ABSTRACT
Background and aims: Neisseria meningitidis (N. meningitidis) is a Gram-negative bacterium that can cause life-threatening invasive infections referred to as invasive meningococcal disease (IMD). In the last decade the incidence of IMD in Israel is about 1/100,000 population annually. We aimed to describe the epidemiology of IMD in Israel combining epidemiological data and characterization of N. meningitidis isolates.
Methods: Invasive infection caused by N. meningitidis is a notifiable disease in Israel. Data were collected by epidemiological investigations and control measures were employed. Laboratory work-up included serogrouping, N. meningitides molecular characterization and whole-genome sequencing.
Results: During 1998–2017, 1349 cases of IMD were notified in Israel (mean annual incidence rate 0.94/100,000). The peak incidence rates were observed in infants under 1 year of age (10.9/100,000). Case fatality rate was 9.7%. The majority of the N. meningitidis isolates were of serogroup B (67.9%). During 2007–2017, three clonal complexes (CC) 32, 41/44 and 23 (hyper-invasive clonal complexes) were the leading CC (61%). CC32 was the leading CC causing meningococcemia and mortality. In 2017, 35 isolates were tested for 4CMenB antigens variants; of the serogroup B isolates tested 46.7% showed a match to one or more antigens (fHbp or PorA:VR1), most were ST32 (CC32).
Conclusions: Preliminary analysis based on limited number of samples suggests that the 4CMenB coverage would be about half the strains; further research is necessary. Integration of clinical, epidemiological and laboratory data is essential to support decision-making on the introduction of the novel MENB vaccines in Israel.
Background
Neisseria meningitidis (N. meningitidis) is a Gram-negative bacterium that can cause life-threatening invasive infections, meningitis and sepsis (meningococcemia) referred to as invasive meningococcal disease (IMD). The worldwide geographic prevalence of N. meningitidis varies, as the serogroups B, C and Y prevail mainly in Europe, America and Australia, while the serogroup A is prevalent mostly in Africa and Asia.Citation1 IMD may emerge as sporadic cases, or as clusters and outbreaks and the reported IMD incidence rates range considerably between countries from 1–6 per 100,000 population annually.Citation2 The public health management of IMD cases is often challenging due to their unpredictability, severity and associated public anxiety.Citation1–Citation5 Invasive meningococcal disease is considered a Vaccine Preventable Disease (VPD) with available commercial vaccines against serogroups A, C, W135 and Y and lately against serogroups B. Because of the diverse geographic epidemiology of N.meningitidis and the different disease burden the target population groups for which meningococcal vaccines are recommended also vary vastly between countries.Citation6,Citation7 The overall annual incidence rate of IMD in Israel, has been about 1 per 100,000 population in the past decade.Citation8,Citation9 Most IMD cases in Israel have been reported in young children; infants under 1-year-old present the highest age-specific incidence rate.Citation10–Citation13 Most IMD cases are sporadic and outbreaks are fairly uncommon. The reported case fatality rate (CFR) among IMD cases is 5–15% depending on the clinical presentation (significantly higher CFR in meningococcemia than in meningitis).Citation8,Citation10,Citation11 About a third of the IMD survivors display long term sequela.Citation11 According to the national surveillance data in children under 15 years (1989–2010), most of the N. meningitidis isolates in Israel were of serogroup B (76.9%) and the remainder distributed between serogroups C (10.9%), Y (8%), W135 (2.9%); 78.6% of all serogroup B isolates were from children under 5 years old.Citation8
Meningococcal vaccines are not included in the routine childhood immunization program in Israel. The recent availability of N. meningitidis serogroup B vaccines may affect future recommendations.Citation13 Vaccines against serogroups A, C, Y and W135 are currently recommended by the Ministry of Health in Israel for immune compromised persons, army recruits and international travelers to endemic areas.Citation14,Citation15 We aimed to describe the epidemiology of invasive meningococcal disease combining public health investigations and molecular characterization of N. meningitidis isolates. The molecular analysis tools of characterizing Neisseria meningitidis strains had been recently embraced in Israel. Routine, population-based pathogen analysis had been implemented as a tool for surveillance of existing strains in the community. The knowledge regarding circulating strains is essential for adopting strain-specific vaccines. The combination of data on the phenotypic and genotypic association should provide a basis for evidence based decision making on use of meningococcal vaccines.
Results
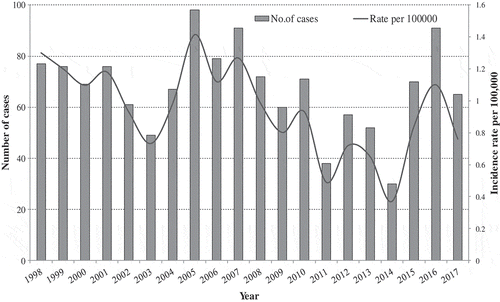
The incidence of Invasive Meningococcal disease in Israel has been around 1 cases per 100,000 population over the last two decades. During 1998–2017, 1349 cases of IMD were notified nationally in all age groups. The mean annual incidence rate was 0.94 ± 0.3 per 100,000. The IMD incidence rates displayed fluctuations over the years (0.37 per 100,000 in 2014 to 1.41 per 100,000 in 2005) and showed a general trend of decline () with an incidence of 0.76/100,000 population in 2017. The annual number of reported IMD cases (mean ± SD) in the years 1998–2017 was 67.5 ± 16.6 nationally (ranging from 30 to 98 notified IMD cases annually).
Figure 1. Number of case notifications and annual incidence rates of Invasive Meningococcal Disease (IMD) per 100,000 population, during the years 1998 – 2017 in Israel.
The age-specific incidence rates of IMD differed between the age groups. The peak incidence rates of IMD were observed among infants under 1 year (10.9/100,000). The rates in infants were significantly higher than the other age groups: 1–4 years (2.6/100,000), 5–14 years (1/100,000) and ≥ 15 years (0.25/100,000), both through multiple pairwise comparisons and while compared to all other age groups combined (RR = 19.03 95%CI = 16.8–21.6, p = 0.0001). Most of the IMD cases were reported in children, 84% were under age 15 years. Among infants under 1 year the median age and the mean± SD age at the disease onset were 4.8 months and 5.1 ± 3 months, respectively.
Overall, a significant seasonal trend had been observed with 45% of the IMD cases occurring during the winter months (December to March, Ratchet circular scan method, p = 0.047). The case fatality rate among IMD cases was 9.7%. In a multiple logistic regression model analysis for mortality including the variables age, gender, ethnicity, seasonality and clinical presentation, the significant variables found were ethnicity (higher risk in Arab cases, OR = 2.8 95%CI = 1.2–6.7, p = 0.018) and the clinical presentation (higher risk among meningococcemia cases, OR = 16.2 95%CI = 3.6–72.1, p = 0.0001).
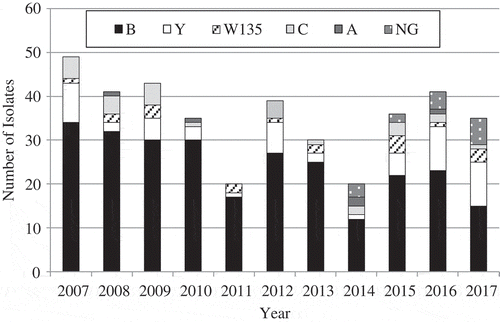
The distribution of the isolates of N. meningitidis serogroups (2007–2017) in Israel is presented in . The majority of the N. meningitidis isolates were of serogroup B (67.9%). The proportion of serogroup B of all isolates showed some decline recently (75.9% in 2007–2013 and 54.5% in 2014–2017) while serogroup Y fraction increased (11.3% and 19.7% in the same years). The proportion of N. meningitidis serogroup B varied among the age group: 76.6% of infants under 1-year, 79.8% at age 1–4 years, 63.2% at age 5–14 years and 42.8% of persons 15 years and above.
Figure 2. The distribution of the isolates of N. meningitidis serogroups during the years 2007–2017 in Israel.
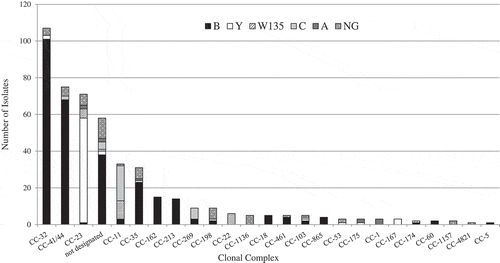
The distribution of the Clonal Complexes (CC) according to the N. meningitidis serogroups (2007–2017) in Israel is presented in . Overall, three CC 32, 41/44 and 23 (referred to as hyper invasive clonal complexes) were responsible for over half of all the IMD isolates in Israel (61%). The leading CC was CC32, detected in 25.8% of all the IMD isolates and also the leading CC causing mortality. The next abundant CCs was CC41/44 (18% of IMD isolates) and CC23 (17%).
Figure 3. The distribution of Clonal Complexes (CC) according to N. meningitidis serogroups during the years 2007–2017 in Israel.
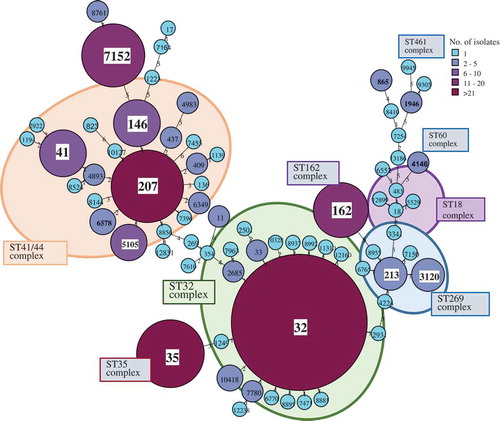
The data on the genetic diversity of IMD isolates including the Sequence types (ST) and the Clonal Complexes (CC), for serogroup B in Israel (2007–2017) are presented in . The leading ST revealed were: ST32, ST41/44, ST35, ST162 and ST269. Altogether, a total of 103 STs and 19 CCs were identified in IMD isolates in Israel and 41 of the STs identified in this study had not previously been reported globally.
Figure 4. The Sequence types (ST) and Clonal Complexes (CC), for N. meningitidis serogroup B during the years 2007–2017 in Israel. Minimum spanning tree of Multilocus sequence typing (MLST) data for 276 N. meningitidis serogroup B isolated from blood and CSF in Israel in 2007–2017. Each circle corresponds to a particular sequence type (ST) indicated on top of the circle and the size of the circle is proportional to the number of isolates sharing the same ST. The length of the branches corresponds to the allelic distance between STs (1–6 alleles). STs belonging to the same clonal complex (CC) are color-shaded and the CC are indicated in boxes.
Whole genome sequencing (WGS) analysis for invasive N. meningitidis isolates in Israel (n = 35, 2017) has been performed and BAST (Bexsero® Antigen Sequence Types) antigens were extracted from these genomes. The N. meningitidis serogroups found were B (15), Y (10), W135 (3), C (1), NG (6). The antigenic variants of the proteins incorporated in the 4CMenB vaccine include: fHbp-1.1, NHBA-2, NadA-2/3.8 and PorA: P1.7–2,4. BAST analysis showed that of the 15 serogroup B isolates tested 3 were found to harbor the fHbp vaccine antigen1.1 (ST32; CC32) and another 3 were found to harbor antigen variants that are considered to be cross reactive with the vaccine antigen (ST7152). Based on the analysis of N. meningitides serogroup B isolates (Israel, 2017), we propose a strain coverage range estimate varying from 20% (3/15 N. meningitides serogroup B isolates, 95%CI 3–39.7%) to 46.7% (7/15 N. meningitides serogroup B isolates, 95%CI 22–71%).
By WGS, none of the isolates was found as carrying the other proteins variants. Furthermore, PorA analysis of 148 isolates using conventional PCR and sanger sequencing indicated only one DNA sample from CSF (ST41; CC41/44) with the PorA vaccine variant (P1.7–2,4). The leading PorA profile was P1.7,16 (25%), all the isolates but 1 were of ST32 and CC32. This isolate was ST12160 which is CC32 and has 1 allele difference from ST32.
Discussion
Invasive meningococcal disease is increasingly becoming a vaccine-preventable disease.Citation6,Citation7 Capsular polysaccharide conjugate vaccines have been effective against disease due to meningococcal serogroups A, C, W, and Y. However, as a result of the inadequate immunogenicity of the MenB capsular polysaccharide, developing safe and effective MenB vaccines has been more challenging.Citation16 The recent technological progress in vaccinology enabled the development of two potentially effective vaccines against N. meningitidis serogroup B: 4CMenB (Bexsero®) and MenB-FHbp (Trumenba®).Citation17 The World Health organization issued recommendations for developing evidence-based vaccination-related policies.Citation18 Decision-making should include disease burden, vaccine safety and efficacy, cost, cost-effectiveness, affordability, feasibility, delivery requirements and public perceptions and demand.Citation18 In a study on the epidemiology of IMD in European countries, in 2004–2014, most cases were caused by serogroup B and the highest notification rate of serogroup B in infants under one year.Citation19 Similarly, N. meningitidis serogroup B has been predominant in IMD morbidity Israel in the last two decades,Citation8–Citation12 as well as in this study. A cost-utility analysis of a serogroup B IMD vaccination program in Israel, assessed cost per averted disability-adjusted life year (DALY).Citation13 The model assumptions were based on previous estimates of 78% N. meningitidis serogroup B strain coverage.Citation20 A national vaccination program was found cost-effective if vaccine costs fall below $19.44 per dose. In high-risk settings, vaccination may be cost-effective due to high burden and defined target population.Citation13
In the current study we combined data on the phenotypic and genotypic IMD aiming to provide a basis for evidence-based decisions on use of meningococcal vaccines. The overall incidence rate of IMD in Israel showed notable fluctuations and a general trend of decline during the last two decades, from 1.3 in 1998 to 0.8 per 100,000 population in 2017. The fraction of the N. meningitidis serogroup B declined from 75.9% in 2007–2013 to 54.5% during 2014–2017. Similarly, in Europe, there was a slight trend of decrease in the overall IMD annual incidence rate during 2008 to 2012, from 0.95 to 0.68 per 100,000 population, with the highest incidence rates of over 2 per 100,000 population observed in the UK, Ireland and Lithuania.Citation21 The Technical Advisory Group on Immunisation Meningococcal Working Party in Australia reported a decline in meningococcal serogroup B disease incidence rates from 1.52 per 100,000 population in 2001 to 0.47 in 2015.Citation2,Citation22 The varying incidence rates of meningococcal serogroup B disease may affect cost-effectiveness equilibrium and decision-making on vaccine introduction into the routine vaccination program.
The risk of IMD after infection is significantly increased in infants probably as a result of susceptibility of the individual due to the gradual maturation of the immune system in early life.Citation23 The majority of IMD cases in our study were observed in children under 15 years of age, similar to prior reports in Israel.Citation8–Citation11 The peak incidence rates of IMD were observed among infants under 1 year (10.9/100,000), similar to data from Europe, with the highest age-specific incidence rates of IMD in infants under 1 year (> 10 per 100,000 per year).Citation21 Based on disease burden, the target population for N. meningitidis serogroup B vaccination in Israel are young children.
N. meningitidis serogroup B accounted for most of the disease burden in Israel. The leading CCs found were 32, 23 and 41/44 (the hyper-invasive clonal complexes). Assessment and prediction of serogroup B strains coverage can be obtained by the molecular methods of whole-genome sequencing, multi-locus sequence typing (MLST) and BAST for the 4CMenB vaccine.Citation24 Meningococcal Antigen Typing System (MATS) has been reported to produce similar predicted vaccine coverage obtained using BAST profiling of serogroup B strains.Citation25 It has been suggested that induction of immunogenicity may be inferred by one of the four vaccine targets that may be sufficient to protect against invasive disease.Citation24 Our preliminary analysis based on limited number of N. meningitides serogroup B isolates (Israel, 2017) suggests an estimated strain coverage for 4CMenB ranging from 20% to 46.7%.
According to several BAST studies performed in various countries, the predicted strain coverage of 4CMenB vaccine according to the BAST antigens, varies between years and between countries.Citation24,Citation25 A large study including 2016 confirmed IMD isolates from Great Britain and Ireland revealed variation of immunogenicity to at least one of 4 vaccine targets from 30.8% in 2010 to 22.8% in 2014.Citation24 A study from Australia showed a difference between 2 geographic regions, Victoria (VIC) and Western Australia (WA) in the years 2008–2012. The fraction of either of the exact antigen variants incorporated in 4CMenB vaccine in the Serogroup B isolates was 36% in VIC, 20% in WA and 30.6% (52/170 isolates) from both regions combined.Citation25 The most recent UK study reporting 3073 IMD confirmed isolates during 2010–2016 showed that 36% of 1820 serogroup B isolates had exact matches to ≥ 1 4CMenB vaccine antigen variants.Citation26 The serogroup B isolates strain coverage range estimate for 4CMenB derived from our data is in line with the range in the above studies.
At present, one of the two commercially available MENB vaccines has been licensed in Israel (4CMenB) and the official vaccination recommendations for vaccine use had not been distributed thus far. Since a genotypic scheme was not available for the other MENB vaccine – MenB-FHbp, bivalent rLP2086 (Trumenba®) antigens, such analysis could not be performed in the present study.
A recent meta-analysis included randomized controlled trials on the immunogenicity and safety of 4CMenB vaccine in children and adolescents. The main findings included an acceptable short-term safety profile, high immunogenicity in the first months post vaccination, and a need for a booster dose to prolong protection against all strains.Citation27 In September 2015 the UK became the first country to include the 4CMenB vaccine in the national infant immunisation programme; a reduction of nearly 50% in MenB cases was reported in the vaccine eligible age-group.Citation28 The strain coverage altered before and after the introduction of routine vaccinations. MATS investigation of meningococcal group B isolates from England, Wales, and Northern Ireland showed an estimated 73% strain coverage in 2007–2008 vs. 66% in 2014–2015. The fraction of isolates with one vaccine antigen was 23% (2007–08) vs. 31% (2014–15) while the fraction with more than one antigen was 50% vs. 35%.Citation29 The 4CMenB vaccine was introduced into the national routine immunisation programme in Ireland in October 2016 and in Italy in January 2017.Citation30 Decision-making in other countries (including Israel) should take into account the real-world experience reported from these countries.
Our study has several limitations due to data incompleteness owing to the availability of data and the sequential implementation of the progressive laboratory methods. Yet, our findings correspond well with the reports from other developed countries.
In conclusion we were able to integrate epidemiological and laboratory data to support decision-making on the introduction of the novel MENB vaccines in Israel. The National Immunization Technical Advisory Group (NITAG) in Israel discussed the possible use of the 4CMenB vaccine among risk groups and in the routine immunization program in September 2014. The advisory group recommendation was not to include the 4CMenB vaccine in any group due to lack of data on effectiveness, the vaccine had not been registered at the time and no other country reported including the vaccine in the routine immunization program. The 4CMenB vaccine was registered in Israel in March 2016. The next discussion on meningococcal vaccines by the NITAG in Israel is scheduled for the summer of 2018 meeting. Further assessment of all IMD isolates received at the reference laboratories on a regular basis and of the historical isolates and analysis by means of WGS, MLST and BAST are essential for comprehensive epidemiology such as isolate characterization and surveillance of circulating clones. Application of these methods is necessary as well in evaluating the future impact of meningococcal vaccination programs for effective protection of children health and public health in Israel.
Methods
Invasive infection caused by Neisseria meningitidis is a notifiable disease in Israel by law, applying both to physicians as well as to microbiological laboratories. IMD cases are informed promptly to the district health office and the bacterial isolates are sent from the hospital laboratory to the reference laboratory. Laboratory case confirmation requires N. meningitidis isolation from the blood, cerebrospinal fluid (CSF) or other sterile site in a patient with a clinically compatible illness. An epidemiological investigation is performed in each IMD case and post-exposure prophylaxis is provided to close contacts in accordance with the Ministry of Health guidelines. The epidemiological IMD data in this study are presented for the years 1998–2017. Data on IMD case notifications are presented in the national weekly epidemiological reports.Citation31 The IMD incidence rates are calculated based on the notifications to the Ministry of Health.Citation31 The population of Israel was 8.5 million persons in 2017; children under 15 years of age comprised 28.3% of the national population.Citation32 The age- specific incidence rates are calculated for the following age groups: infants < 1 year (182,000 in 2017), toddlers 1–4 years (696,200), children 5–14 years (1,537,400), and persons ≥ 15 years of age (6,130,300).
Laboratory methods
N. meningitidis isolates are routinely submitted to the national Ministry of Health reference center for meningococci for verification, serogrouping and antimicrobial susceptibility testing. The N. meningitidis isolates or DNA (when confirmed by PCR without isolate) are sent to the national Ministry of Health molecular reference laboratory.
Molecular characterization includes: Multi Locus Sequence Typing (MLST) genotyping (since 2007, n = 393); Fine typing (since 2014, n = 148); Whole Genome Sequencing (WGS) (since 2017, n = 35). MLST is performed according to the standard N. meningitidis protocol (http://pubmlst.org/neisseria/info). The allele number, sequence type (ST) and clonal complex (CC) is determined at the Neisseria MLST database. Finetyping of the antigens FetA and PorA of 3 variable regions (PorA: VR1, VR2, VR3), is performed according to the standard protocol for N. meningitidis target genes and antigens. (http://pubmlst.org/neisseria).
These antigens are outer membrane proteins that are part of the antigenic profile of a meningococcal strain and comprise the serogroup. The sequence types of the variable regions of PorA, and of the immunodominant of FetA were recommended by the European Monitoring Group on Meningococci to be implemented as a standard typing method for additional resolution between the MLST sequence types.Citation33
For WGS analysis, genomic DNA was extracted using the QiaSymphony platform (QIAGEN) and quantified with the Qubit fluorimetry, 1.0 ng of DNA was used for Illumina Nextera XT library preparation. Short sequence reads were generated on HiSeq (Illumina) aiming at > 100X coverage. The reads were de novo assembled by SPAdes. Embedded tools within PubMLST were used to analyze the presence of 4CMenB (Bexsero®) vaccine antigens (fHbp, NadA, NHBA and PorA) and Bexsero Antigen Sequence Types (BAST).Citation24,Citation25 All Sequence analysis was performed with Bionumerics 7.6 (Applied Maths, Belgium)
Statistical analysis
The statistical analysis was performed with SPSSTM version 23.0 software for Windows (IBM SPSS, Chicago, IL, USA) and WINPEPI (PEPI-for-Windows): computer programs for epidemiologists. The incidence rates were compared by Rate Ratio (RR) with 95% Confidence Interval (95%CI). The age- specific incidence rates were compared by multiple pairwise comparisons (Tukey procedure). The Ratchet circular scan method was used to assess seasonality. (The Ratchet circular scan test is based on the maximum number of events in two or three consecutive months. It is sensitive to an increase in incidence for a season, superimposed on a constant incidence over the entire year).Citation34
A multiple regression model was implemented to assess risk markers for mortality. Associations between variables and mortality were presented as Odds Ratio (OR) and 95%CI. A p-value < 0.05 was considered significant for all comparisons.
Disclosure of potential conflicts of interest
No potential conflict of interest was reported by the authors.
Acknowledgments
We thank the microbiological laboratories of the Medical Centers in Israel for sending the Neisseria meningitidis isolates to the National Center for Meningococci. We thank the District Health offices epidemiologic teams for the investigation and control of the IMD cases. This investigation made use of the Neisseria Multi Locus Sequence Typing Website (http://pubmlst.org/neisseria/) developed by Keith Jolley and sited at the University of Oxford (Jolley & Maiden 2010, BMC Bioinformatics, 11:595). The development of this site has been funded by the Welcome Trust and the European Union.
References
- Harrison LH, Pelton SI, Wilder-Smith A, Holst J, Safadi MA, Vazquez JA, Taha MK, LaForce FM, Von Gottberg A, Borrow R, et al. The Global Meningococcal Initiative: recommendations for reducing the global burden of meningococcal disease. Vaccine. 2011 Apr 18;29(18):3363–3371. doi:10.1016/j.vaccine.2011.02.058.
- Jafri RZ, Ali A, Messonnier NE, Tevi-Benissan C, Durrheim D, Eskola J, Fermon F, Klugman KP, Ramsay M, Sow S, et al. Global Epidemiology of invasive meningococcal disease. Popul Health Metr. 2013 Sep 10;11(1):17. doi:10.1186/1478-7954-11-17.
- Borrow R, Da C, Ceyhan M, Christensen H, Ec D, Findlow J, Glennie L, Von GA, Kechrid A, Vázquez MJ, et al. Global Meningococcal Initiative (GMI). Meningococcal disease in the Middle East and Africa: findings and updates from the global meningococcal initiative. J Infect. 2017 Jul;75(1):1–11. doi:10.1016/j.jinf.2017.04.007.
- Vázquez JA, Taha MK, Findlow J, Gupta S, Borrow R. Global Meningococcal Initiative: guidelines for diagnosis and confirmation of invasive meningococcal disease. Epidemiol Infect. 2016 Oct;144(14):3052–3057. doi:10.1017/S0950268816001308.
- Sridhar S, Greenwood B, Head C, Plotkin SA, Sáfadi MA, Saha S, Taha MK, Tomori O, Gessner BD. Global incidence of serogroup B invasive meningococcal disease: a systematic review. Lancet Infect Dis. 2015 Nov;15(11):1334–1346. doi:10.1016/S1473-3099(15)00217-0.
- Vuocolo S, Balmer P, Gruber WC, Jansen KU, Anderson AS, Perez JL, York LJ. Vaccination strategies for the prevention of meningococcal disease. Hum Vaccin Immunother. 2018 Mar;15:1–48.
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013 Mar 22;62(RR–2):1–28.
- Ben-Shimol S, Dagan R, Schonmann Y, Givon-Lavi N, Keller N, Block C, Kassis I, Ephros M, Greenberg D. Dynamics of childhood invasive meningococcal disease in Israel during a 22-year period (1989-2010). Infection. 2013 Aug;41(4):791–798. doi:10.1007/s15010-013-0439-6.
- Israel Center for Disease Control (2012) Notifiable infectious diseases in Israel, 60 years of surveillance 1951–2010. Publication No.342
- Stein-Zamir C, Abramson N, Zentner G, Shoob H, Valinsky L, Block C. Invasive meningococcal disease in children in Jerusalem. Epidemiol Infect. 2008 Jun;136(6):782–789. doi:10.1017/S0950268807009259.
- Stein-Zamir C, Shoob H, Sokolov I, Kunbar A, Abramson N, Zimmerman D. The clinical features and long-term sequelae of invasive meningococcal disease in children. Pediatr Infect Dis J. 2014 Jul;33(7):777–779. doi:10.1097/INF.0000000000000282.
- Valinsky L, Jaffe J, Keller N, Block C, Abramson N, Stein-Zamir C. A cluster of invasive meningococcal disease revealed by the characterization of a novel serogroup B meningococcal clone. Epidemiol Infect. 2016 Jan;144(1):183–188. doi:10.1017/S0950268815001296.
- Ginsberg GM, Block C, Stein-Zamir C. Cost-utility analysis of a nationwide Vaccination programme against serogroup B meningococcal disease in Israel. Int J Public Health. 2016 Jul;61(6):683–692. doi:10.1007/s00038-016-0821-0.
- Ministry of Health, Israel. Immunizations recommendations for travel abroad; Updated 2017 Mar. https://www.health.gov.il/Subjects/vaccines/Vaccinesabroad/Documents/travel2013.pdf
- Ministry of Health, Israel. Immunizations recommendations, special circumstances; Updated 2017 Aug. https://www.health.gov.il/UnitsOffice/HD/PH/epidemiology/td/docs/110_Tochen.pdf
- Donald RG, Hawkins JC, Hao L, Liberator P, Jones TR, Harris SL, Perez JL, Eiden JJ, Jansen KU, Anderson AS. Meningococcal serogroup B vaccines: estimating breadth of coverage. Hum Vaccin Immunother. 2017 Feb;13(2):255–265. doi:10.1080/21645515.2017.1264750.
- Crum-Cianflone N, Sullivan E. Meningococcal Vaccinations. Infect Dis Ther. 2016 Jun 5;2:89–112. doi:10.1007/s40121-016-0107-0.
- World Health Organization, Guidance for the development of evidence-based vaccination -related recommendations, 2017 Jan. http://www.who.int/immunization/sage/Guidelines_development_recommendations.pdf
- Whittaker R, Dias JG, Ramliden M, Ködmön C, Economopoulou A, Beer N, Pastore Celentano L. ECDC network members for invasive meningococcal disease. The epidemiology of invasive meningococcal disease in EU/EEA countries, 2004-2014. Vaccine. 2017 Apr 11;35(16):2034–2041. doi:10.1016/j.vaccine.2017.03.007.
- Findlow J. Meningococcal group B vaccines. Hum Vaccin Immunother. 2013 Jun;9(6):1387–1388. doi:10.4161/hv.24689.
- Sadarangani M, Pollard AJ. Can we control all-cause meningococcal disease in Europe? Clin Microbiol Infect. 2016 Dec 1;22(Suppl 5):S103–S112. doi:10.1016/j.cmi.2016.03.006.
- Archer BN, Chiu CK, Jayasinghe SH, Richmond PC, McVernon J, Lahra MM, Andrews RM, McIntyre PB. Australian Technical Advisory Group on Immunization (ATAGI) meningococcal working party. epidemiology of invasive meningococcal B disease in Australia, 1999-2015: priority populations for vaccination. Med J Aust. 2017 Nov 6;207(9):382–387.
- Deasy A, Read RC. Challenges for development of meningococcal vaccines in infants and children. Expert Rev Vaccines. 2011 Mar;10(3):335–343. doi:10.1586/erv.11.3.
- Brehony C, Rodrigues CMC, Borrow R, Smith A, Cunney R, Moxon ER, Maiden MCJ. Distribution of Bexsero® Antigen Sequence Types (BASTs) in invasive meningococcal disease isolates: implications for immunisation. Vaccine. 2016 Sep 7;34(39):4690–4697. doi:10.1016/j.vaccine.2016.08.015.
- Mowlaboccus S, Mullally CA, Richmond PC, Howden BP, Stevens K, Speers DJ, Keil AD, Bjørnstad ON, Perkins TT, Kahler CM. Differences in the population structure of Neisseria meningitidis in two Australian states: victoria and Western Australia. PLoS One. 2017 Oct 24;12(10). doi:10.1371/journal.pone.0186839.
- Rodrigues CMC, Lucidarme J, Borrow R, Smith A, Cameron JC, Moxon ER, Maiden MCJ. Genomic surveillance of 4CMenB vaccine antigenic variants among disease-causing Neisseria meningitidis isolates, United Kingdom, 2010-2016. Emerg Infect Dis. 2018 Apr;24(4):673–682. doi:10.3201/eid2404.171480.
- Flacco ME, Manzoli L, Rosso A, Marzuillo C, Bergamini M, Stefanati A, Cultrera R, Villari P, Ricciardi W, Ioannidis JPA, et al. Immunogenicity and safety of the multicomponent meningococcal B vaccine (4CMenB) in children and adolescents: a systematic review and meta-analysis. Lancet Infect Dis. 2018 Apr;18(4):461–472. doi:10.1016/S1473-3099(18)30048-3.
- Ladhani SN, Borrow R, Andrews NJ. Growing evidence supports 4CMenB effectiveness. Lancet Infect Dis. 2018 Apr;18(4):370–371. doi:10.1016/S1473-3099(18)30051-3.
- Parikh SR, Newbold L, Slater S, Stella M, Moschioni M, Lucidarme J, De Paola R, Giuliani M, Serino L, Gray SJ, et al. Meningococcal serogroup B strain coverage of the multicomponent 4CMenB vaccine with corresponding regional distribution and clinical characteristics in England, Wales, and Northern Ireland, 2007-08 and 2014-15: a qualitative and quantitative assessment. Lancet Infect Dis. 2017 Jul;17(7):754–762. doi:10.1016/S1473-3099(17)30170-6.
- European Centre for Disease Prevention and Control. Expert opinion on the introduction of the meningococcal B (4CMenB) vaccine in the EU/EEA. Stockholm. ECDC; 2017 Dec.
- Ministry of health Israel. https://www.health.gov.il/UnitsOffice/HD/PH/epidemiology/Pages/epidemiology_report.aspx
- Population of Israel and Jerusalem district by Population Group (table 2.19). In: Statistical abstract of Israel Jerusalem, Israel 2017. http://www.cbs.gov.il/shnaton68/shnaton68.pdf
- Elias J, Harmsen D, Claus H, Hellenbrand W, Frosch M, Vogel U. Spatiotemporal analysis of invasive meningococcal disease, Germany. Emerg Infect Dis. 2006 Nov;12(11):1689–1695. doi:10.3201/eid1211.060682.
- Abramson J. 2011. WINPEPI updated: computer programs for epidemiologists, and their teaching potential. Epidemiol Perspect Innovation. 8:1. doi:10.1186/1742-5573-8-1.
