even the full-dose group (AS03 group, p1 = 0.011, and p2 = 0.001; AS03/BW006 group, p1 = 0.011, and p2 = 0.001). After the second immunization, the difference in seroconversion rates within these groups was not significant (). The seroconversion rate results indicated that AS03, BW006 and AS03/BW006 adjuvanted groups can provide a similar level of protection to that of the full-dose group, indicating that adding AS03 and BW006 or both to 1/4-dose sIPV can provide satisfactory protection as early as28 days after the first immunization in rats.
Table 1a. Analyzed seroconversion of trivalent sIPV with different dose and adjuvanted group after the first immunization.
Table 1b. Analyzed seroconversion of trivalent sIPV with different dose and adjuvanted group after second immunization.
The neutralizing antibody level
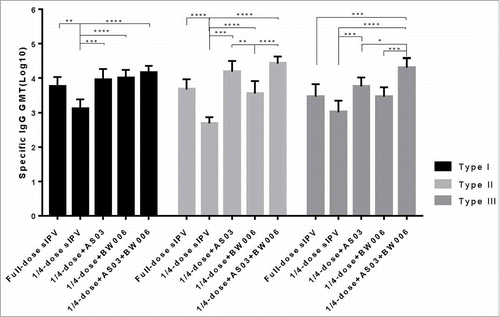
To evaluate the antigen sparing capacity of AS03, BW006 and PolyI:C, rats received an intramuscular (i.m.) immunization twice (with an interval of 28 days) with full-dose sIPV (30 DU,32 DU, and 45 DU for type I, type II, and type III, respectively), or 1/4-dose sIPV (7.5 DU,8 DU, or 11.25 DU for type I, type II, and type III, respectively) alone or admixed with adjuvant. Sera obtained at day 28 and day 56 from each group were used to measure the neutralizing antibody titers. After the first immunization, the neutralizing titers for the 3 types of poliovirus induced by different adjuvants or different vaccine doses varied (type I F = 16.479, p<0.05; type II F = 9.834,p<0.05;type III F = 13.246,p<0.05). As shown in , the 1/4-dose group did not yield effective neutralizing antibody for type II poliovirus. 1/4-dose with PolyI:C was not able to induce effective neutralizing antibodies for type II or type III poliovirus. Neutralizing antibody for type I poliovirus was induced in all groups, there was no significant difference between the full-dose sIPV group and the 1/4-dose with one adjuvant groups, and the differences among the 3 groups given a 1/4-dose with a single adjuvant were not significant. When AS03 and BW006 were combined, the neutralizing antibody enhancement for type I poliovirus was remarkable. The addition of AS03 or BW006 alone with a 1/4-dose sIPV induced a type II neutralizing antibody titer corresponding to the full-dose antigen group (p = 1.000, p = 1.000). The neutralizing antibody titer for type III poliovirus was similar to that of type II; 1/4-dose with BW006 could induce a neutralizing antibody level equivalent to the full-dose sIPV group (p = 1.000). In the 1/4-dose admix, which included AS03/BW006, the difference was not significant. After the second immunization, the levels of neutralizing antibody rose in all groups, and the geometric titer (GMT) in each group increased from 6- to 10- fold (). Groups with 1/4-dose combined with AS03, BW006 or PolyI:C all achieved the equivalent effect induced by full-dose sIPV. AS03 exhibited the most convincing dose-sparing adjuvant results by simultaneously enhancing all 3 types of neutralizing antibody, whereas compared with AS03 and BW006, PolyI:C was inferior. The GMT in the group administered a 1/4-dose together with AS03/BW006 was higher than that in the single adjuvant group.
Table 2a. The geometric mean titers(GMTs) against Sabin strains of poliovirus after first immunization.
Table 2b. The geometric mean titers(GMTs) against Sabin strains of poliovirus after second immunization.
Poliovirus-specific IgG antibody and the subclasses
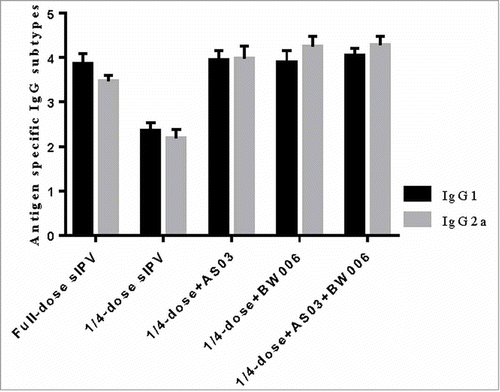
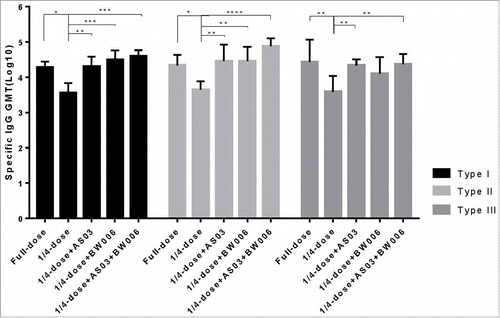
As shown in , after the first immunization, in groups without adjuvant, all 3 types of IgG in the full-dose sIPV group were significantly higher than those in the 1/4-dose sIPV group. In groups receiving the same dose, the IgG of the 1/4-dose without adjuvant was significantly lower than that of the other groups with adjuvant addition(). All 1/4-dose adjuvanted groups achieved a type I IgG level similar to that of the full-dose sIPV group (). For type II and III, the IgG levels of the 1/4-dose AS03 and 1/4-dose BW006 groups were as high as that of the full-dose sIPV group's, but the IgG level of the 1/4-dose AS03/BW006 group was significantly higher than that of the full-dose sIPV (p = 0.001, p = 0.000). The differences in the levels of all 3 IgG types between the 1/4-dose AS03 group and the 1/4-dose BW006 group were not significant.
Figure 1a. Poliovirus antigen specific IgG after the first immunization.
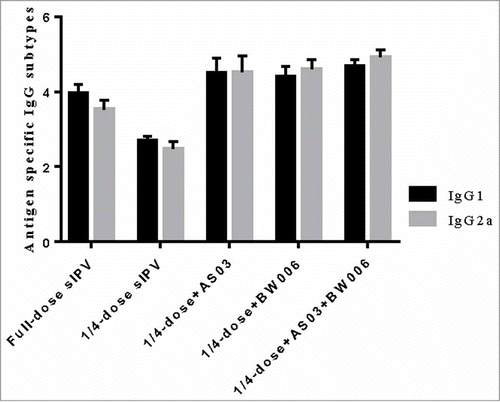
Compared with the levels after the first immunization, the antigen-specific IgG levels in all the groups increased nearly 10-fold after the second immunization ().. Significantly lower type I, II and III specific IgG levels were found in the 1/4-dose unadjuvanted group than in the other groups after the second immunization (). There was no significant difference in type of IgG level between the full-dose sIPV group and the 1/4-dose adjuvanted groups.
Figure 1b. Poliovirus antigen specific IgG after the second immunization.
Additionally, we analyzed the antigen-specific IgG subtype for type II poliovirus (, ). IgG1 and IgG2a levels in the 1/4-dose unadjuvanted group did not reach the same level as those in the other groups (p = 0.000 for all comparisons) after each immunization.
Compared with isotypes levels in the full-dose sIPV group IgG1, the 1/4-dose adjuvanted groups showed no significant difference isotype levels after the first immunization. However, after the second immunization, IgG1 levels in the 1/4-dose AS03 group and the 1/4-dose AS03/BW006 group were significantly higher than those in the full-dose group (p = 0.022, p = 0.000). IgG1 levels in the 1/4-dose BW006 group were similar to those in the full-dose sIPV group. IgG2a isotype levels in the 1/4-dose adjuvanted groups were significantly higher than those in the full-dose group after 2 immunizations (p = 0.000 for all comparisons).
Vaccine and adjuvant safety
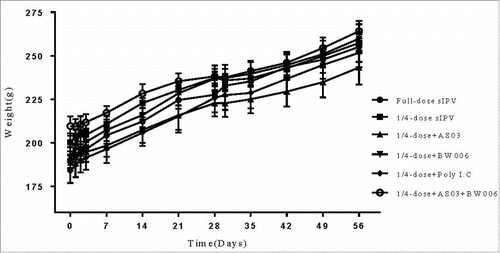
Animal conditions were frequently monitored, specifically platelet and peripheral white blood cell (WBC) count and animal activity during the 7 days after the first immunization; rat weights were also monitored through the whole experiment. The platelet and WBC count were not altered in either the antigen group or adjuvant combined group (data not shown). There was no record of convulsion, shock, death or other serious effects in any of the animal groups. The weights of the rats were continuously recorded one week before the first immunization(, shown from day 0). Three days after each immunization, the rat weights temporarily remained stable or increased slowly, and three days later, the growth rate in all groups returned to normal. The recovery time was shorter after the second immunization. Based on the data presented in , none of the experimental groups showed significant differences (p>0.5).
Discussion
In the near future, according to the Polio Eradication and Endgame Strategic Plan, worldwide demand for IPV will increase, mainly in developing countries. Therefore, it is important to improve vaccine manufacturing strategies and reduce its cost by developing new techniques or reducing antigen dose. Admixing adjuvant with a vaccine is an effective and efficient approach for antigen sparing, and consequently vaccine production capacity is improved. In this study, we evaluated both the humoral and cellular effects induced by adjuvants such as AS03, BW006 and PolyI:C in sIPV. Compared with PolyI:C adjuvanted group, the AS03 adjuvanted 1/4-dose group improved neutralizing antibody GMT production by nearly 9-, 16-, and 44-fold, and BW006 increased it by4.5-, 3.8-, 10-fold for types I, II and III of Sabin-strain poliovirus, respectively. Thus, we concluded that the use of AS03 or BW006is more effective than PolyI:C as an adjuvant to induce neutralizing antibodies for sIPV. In addition, according to the seroconversion rate results, AS03 or AS03/BW006adjuvanted groups provided an appropriate protection rate (100%) for rats as early as 28 days after the first(prime) immunization; this level of protection, normally requires two full doses of sIPV(prime and boost). This finding indicates that AS03 is a new option for a single shot of sIPV that, could reduce vaccine cost, improve compliance, and importantly, boost population immunity in developing countries. In subsequent analysis of the cellular immune response, we studied antigen-specific IgG and IgG isotypes and found that, the antigen-specific IgG results were similar to the neutralizing antibody results. Antigen-specific IgG in the adjuvanted groups reached the same level induced by full-dose sIPV. We also evaluated antigen-specific IgG1 and IgG2a for type II poliovirus. The 1/4-dose AS03 and 1/4-dose AS03/BW006 adjuvanted sIPV effectively enhanced both IgG1 and IgG2a to higher levels than those induced by full-dose sIPV in a balanced manner. These results may be related to the α-tocopherol-dependent stimulation of monocytes and cytokine production.Citation24 Similar results have been observed by GSK Biologicals in several other AS03-adjuvanted vaccines(e.g., the influenza vaccine, HIV vaccine and malaria vaccine).Citation15,Citation33 Moreover, BW006 is a B type CpG-ODN, which after being recognized by Toll-like receptor 9, improved B cell activation, inhibited B cells apoptosis, and enhanced immunoglobulin G (IgG) class switch.Citation34,Citation35 A previous study on the rabies vaccine showed that BW006 induces a more balanced Th1/Th2 immune response than that from antigen alone.Citation36 This finding was also verified by our study of BW006 with sIPV. In rats, the different Th1 and Th2 immune responses are divided in two distinct major subsets of T-cells, characterized by their pattern of cytokine production. Th1 responses are correlated with the induction of cell-mediated immunity and are usually associated with protection against intracellular pathogens, enhancement of IgG2a and 2b and IgG3 isotypes and production of the cytokines IL-2, TNF- β and IFN-γ.Citation37,Citation38 Th2 responses are mainly correlated with humoral immune response through the triggering of B cell proliferation and differentiation, with stimulation of production of IgG1 and the cytokines IL-4 and IL-1037. The results of our study showed that AS03 or AS03 mixed with BW006 enhanced both Th1 and Th2 responses, leading to a more balanced immune event than antigen alone, and ultimately to strong humoral immunity. Hence, the combination of AS03 or AS03/BW006 has the potential to increase IPV dose availability by 4-fold.
There are several limitations to our study. In our previous study on by intradermal delivery of fractional doses in rats, the antigen dose could be reduced to as little as 1/514, whereas in this study, we only considered the routine doses (full-dose and 1/4-dose) used in adjuvant(e.g., AS03) evaluation studies for influenza. According to the results, the use of AS03 or AS03/BW006 can spare more antigen dose in a rat model. Therefore, it is important for us to further determine the lowest antigen dose that can be achieved by AS03 or AS03/BW006. Additionally, whether these adjuvant can induce mucosal immunity has still not been elucidated. In future research, we should determine the minimal dose required for both sIPV and adjuvant, especially AS03, because we may be able to improve the existing regimen and its cost-effectiveness (AS03 per dose costs 0.10 USD).Citation39-41 Furthermore, a more elaborate adjuvant toxicity study is required before these adjuvants can be considered for human use, especially in children.
Consistent with these results, the combination of AS03 or AS03/BW006 is a potential feasible adjuvant for sIPV dose-sparing that would not compromise immune response. Furthermore, 1/4-dose sIPV could be induced to produce sufficient antibody protection in rats after the first immunization by triggering a cellular immune effect. Adjuvanted sIPV could reduce current costs and fulfill the growing demand in developing countries, which is in accordance with the Polio Eradication and Endgame Strategic Plan.
Materials and methods
Vaccine preparation
Sabin-IPV was prepared by the Institute of Medical Biology, Chinese Academy of Medical Science(Kunming, China) and generated from types I and II of the Sabin Original(SO+2) strain and type III of the RSO2(Pfizer) strain.Citation42 The indirect enzyme-linked immuno sorbent assay (ELISA) method for D-Ag detection was established in our laboratory. The D-Ag content in one full-dose sIPV was 30 DU, 32 DU and 45 DU for types I, II and III, respectively, in a 0.5-ml volume. Then, 1/4-dose of sIPV (7.5 DU, 8 DU and 11.25 DU for types I, II and III, respectively) was diluted in M199 to a final volume of 0.5 ml (without antigen) or 0.25 ml (adjuvant addition).
Adjuvant formulation
The AS03 used in this study contained 23.72 mg/ml α-tocopherol, 21.38 mg/ml squalene and 9.72mg/ml polysorbate-80 in PBS (pH 6.8).Citation24,Citation43 The mean particle sizes of the adjuvant emulsions were determined with a Zeta-Nanosizer instrument (Malvern instruments). The amount of AS03 used for each rat was 0.25 ml in a final volume of 0.5 ml. The CpG ODN used in this study was B type CpG ODN BW006 with a sequence of 5′-TCGACGTTCGTC GTTCGTCGTTC-3′,Citation44 synthesized with a nuclease-resistant phosphorothioate backbone by Sangon Biotech Company(Shanghai, China). BW006 was injected at 50 μg per injection. PolyI:C was obtained from Invivogen (USA) and used at 50 μg per injection.
Rats and immunizations
Wistar rats (SPF grade, weighting 180–220 g) were purchased from Institute of Medical Biology and used for all immunization experiments. The experiments were performed using male and female(1:1) rats, which were evenly distributed between all groups. Groups of 10 rats were administered an i.m. immunization twice (4 weeks apart), with a 0.5-ml total volume containing various IPV vaccine doses(full or, 1/4-dose) and adjuvants (AS03, BW006, PolyI:C or in combinations) in the hind limb. Serum samples were collected twice every 4 weeks after the first and second immunization and stored at −20°C until use.
Neutralizing assay
Neutralizing antibodies against all three poliovirus types were measured separately using a standard micro-neutralization assay in 96-well plates. Briefly, pooled sera from each group were diluted 4-fold in MEM and treated at 56 °C for 30 min to inactivate complement. Serum samples were made from 2-fold serial dilutions from 23 (1:8) – 214 (1:16384) and incubated at 37°C and 5% CO2 for 3h with 100 TCID50/50 μl of poliovirus Sabin strains. Then, 5 × 103/ml Hep-2 cells were added to each well, and seven days later, the cytopathic effect was monitored by staining the plate with crystal violet dye. The neutralizing antibody titers are expressed as the last serum dilution that had an intact monolayer and were calculated using Reed-Muench formula.Citation45
Antigen-specific IgG analysis using an ELISA
Anti-poliovirus IgG(total), IgG1, and IgG2a were quantified in each serum sample using an indirect ELISA. High-binding 96-wells plates (Corning) were coated with diluted Sabin strain (1:300, v/v) in carbonate-bicarbonate buffer (pH 9.6) and incubated for 16 h at 4°C. After adsorption of the antigen, plates were washed once with 100 μl of PBS (pH 7.4) containing 0.05% Tween20 (PBS-T) and blocked with 200 μl of PBS-T containing 5% calf serum (PBST-C) at 37°C for 30 min. Subsequently, the plates were washed twice with PBS-T. Sera were diluted by serial 2-fold dilutions with PBS-T, added to the plates in duplicate and incubated at 37°C for 1 h. After three washes with PBS-T, the plates were incubated with appropriate dilutions of HRP-conjugated anti-rat IgG (Abcam), anti-rat IgG1 (Abcam) or anti-rat IgG2a (Abcam) for 1 h at 37°C. After extensive washing, 100 μl of O-phenylene diamine was added to each well. After 15 min of incubation in the dark at room temperature, the reaction was stopped by adding 1 M H2SO4(50μl/well). The antibody titers of each sample were determined by end-point dilution with a cutoff OD450 value of 0.100. Antibody titers from each group were transformed logarithmically and are expressed as the geometric means ± standard errors.
Statistical analysis
Comparisons of the GMT of neutralizing antibody were performed using ANOVA followed by a Bonferroni test, whenever appropriate, with the statistics package SPSS 25.0. The GMT of antigen-specific IgG or IgG isotypes was assessed via ANOVA using prim 7.0 software. Otherwise, seroconversion refers to a reciprocal antibody titer of 8 or higher. Serum seroconversion rates were measured with Fisher's Exact tests. All p-values <0.05 were considered to be statistically significant and p-values <0.01 were considered to be highly statistically significant.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work is supported by National Cooperation Project (2014DFA32870); Cooperative innovation program (2016-I2M-3-026); Innovation team project (2015HC027) and Technology Major research project (2017IB008) in Yunnan Province.
References
- Home C. Laboratory surveillance for wild and vaccine-derived polioviruses--worldwide, January 2007-June 2008. Mmwr Morbidity & Mortality Weekly Report. 2008;57:967.
- Roberts L. A one-two punch against polio. Petroleum Rock Mechanics. 2014;345:63–76.
- Bahl S, Bhatnagar P, Sutter RW, Roesel S, Zaffran M. Global Polio Eradication - Way Ahead. The Indian Journal of Pediatrics. 2018;85:124–131. doi:10.1007/s12098-017-2586-8. PMID:29302865.
- Burns CC, Diop OM, Sutter RW, Kew OM. Vaccine-Derived Polioviruses. Journal of Infectious Diseases. 2014;210(Suppl 1):S283. doi:10.1093/infdis/jiu295. PMID:25316847.
- Platt LR, Estívariz CF, Sutter RW. Vaccine-Associated Paralytic Poliomyelitis: A Review of the Epidemiology and Estimation of the Global Burden. Journal of Infectious Diseases. 2014;210(Suppl 1):S380. doi:10.1093/infdis/jiu184. PMID:25316859.
- Initiative GPE. Polio Eradication and Endgame Strategic Plan 2013–2018. Immunization Programs. 2013. http://www.who.int/immunization/sage/meetings/2012/november/1_DRAFTGPEI_EndgameStrategicPlan23Oct12.pdf.
- http://www.who.int/wer/2012/wer8721.pdf?ua=1._Weekly_epidemiological_record. 2012.
- Okayasu H, Sutter RW, Jafari HS, Takane M, Aylward RB. Affordable inactivated poliovirus vaccine: strategies and progress. Journal of Infectious Diseases. 2014;210(Suppl 1):S459. doi:10.1093/infdis/jiu128. PMID:25316868.
- Shulman LM, Manor Y & Sofer D. Poliovirus vaccine and vaccine-derived polioviruses. N Engl J Med. 2010;363:1870–1. doi:10.1056/NEJMc1009551. PMID:21047241.
- Cadorna-Carlos J, Vidor E, Bonnet MC. Randomized controlled study of fractional doses of inactivated poliovirus vaccine administered intradermally with a needle in the Philippines. Int J Infect Dis. 2012;16:110–6. doi:10.1016/j.ijid.2011.10.002.
- Resik S, Tejeda A, Sutter RW, Diaz M, Sarmiento L, Alemañi N, Garcia G, Fonseca M, Hung LH, Kahn AL, et al. Priming after a fractional dose of inactivated poliovirus vaccine. New England Journal of Medicine. 2013;368:416–24. doi:10.1056/NEJMoa1202541. PMID:23363495.
- Anand A, Zaman K, Estívariz CF, Yunus M, Gary HE, Weldon WC, Bari TI, Steven Oberste M, Wassilak SG, Luby SP, et al. Early priming with inactivated poliovirus vaccine (IPV) and intradermal fractional dose IPV administered by a microneedle device: A randomized controlled trial. Vaccine. 2015;33:6816. doi:10.1016/j.vaccine.2015.09.039. PMID:26476367.
- Resik S, Tejeda A2, Diaz M, Okayasu H, Sein C, Molodecky NA, Fonseca M, Alemany N, Garcia G, Hung LH, et al. Boosting Immune Responses Following Fractional-Dose Inactivated Poliovirus Vaccine: A Randomized, Controlled Trial. Journal of Infectious Diseases. 2017;215:175. PMID:28073858.
- Ma L, Cai W, Sun M, Cun Y, Zhou J, Liu J, Hu W, Zhang X, Song S, Jiang S, et al. Analyzed immunogenicity of fractional doses of Sabin-inactivated poliovirus vaccine (sIPV) with intradermal delivery in rats. Human Vaccines. 2016;12:3125–31. doi:10.1080/21645515.2016.1214347.
- Giarolasilva S, Coelho-Dos-Reis JGA, Mourão MM, Campi-Azevedo AC, Nakagaki Silva EE, Luiza-Silva M, Martins MA, Silveira-Cassette ACO, Batista MA, Peruhype-Magalhães V, et al. Distinct patterns of cellular immune response elicited by influenza non-adjuvanted and AS03-adjuvanted monovalent H1N1(pdm09) vaccine. Antiviral Research. 2017;144:70–82. doi:10.1016/j.antiviral.2017.05.009. PMID:28549970.
- Durando P, Iudici R, Alicino C, Alberti M, de Florentis D, Ansaldi F, Icardi G. Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. Human Vaccines. 2011;7(Suppl):29. doi:10.4161/hv.7.0.14560. PMID:21245655.
- Patel S, Faraj Y, Duso DK, Reiley WW, Karlsson EA, Schultz-Cherry S, Vajdy M. Comparative Safety and Efficacy Profile of a Novel Oil in Water Vaccine Adjuvant Comprising Vitamins A and E and a Catechin in Protective Anti-Influenza Immunity. Nutrients. 2017;9:516. doi:10.3390/nu9050516.
- Vesikari T, Pellegrini M, Karvonen A, Groth N, Borkowski A, O'Hagan DT, Podda A. Enhanced immunogenicity of seasonal influenza vaccines in young children using MF59 adjuvant. Pediatric Infectious Disease Journal. 2009;28:563. doi:10.1097/INF.0b013e31819d6394. PMID:19561422.
- Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Science Translational Medicine. 2011;3:85ra48. doi:10.1126/scitranslmed.3002336. PMID:21632986.
- Westdijk J, Koedam P, Barro M, Steil BP, Collin N, Vedvick TS, Bakker WA, van der Ley P, Kersten G. Antigen sparing with adjuvanted inactivated polio vaccine based on Sabin strains. Vaccine. 2013;31:1298. doi:10.1016/j.vaccine.2012.12.076. PMID:23313617.
- Ghendon Y, Markushin S, Akopova I, Koptiaeva I & Krivtsov G. Chitosan as an adjuvant for poliovaccine. Journal of Medical Virology. 2011;83:847–52. doi:10.1002/jmv.22030. PMID:21412793.
- Verdijk P, Rots NY, van Oijen MG, Weldon WC, Oberste MS, Okayasu H, Sutter RW, Bakker WA. Safety and immunogenicity of a primary series of Sabin-IPV with and without aluminum hydroxide in infants. Vaccine. 2014;32:4938–44. doi:10.1016/j.vaccine.2014.07.029. PMID:25043278.
- Rivera L, Pedersen RS, Peña L, Olsen KJ, Andreasen LV, Kromann I, Nielsen PI, Sørensen C, Dietrich J, Bandyopadhyay AS, et al. Immunogenicity and safety of three aluminium hydroxide adjuvanted vaccines with reduced doses of inactivated polio vaccine (IPV-Al) compared with standard IPV in young infants in the Dominican Republic: a phase 2, non-inferiority, observer-blinded, randomised, and controlled dose investigation trial. The Lancet Infectious Diseases. 2017;17:745–53. doi:10.1016/S1473-3099(17)30177-9. PMID:28454674.
- Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi:10.1016/j.vaccine.2011.01.011. PMID:21256188.
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740. doi:10.1038/35047123. PMID:11130078.
- Ishii KJ & Akira S. Toll or toll-free adjuvant path toward the optimal vaccine development. Journal of Clinical Immunology. 2007;27:363. doi:10.1007/s10875-007-9087-x. PMID:17370119.
- Minang JT, Inglefield JR, Harris AM, Lathey JL, Alleva DG, Sweeney DL, Hopkins RJ, Lacy MJ, Bernton EW. Enhanced early innate and T cell-mediated responses in subjects immunized with Anthrax Vaccine Adsorbed Plus CPG 7909 (AV7909). Vaccine. 2014;32:6847–54. doi:10.1016/j.vaccine.2014.01.096. PMID:24530403.
- Hopkins RJ, Kalsi G, Montalvo-Lugo VM, Sharma M, Wu Y, Muse DD, Sheldon EA, Hampel FC, Lemiale L. Randomized, double-blind, active-controlled study evaluating the safety and immunogenicity of three vaccination schedules and two dose levels of AV7909 vaccine for anthrax post-exposure prophylaxis in healthy adults. Vaccine. 2016;34:2096–105. doi:10.1016/j.vaccine.2016.03.006. PMID:26979136.
- Robinson RA, DeVita VT, Levy HB, Baron S, Hubbard SP, Levine AS. A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patieonts with leukemia or solid tumors. Journal of the National Cancer Institute. 1976;57:599–602. doi:10.1093/jnci/57.3.599. PMID:978771.
- Zhang Y, Zhang S, Li W, Hu Y, Zhao J, Liu F, Lin H, Liu Y, Wang L, Xu S, Hu R, et al. A novel rabies vaccine based-on toll-like receptor 3 (TLR3) agonist PIKA adjuvant exhibiting excellent safety and efficacy in animal studies. Virology. 2016;489:165. doi:10.1016/j.virol.2015.10.029. PMID:26765968.
- Zhang A, Lai H, Xu J, Huang W, Liu Y, Zhao D, Chen R. Evaluation of the Protective Efficacy of Poly I:C as an Adjuvant for H9N2 Subtype Avian Influenza Inactivated Vaccine and Its Mechanism of Action in Ducks. Plos One. 2017;12:e0170681. doi:10.1371/journal.pone.0170681. PMID:28135294.
- Moriyama M, Chino S, Ichinohe T. Consecutive inoculations of influenza virus vaccine and poly(I:C) protects mice against homologous and heterologous virus challenge. Vaccine. 2017;35:1001–7. doi:10.1016/j.vaccine.2017.01.025. PMID:28111142.
- Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Review of Vaccines. 2012;11:349–66. doi:10.1586/erv.11.192. PMID:22380826.
- Hartmann G, Weiner GJ, Krieg AM. CpG DNA: A potent signal for growth, activation, and maturation of human dendritic cells. Proc Natl Acad Sci U S A. 1999;96:9305–10. doi:10.1073/pnas.96.16.9305. PMID:10430938.
- He B, Qiao X & Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. Journal of Immunology. 2004;173:4479. doi:10.4049/jimmunol.173.7.4479.
- Wang X, Bao M, Wan M, Wei H, Wang L, Yu H, Zhang X, Yu Y, Wang L. A CpG oligodeoxynucleotide acts as a potent adjuvant for inactivated rabies virus vaccine. Vaccine. 2008;26:1893–901. doi:10.1016/j.vaccine.2008.01.043. PMID:18321616.
- TR M & S S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunology today. 1996;17:138. doi:10.1016/0167-5699(96)80606-2. PMID:8820272.
- Broere F, Apasov SG, Sitkovsky MV, Eden WV. A2 T cell subsets and T cell-mediated immunity. Principles of Immunopharmacology. Birkhäuser Basel. 2011;15–27.
- DL-alpha-Tocopherol. http://www.sigmaaldrich.com/catalog/product/supelco/47786?lang=en®ion=US.
- Squalene. http://www.sigmaaldrich.com/catalog/substance/squalene4107211102411?lang=en®ion=US.
- Polysorbate80. http://www.sigmaaldrich.com/catalog/substance/polysorbate8012345900565611?lang=en®ion=US.
- Annex 2: Guidelines for the safe production and quality control of inactivated poliomyelitis vaccine manufactured from wild polioviruses (Addendum, 2003, to the Recommendations for the Production and Quality Control of Poliomyelitis Vaccine. Who Technical Report No. 926, 2004.
- Haensler J. Manufacture of Oil-in-Water Emulsion Adjuvants. New York: Springer; 2017.
- Zhou X, Wei H, Sun P, Wu X, Wan M, Zhang P, Guo S, Zhao T, Yu Y, Wang L. Recombinant hepatitis B virus surface antigen formulated with B-type CpG oligodeoxynucleotide induces therapeutic immunity against hepatitis B virus surface antigen-expressing liver cancer cells in mice. Cancer Biotherapy & Radiopharmaceuticals. 2012;27:234. doi:10.1089/cbr.2011.1127.
- Reed LJ, Muench H. A SIMPLE METHOD OF ESTIMATING FIFTY PER CENT ENDPOINTS. American Journal of Tropical Medicine & Hygiene. 1938;27.