ABSTRACT
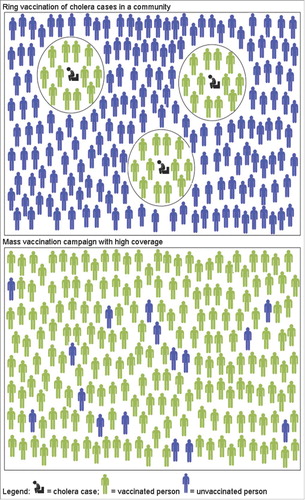
Ring vaccinations create a zone of immune contacts around a case to prevent further disease transmission and have been successfully employed in the eradication of smallpox and the control of other infections. Millions of oral cholera vaccine (OCV) doses have been effectively deployed through mass vaccination campaigns. But there are situations when the OCV supply, resources, and time are limited and alternative strategies need to be considered. People living in close proximity of cholera cases often share risk factors such as contaminated water supply and poor sanitation. Targeting people within a given radius around a cholera case for intervention including vaccination, improved water supply and sanitation may be a practical and effective approach. A ring oral cholera vaccination strategy could be considered before, after or as an alternative to a mass vaccination approach. We review here the use of the ring vaccinations in general and specifically during cholera outbreaks.
Background
In any community, including one in which an infection is spreading, people do not mix homogeneously. Individuals interact in geographic patterns and disease spreads from contact to contact within these networks.Citation1 The purpose of ring vaccinations is to create and ensure the presence of a buffer of immunity around a case that would contain the spread of the disease. The ring vaccination strategy starts with the identification of infectious index cases, followed by immunization of those in contact with them.
There are several prerequisites for a ring vaccination.Citation2 First, an effective vaccine has to be available; the vaccine should confer full or partial protection within days of administration of a single dose. Second, timely and thorough surveillance has to be in place to assure that all cases in the community are detected. Cases have to be quickly identified; typical clinical features or a rapid and accurate laboratory test is essential, as this will be used to trigger the ring vaccination response. Third, it must be possible to visit and vaccinate all contacts of the index case within days. Several layers of contacts extending from the index case could be considered for vaccination, depending on the disease, the vaccine, the population density and other factors. Even if the vaccine does not protect close contacts of the index case who are already incubating the disease, vaccination of secondary and tertiary contacts may prevent transmission of the disease to the rest of the population.
Experience with the ring vaccination strategy
Ring vaccination has been utilized under various situations and for different purposes. Perhaps the best known example is the Smallpox Eradication Program which began in 1966 under the overall leadership of Donald Henderson.Citation3 Early eradication efforts were based on mass smallpox vaccinations of entire populations.Citation4 From the start, the program emphasized surveillance to monitor progress and better understand the epidemiology of smallpox. In Western Nigeria where 90% of the population had been vaccinated, a smallpox outbreak occurred among a religious sect resisting vaccination.Citation5 With a delay in delivery of supplies, campaign staff were forced into action and rapidly learned to isolate infected individuals and identify and vaccinate their contacts. The prominent clinical features of smallpox made this approach highly practical. Further observations indicated that smallpox did not spread quickly such that isolation of patients and vaccination of their contacts halted the transmission of the disease.Citation4,Citation6 During the final stages of the eradication project, specifically in India, efforts shifted from mass vaccination campaigns to the “surveillance and containment” strategy, devised by William Foege and later known as ring vaccination. This consisted of rigorous investigation to find smallpox cases, immediate and strict isolation of patients and vaccination of the patient's primary contacts (relatives, neighbours, co-workers) and secondary contacts (the contacts of contacts). The ring vaccination strategy combined with technological advancements such as the invention by Benjamin Rubin of the bifurcate needle, an easy to produce and use instrument which required only 25% of the standard amount of vaccine, played a major role in the acceleration and ultimate success of smallpox eradication.Citation3 Even with delays in identifying index cases, there were several factors that enhanced the effectiveness of the ring vaccination strategy against smallpox.Citation7 First, smallpox is clinically apparent in almost every infection and its characteristic rash facilitated surveillance and confirmation of cases. Second, smallpox is generally transmitted through fairly prolonged person-to-person contact, by bodily fluids or through contaminated objects such as bedding or clothing, making containment feasible. Third, there is no carrier state or animal reservoir of the disease. Fourth, the smallpox vaccine used was heat stable, required only one dose and was easy to transport and administer.
In 1977, during an epidemic of group A Neisseria meningitides infection in Zaria, Nigeria a study was conducted to assess the effect of meningococcal vaccination of household contacts in preventing secondary infections, when given on the day after admission of the index case to hospital.Citation8 Household contacts of patients with group A meningococcal infection were vaccinated with either group A and C polysaccharide meningococcal vaccine, of which only a limited number of doses were available, or tetanus toxoid vaccine. Five of 523 subjects who received tetanus toxoid developed meningococcal meningitis and another four probably had meningococcal disease (secondary attack rate of about 18 per 1000 household contacts). Only one possible case of meningococcal infection occurred among 520 contacts vaccinated with meningococcal vaccine. Vaccination had no effect on nasopharyngeal carriage of meningococci. The investigators concluded that meningococcal vaccination of household contacts is an effective way of using the vaccine when only limited doses are available. However, the meningococcal ring vaccination strategy was considered insufficient to control epidemics since proximity to a case is only one of several risk factors for meningococcal disease and only a small proportion of cases occur in the families of affected patients.Citation8 Furthermore, the potential of ring vaccination for meningococcal control has been superseded by the wide introduction of a meningococcal serogroup A conjugate vaccine in the sub-Saharan African meningitis belt starting in December 2010.Citation9 The phased deployment was done through mass vaccination campaigns in 26 African countries targeting 1 to 29 year olds. Starting in 2017 the vaccine will be introduced in the routine Expanded Programme on Immunization of children aged 9 to 18 months in these countries.Citation9
More recently, the ring vaccination strategy has been used to control mumps outbreaks in Israel. Despite high childhood vaccine coverage in the country, there have been mumps outbreaks in confined settings, such as in schools and in military personnel.Citation10 The outbreaks have been attributed to waning vaccine immunity and reduced natural exposure that could boost protection. In response, a comprehensive ring strategy that involved vaccinating people sharing the confined settings of a case (without necessarily direct contact with the case) was carried out. A single class or a single military company was vaccinated in response to one mumps case in the school or battalion, respectively, whereas the entire school or whole battalion was vaccinated when two or more cases were detected. It was reasoned that even if the mumps vaccine may not prevent infection in those already exposed, it could shorten the duration of virus shedding, avert severe disease, lower complication rates and stop transmission of mumps to the contacts' contacts. The overall aim of the strategy was to interrupt transmission and limit the number of those affected.Citation10
During a prolonged measles outbreak in Greater Manchester in the United Kingdom from 2012 to 2013, vaccination of close contacts was used to contain spread of the disease.Citation11 Where probable or confirmed cases were reported, a complete course of vaccine was advised for all under-immunised household contacts. In the response to some local outbreaks, schools with a high number of susceptible children were identified. Vaccine was proactively offered in these high-risk schools, especially when they had links to affected schools.Citation8
Towards the end of the 2013–2016 West African Ebola virus disease (EVD) epidemic the protection conferred by the recombinant vesicular stomatitis virus Ebola vaccine (rVSV-ZEBOV) when given through a ring vaccination strategy in Guinea was assessed under time pressure and with limited availability of vaccine.Citation12 In the trial, epidemiologically-defined rings around newly diagnosed EVD index cases were formed.Citation13 The rings consisted of contacts of the case and contacts of contacts. Contacts were defined as those who within the previous 21 days lived in the same household as the index case, were visited by the index case or were in close physical contact with the index case's body or body fluids, linen, or clothes.Citation14 Contacts of contacts included neighbours or extended family members living within the nearest geographic boundary plus household members of any high-risk contacts. The rings were randomised to the intervention arm of immediate vaccination or the control arm of delayed vaccination. The results showed high efficacy of a single injection of rVSV-ZEBOV in preventing EVD when delivered through the ring approach.Citation12
Mass versus ring vaccination
There are several factors that favour either mass or ring vaccination for disease control (). The size of the population at risk relative to the available number of vaccine doses, logistics and manpower are important considerations. Mass vaccination campaigns protect millions against poliomyelitis, measles and meningococcal meningitis in different impoverished settings.Citation15 Mass vaccination campaigns targeting other diseases may not always be feasible. In large populations with sporadic occurrence of the disease and especially with limited vaccine supply, a ring vaccination approach could be considered. For ring vaccinations, resources are required in terms of on-going surveillance, case confirmation and identification of contacts. On the other hand, compared to mass campaigns, less resources are needed for community engagement because of the smaller numbers targeted for vaccination and because individuals and households around an index case may be more motivated to take part in vaccinations than people who do not consider themselves at high risk.
Table 1. Comparison factors for and against mass campaigns or ring vaccination strategy for disease control.
When considering the mass versus the ring vaccination approach, timing is crucial. In the middle of an outbreak involving a large number of cases, mass vaccination will prevent more deaths than ring vaccinations, particularly in the context of limited resources for tracing and vaccinating contacts.Citation16 The ring approach may be most useful in the initial or final phases of an outbreak. Simulation studies suggest that the addition of ring vaccination earlier during the West African Ebola epidemic may not have contained the outbreak because of failure to detect early cases; in later stages of the epidemic, ring vaccinations could help eliminate the disease.Citation17 Whether in the initial or final stages of an outbreak, the success of a potential ring vaccination approach rests on the early and complete detection of cases.
There are clear instances when the ring approach is not a viable option. For example, the ring vaccination strategy is unlikely to work for vector-borne diseases like malaria because vectors do not stay within defined rings.Citation18 The ring vaccination strategy is not used in the Global Polio Eradication Initiative (GPEI) because subclinical poliovirus infection is typically far more widespread than the immediate social network of children with poliomyelitis.Citation19 The combined strategy of oral poliovirus vaccine (OPV) mass vaccination and the surveillance of children with acute flaccid paralysis (AFP) has eliminated wild-type polioviruses from much of the world but AFP surveillance is inherently limited because paralytic disease develops in only one of every 100 to 1000 infected individuals.Citation20 After 2 to 5 years without paralytic cases detected in a population of 200,000, the probability for the presence of silent poliovirus transmission has been estimated to still be between 38% to 1%.Citation21 Thus, the detection of even a single case of poliomyelitis due to wild poliovirus in a “polio free” country (i.e. free from polio for at least six months) is thought to require vaccinating a minimum of 2 million individuals using monovalent OPV within four weeks.Citation22
An oral cholera vaccination strategy?
A global cholera vaccine stockpile was created in 2011 for a rapid response to cholera outbreaks.Citation23 Through the stockpile mechanism, oral cholera vaccine (OCV) has been deployed in large mass vaccinations under diverse circumstances. From 2013 to 2017, over 25 million doses have been requested from the cholera vaccine stockpile, of which only 51% were shipped to countries for 46 deployments.Citation24 As the stockpile aims to expand the availability and distribution of cholera vaccine to more affected populationsCitation25 and with increasing awareness of policymakers, demand will continue to outstrip supply.
Although there are environmental reservoirs of V. cholerae in areas that give rise to cholera cases, when outbreaks occur cases tend to cluster.Citation26,Citation27 This may be due to a common source of infection, direct person-to-person transmission or spread from the local environment that has been contaminated by a case. Previous studies have shown that those living close to a cholera patient have an increased risk for developing cholera.Citation28-30 Direct exposure contributes significantly to endemic transmission of symptomatic cholera leading to increased risk within households.Citation31 One study showed that 24% of 294 household contacts of a cholera case had V. cholerae infection and bacterial shedding for an average of 2 days, with 5% of contacts shedding for more than 4 days.Citation32 If surveillance and contact tracing is in place prior to the start of the outbreak, then ring oral cholera vaccination could be considered as a preliminary control strategy, which could be followed by a wider mass vaccination campaign, if needed. A ring OCV strategy could also be considered following a mass campaign targeting contacts of break-through cases. A cholera rapid diagnostic test is now commercially available, which may be sufficient for the purpose of identifying index cases.Citation33 A single OCV dose given immediately to the contacts of a cholera case may be sufficient to elicit an immunity buffer, reduce short-term risk and limit the size of the outbreak until a second dose can be given.Citation34
Previous data from a cluster-randomised study was used to model a potential ring OCV strategy in a cholera-endemic site in Kolkata and found that high-level protection can be achieved for those living close to cholera cases.Citation30 More recently, case-area targeted interventions, which can include improved water quality and supply, sanitation, hand washing, oral cholera vaccine, and prophylactic antibiotics, was modelled.Citation35 The authors simulated the impact of such targeted interventions in N'Djamena, Chad. They found that vaccinating people within 100 meters around index case households and improving their water source early in epidemics would reduce the number of cases by 82% (IQR 71 to 88) compared to uncontrolled epidemics. The simulated, additional antibiotic treatment of neighbours within a 30- to 45-meter radius around the index case was helpful but only in the short term.
There is very little actual experience with using the ring oral cholera vaccination strategy (). In 2015, after a single dose mass oral cholera vaccination campaign that targeted most affected neighbourhoods in Juba, South Sudan, a single dose case-centred oral cholera vaccination strategy targeted remaining sporadic cases.Citation36 In 2016, the South Sudan Ministry of Health used a similar approach to target reported cases early in an epidemic.Citation37
As with all ring vaccination strategies there are several challenges of the ring OCV strategy. Cholera cases have to be detected quickly, sufficient vaccine doses must be stored (although less than in a mass vaccination campaign) and be available on site when the first cases are detected and manpower and the logistics for the contact tracing and vaccination have to be set-up. The cost and feasibility of establishing such a surveillance and response system integrated into the government health infrastructure so that it is sustainable, has yet to be estimated. Another challenge is defining the appropriate ring size to stop the transmission of cholera.Citation30 The radius may vary by location, population density, water supply and sanitation facilities. A large-ring approach, in which entire hamlets or villages are vaccinated in response to detected cases, may be considered. As with the mumps ring vaccination strategy described above, if the cholera vaccine does not prevent infection among the direct contacts of the case, it may stop transmission of cholera to the contacts of contacts, if the rings are large enough.
Conclusions
In some of the cholera endemic areas around the world, thousands if not millions of people are at risk of cholera – protecting all of them through mass vaccination would probably not be feasible especially with a vaccine that does not confer life-long protection. A more case-based approach needs to be considered. Even with the availability of an increased supply of OCV through the introduction of additional internationally-licensed products into the market, the ring strategy may still be favoured in some circumstances. The ring strategy is potentially cost effective and likely to be cheaper than a mass vaccination campaign, in terms of number of cases averted per doses administered.Citation38 All assumptions of feasibility, effectiveness and cost-effectiveness of an oral cholera ring vaccination strategy will need to undergo rigorous assessment in real-life public health settings.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Greenhalgh D. Optimal control of an epidemic by ring vaccination. Commun Stat Stoch Models. 1986;2(3):339–63. doi:10.1080/15326348608807041.
- Wells CR, Tchuenche JM, Meyers LA, Galvani AP, Bauch CT. Impact of imitation processes on the effectiveness of ring vaccination. Bull Math Biol. 2011;73(11):2748–72. Epub 2011/03/17. doi:10.1007/s11538-011-9646-4. PMID:21409511.
- Henderson DA. The eradication of smallpox–an overview of the past, present, and future. Vaccine. 2011;29(Suppl 4):D7–9. Epub 2011/12/23. doi:10.1016/j.vaccine.2011.06.080. PMID:22188929.
- Lane JM. Mass vaccination and surveillance/containment in the eradication of smallpox. Curr Topics Microbiol Immunol. 2006;304:17–29. Epub 2006/09/23. PMID:16989262.
- Hopkins JW. The eradication of smallpox: organizational learning and innovation in international health administration. J Dev Areas. 1988;22(3):321–32. PMID:12342353.
- Strassburg MA. The global eradication of smallpox. Am J Infect Control. 1982;10(2):53–9. Epub 1982/05/01. doi:10.1016/0196-6553(82)90003-7. PMID:7044193.
- In: Knobler S, Lederberg J, Pray LA, editors. Considerations for Viral Disease Eradication: Lessons Learned and Future Strategies: Workshop Summary. Washington (DC): The National Academies Collection: Reports funded by National Institutes of Health; 2002.
- Greenwood BM, Hassan-King M, Whittle HC. Prevention of secondary cases of meningococcal disease in household contacts by vaccination. Br Med J. 1978;1(6123):1317–9. doi:10.1136/bmj.1.6123.1317. PMID:417754.
- Trotter CL, Lingani C, Fernandez K, Cooper LV, Bita A, Tevi-Benissan C, Ronveaux O, Préziosi MP, Stuart JM. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010–15: an analysis of surveillance data. Lancet Infect Dis. 2017;17(8):867–72. doi:10.1016/S1473-3099(17)30301-8. PMID:28545721.
- Levine H, Rishpon S, Huerta-Hartal M, Davidovitch N. Preventing mumps outbreaks in confined settings: comprehensive ring vaccination as a containment strategy. Hum Vaccin. 2011;7(12):1389–93. Epub 2011/11/24. doi:10.4161/hv.7.12.18111. PMID:22108037.
- Pegorie M, Shankar K, Welfare WS, Wilson RW, Khiroya C, Munslow G, Fiefield D, Bothra V, McCann R. Measles outbreak in Greater Manchester, England, October 2012 to September 2013: epidemiology and control. Euro Surveill. 2014;19(49):20982. PMID:25523970.
- Henao-Restrepo AM, Longini IM, Egger M, Dean NE, Edmunds WJ, Camacho A, Carroll MW, Doumbia M, Draguez B, Duraffour S, et al. Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet (London, England). 2015;386(9996):857–66. Epub 2015/08/08. doi:10.1016/S0140-6736(15)61117-5. PMID:26248676.
- Ebola ça suffit ring vaccination trial consortium. The ring vaccination trial: a novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ (Clinical Research Ed). 2015;351:h3740. Epub 2015/07/29. PMID:26215666.
- WHO. Contact tracing during an outbreak of Ebola virus disease. http://www.who.int/csr/resources/publications/ebola/contacttracing-during-outbreak-of-ebola.pdf 2014.
- Heymann DL, Aylward RB. Mass vaccination: when and why. Curr Topics Microbiol Immunol. 2006;304:1–16. Epub 2006/09/23. PMID:16989261.
- Kaplan EH, Craft DL, Wein LM. Emergency response to a smallpox attack: the case for mass vaccination. Proc Natl Acad Sci U S A. 2002;99(16):10935–40. doi:10.1073/pnas.162282799. PMID:12118122.
- Kucharski AJ, Eggo RM, Watson CH, Camacho A, Funk S, Edmunds WJ. Effectiveness of ring vaccination as control strategy for ebola virus disease. Emerging Infect Dis. 2016;22(1):105–8. Epub 2015/12/23. doi:10.3201/eid2201.151410. PMID:26691346.
- Parker DM, Landier J, von Seidlein L, Dondorp A, White L, Hanboonkunupakarn B, Maude RJ, Nosten FH. Limitations of malaria reactive case detection in an area of low and unstable transmission on the Myanmar-Thailand border. Malar J. 2016;15(1):571. doi:10.1186/s12936-016-1631-9. PMID:27887652.
- Grassly NC. The final stages of the global eradication of poliomyelitis. Philos Trans R Soc Lond B Biol Sci. 2013;368(1623):20120140. Epub 2013/06/27. doi:10.1098/rstb.2012.0140. PMID:23798688.
- Marx A, Glass JD, Sutter RW. Differential diagnosis of acute flaccid paralysis and its role in poliomyelitis surveillance. Epidemiol Rev. 2000;22(2):298–316. doi:10.1093/oxfordjournals.epirev.a018041. PMID:11218380.
- Eichner M, Dietz K. Eradication of poliomyelitis: when can one be sure that polio virus transmission has been terminated? Am J Epidemiol. 1996;143(8):816–22. doi:10.1093/oxfordjournals.aje.a008820. PMID:8610692.
- GPEI. Responding to a polio virus outbreak: Standard Operating Procedures for a ne polio outbreak in a polio-free country. http://www.polioeradication.org/Portals/0/Document/Resources/PolioEradicators/1a.PolioOutbreakGuideline20150220.pdf 2015.
- Martin S, Costa A, Perea W. Stockpiling oral cholera vaccine. Bull World Health Organ. 2012;90(10):714. doi:10.2471/BLT.12.112433. PMID:23109735.
- Deployments from the oral cholera vaccine stockpile, 2013–2017. Wkly Epidemiol Rec. 2017;92(32):437–42. PMID:28799734.
- Desai SN, Pezzoli L, Martin S, Costa A, Rodriguez C, Legros D, Perea W. A second affordable oral cholera vaccine: implications for the global vaccine stockpile. Lancet Global Health. 2016;4(4):e223–4. doi:10.1016/S2214-109X(16)00037-1. PMID:27013303.
- You YA, Ali M, Kanungo S, Sah B, Manna B, Puri M, Nair GB, Bhattacharya SK, Convertino M, Deen JL, et al. Risk map of cholera infection for vaccine deployment: the eastern Kolkata case. PloS One. 2013;8(8):e71173. doi:10.1371/journal.pone.0071173. PMID:23936491.
- Luquero FJ, Banga CN, Remartinez D, Palma PP, Baron E, Grais RF. Cholera epidemic in Guinea-Bissau (2008): the importance of “place”. PloS One. 2011;6(5):e19005. doi:10.1371/journal.pone.0019005. PMID:21572530.
- Weil AA, Khan AI, Chowdhury F, Larocque RC, Faruque AS, Ryan ET, Calderwood SB, Qadri F, Harris JB. Clinical outcomes in household contacts of patients with cholera in Bangladesh. Clin Infect Dis. 2009;49(10):1473–9. doi:10.1086/644779. PMID:19842974.
- Mosley WH, Ahmad S, Benenson AS, Ahmed A. The relationship of vibriocidal antibody titre to susceptibility to cholera in family contacts of cholera patients. Bulletin World Health Organization. 1968;38(5):777–85. PMID:5303331.
- Ali M, Debes AK, Luquero FJ, Kim DR, Park JY, Digilio L, Manna B, Kanungo S, Dutta S, Sur D, et al. Potential for controlling cholera using a ring vaccination strategy: Re-analysis of data from a cluster-randomized clinical trial. PLoS Med. 2016;13(9):e1002120. doi:10.1371/journal.pmed.1002120. PMID:27622507.
- Sugimoto JD, Koepke AA, Kenah EE, Halloran ME, Chowdhury F, Khan AI, LaRocque RC, Yang Y, Ryan ET, Qadri F, et al. Household transmission of Vibrio cholerae in Bangladesh. PLoS Neglected Tropical Dis. 2014;8(11):e3314. doi:10.1371/journal.pntd.0003314. PMID:25411971.
- Weil AA, Begum Y, Chowdhury F, Khan AI, Leung DT, LaRocque RC, Charles RC, Ryan ET, Calderwood SB, Qadri F, et al. Bacterial shedding in household contacts of cholera patients in Dhaka, Bangladesh. The American Journal Of Tropical Medicine And Hygiene. 2014;91(4):738–42. doi:10.4269/ajtmh.14-0095. PMID:25114012.
- Ley B, Khatib AM, Thriemer K, von Seidlein L, Deen J, Mukhopadyay A, Chang NY, Hashim R, Schmied W, Busch CJ, et al. Evaluation of a rapid dipstick (Crystal VC) for the diagnosis of cholera in Zanzibar and a comparison with previous studies. PLoS One. 2012;7(5):e36930. doi:10.1371/journal.pone.0036930. PMID:22662131.
- Lopez AL, Deen J, Azman AS, Luquero FJ, Kanungo S, Dutta S, von Seidlein L, Sack DA. Immunogenicity and protection from a single dose of internationally available killed oral cholera vaccine: a systematic review and meta-analysis. Clin Infect Dis. 2017. doi:10.1093/cid/cix1039. [Epub ahead of print]
- Finger F, Bertuzzo E, Luquero FJ, Naibei N, Toure B, Allan M, Porten K, Lessler J, Rinaldo A, Azman AS. The potential impact of case-area targeted interventions in response to cholera outbreaks: A modeling study. PLoS Medicine. 2018;15(2):e1002509. doi:10.1371/journal.pmed.1002509. PMID:29485987.
- Azman AS, Parker LA, Rumunu J, Tadesse F, Grandesso F, Deng LL, Lino RL, Bior BK, Lasuba M, Page AL, et al. Effectiveness of one dose of oral cholera vaccine in response to an outbreak: a case-cohort study. Lancet Glob Health. 2016;4(11):e856–e63. doi:10.1016/S2214-109X(16)30211-X. PMID:27765293.
- United Nations Office for the Coordination of Humanitarian Affairs (OCHA). Cholera cases increase. Humanitarian Bulletin South Sudan http://reliefweb.int/sites/reliefweb.int/files/resources/160808_OCHA_SouthSudan_humanitarian_bulletin_11.pdf Accessed 4 December 2017.
- Troeger C, Sack DA, Chao DL. Evaluation of targeted mass cholera vaccination strategies in Bangladesh: A demonstration of a new cost-effectiveness calculator. Am J Trop Med Hyg. 2014;91(6):1181–9. doi:10.4269/ajtmh.14-0159. PMID:25294614.