?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The live-attenuated Japanese encephalitis chimeric virus vaccine JE-CV (IMOJEV®, Sanofi Pasteur) elicits a robust antibody response in children, which wanes over time. Clinical efficacy is based on a correlate of protection against JE infection defined as neutralizing antibody levels equal to or greater than the threshold of 10 (1/dil). Information on the duration of persistence of the JE antibody response above this threshold is needed. We constructed statistical models using 5-year persistence data from a randomised clinical trial (NCT00621764) in children (2–5 years old) primed with inactivated JE vaccine who received a booster dose of JE-CV, and in JE-naïve toddlers (12–24 months) who received a JE-CV single dose primary vaccination. Models were constructed using a Bayesian Monte-Carlo Markov Chain approach and implemented with OpenBugs V3.2.1. Antibody persistence was predicted for up to 10 years following JE-CV vaccination. Findings from a piecewise model with 2 phases (children) and a classic linear model (toddlers) are presented. For children, predicted median antibody titers (77 [2.5th–97.5th percentile range 41–144] 1/dil) remained above the threshold for seroprotection over the 10 years following booster JE-CV vaccination; the predicted median duration of protection was 19.5 years. For toddlers, 10 years after JE-CV primary vaccination median antibody titers were predicted to wane to around the level required for seroprotection (10.8 [5.8–20.1] 1/dil). A booster dose of JE-CV in children is predicted to provide long-term protection against JE. Such data are useful to facilitate decisions on implementation of and recommendations for future vaccination strategies.
Introduction
Japanese encephalitis (JE) virus is the most common cause of viral encephalitis, transmitted predominantly by the mosquito Culex tritaeniorhynchus and endemic to many countries across Asia and the Western Pacific.Citation1 About 25−30% of reported cases are fatal and 50% result in permanent neuropsychiatric sequelae.Citation2
Previous estimates of JE incidence in these regions vary.Citation3 Estimates range from 0.003 per 100,000 in a passive surveillance study in Japan (1992–2004)Citation4 to 15 per 100,000 among 5–9-year-olds in India.Citation5 In Thailand, JE incidence varies between the north and south of the country. In northern Thailand huge epidemics occur during the summer months, whereas in southern Thailand JE tends to be endemic, with a peak in the number of cases reported after the start of the rainy season. Reported incidence rates before introduction of routine vaccination against JE in the 1990s were as high as 8.5/100,000 in some northern provinces; with the introduction of routine vaccination to those areas, the highest rates (2/100,000) are now in southern provinces.Citation6 JE is considered to primarily affect the young. However, in countries that have achieved high vaccine coverage, JE cases are reported mainly in unvaccinated elderly people.Citation7 In Taiwan, where a vaccine campaign was launched in 1968, over 90% of JE cases are reported in people older than 20 years.Citation5
The live attenuated vaccine against JE, SA14-14–2, is available in China and some endemic countries in Asia Pacific.Citation8 A comparable immune response was observed in infants and toddlers in Thailand who received a single dose of JE-CV versus SA14-14–2. Fewer solicited reactions were reported following JE-CV compared with SA14-14–2 administration.Citation9
Newer one-dose primary and booster vaccination with live attenuated JE vaccines are replacing the 3-dose primary immunization and booster vaccination (mouse-brain derived inactivated JE vaccine, MBDV) due to the more favorable reactogenicity profile, improved safety, and a simpler immunization schedule of the live attenuated JE vaccines. JE-CV (IMOJEV®, Sanofi Pasteur) is a live-attenuated Japanese encephalitis chimeric virus vaccine indicated for prophylaxis of JE caused by JE virus in individuals from 9 months of age and over.
Primary vaccination with a single dose of JE-CV elicits a rapid and robust immune response in adults, toddlers and children.Citation10–Citation12 In adults, a protective response against JE was documented to persist for at least five years after a single dose of JE-CV.Citation13 A previous modeling exercise predicted that this protective response in adults would persist for up to 21.4 years (95% confidence interval [CI]: 7.3–34.0 years) in a non-endemic setting, with predicted median antibody titers at 10 years of 38 1/dil (95% CI: < 10–174) corresponding to seroprotection levels at 10 years of 85.5% (95% CI: 72.7–94.9).Citation14 Single-dose primary immunization of JE-vaccine-naïve toddlers (12–24 months old) elicits a seroprotective response that has been shown to wane to levels of approximately 60% at 5 years after vaccination.Citation15,Citation16 These findings prompted the recommendation in the product label by the company for use of booster vaccination at 1 to 2 years after the primary dose in children.Citation17 Indeed, a very strong booster response is observed following a single booster dose in 2–5 year olds primed with JE vaccination,Citation10,Citation18 with high seroprotection levels persisting for at least 5 years post-booster.Citation15
Information on the longer term duration of seroprotection after single-dose primary and booster JE vaccination in children is needed to provide insight into the potential long-term benefits, and to help make informed decisions about immunization programs. Here, we present the results of statistical modelling to predict long-term antibody responses based on previously published observed antibody titers up to 5 years after vaccination following i) a booster dose of JE-CV in children (2–5 years old) primed with two doses of MBDV and ii) a single-dose primary administration of JE-CV in toddlers (12–24 months old).Citation10,Citation15 These data are discussed in the context of a comprehensive review of the literature on the long-term persistence of JE neutralizing antibody response following JE-CV vaccination.
Results
Children – 2 to 5 years old
Model fit
Median parameter estimates and 2.5th–97.5th percentile ranges based on 97 subjects using each of the models tested are shown in . For children, the piecewise linear model showed the best fit (Supplementary Figure S1).
Table 1A: Parameter estimates and fit statistics for the 3 models in children (2–5 Years old)
Predicted antibody persistence using the piecewise model
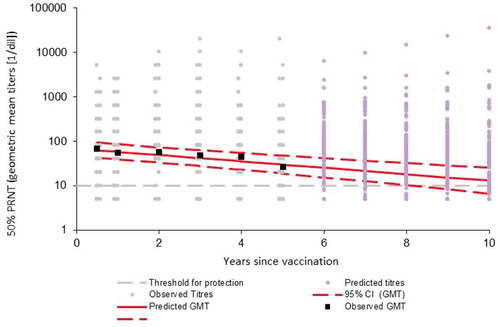
Predicted median antibody titers remained well above seroprotective levels (≥ 10 [1/dil]) for at least 10 years following JE-CV booster vaccination, decreasing from 2684 (2.5th–97.5th percentile range 1778–4056) at day (D) 28 to 539 (357–816) at year (Y) 1, 225 (145–351) at Y5 and 77 (41–144) at Y10 (; ). The estimated median duration of the initial period of rapid antibody decay (from vaccination to the change point) was 0.81 years (2.5th–97.5th percentile range: 0.67–0.98 years), or 9.7 months (8.04–11.8 months) ().
Toddlers – 12 to 24 months old
Model fit
For toddlers, following JE-CV single dose primary vaccination, all tested models showed poor convergence for D28–Y5 data (Table 1B), and so a classical linear model was constructed using data from M6 (Supplementary Figure S2). Median parameter estimates and 2.5th–97.5th percentile ranges based on data from 187 subjects using the linear model are shown in Table 1B.
Predicted antibody persistence using the linear model
Predicted median antibody titers declined to levels only just above seroprotective levels (≥ 10 [1/dil]) at Y10, decreasing from 65.3 (2.5th–97.5th percentile range 44.2–96.2) at M6 to 27.8 (17.8–43.5) at Y5 and 10.8 (5.8–20.1) at Y10 (; ).
Table 1B: Parameter estimates and fit statistics for the models in toddlers (12–24 months)
Table 2: Predicted antibody titers and seroprotection rates for up to 10 years (A) following booster vaccination with JE-CV in children (2–5 years old) who had received primary immunization with inactivated JE vaccine, and (B) following primary vaccination with JE-CV in toddlers (12–24 months)
Predicted proportion of seroprotected subjects
For the toddler (12–24 months old) population, despite a good prediction for the GMT over time, the predicted seroprotection appears to be overestimated (data not presented).
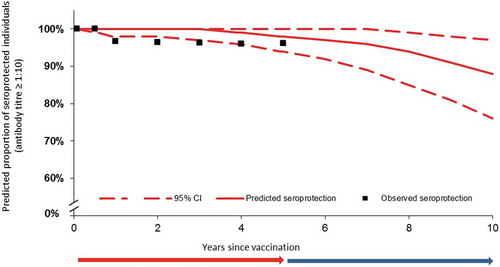
For children (2–5 years old), predicted seroprotection appears better than for the toddlers (closer to the observed seroprotection). The estimated proportion of seroprotected children remained stable between Y1 and Y4, and nearly all children remained seroprotected. After Y5, there was a slight acceleration in decline of overall seroprotection. At Y10, the predicted proportion of seroprotected children was 88% (76–97%) (; Table 2A). The predicted median duration of protection was 19.5 years.
Figure 3. Observed and predicted seroprotection rates from D28 to Y10 in children, using the piecewise linear model. The red and blue arrows indicate the period of time for which real-life data were collected (red) and the period of time over which the model is extrapolated (blue).
Different phases of antibody decay were observed in the 2 populations of children and toddlers; a rapid decay from D28 to year 1 and slower decay after year 1 in children; in contrast to the toddlers with a rapid decay from D28 to month 6 and slower decay after month 6.
Following imputation, the observed data showed two distinct periods of antibody decay for both age groups (Supplementary Figure S3). Antibody titers decayed rapidly between D28 and Y1 for the children and between D28 and M6 for toddlers, followed by a slower phase of decay.
Discussion
We constructed statistical long-term models based on real-life antibody persistence data of 5 years to predict the mean geometric titers and duration of seroprotection following immunisation with JE-CV, administered as a single-dose booster in children previously vaccinated with inactivated JE-vaccine, or as a single-dose for primary vaccination in JE-vaccine naïve toddlers. Antibody responses against JE were predicted to persist for several years in both age groups. Predicted antibody titers and the median duration of antibody persistence were greater in children who had received an additional booster vaccine as compared to children who received only a single dose.
Primary JE-CV vaccination in JE vaccine-naïve toddlers has previously been shown to elicit a robust antibody response that wanes over several years. In the real-life 5-year persistence study in Thailand, JEC01, a single dose of JE-CV elicited a robust immune response in toddlers who had not been previously vaccinated against JE, with protective antibody titers (≥ 10 [1/dil]) in 96% of toddlers at D28.Citation10 Follow-up of these toddlers over 5 years showed a decline in geometric mean titers and seroprotection levels. Among toddlers seroprotected on day 28, 64.0% (Kaplan-Meier analysis) were estimated to remain seroprotected at year 5.Citation16 In another study (JEC02), JE vaccine-naïve toddlers in Thailand and the Philippines similarly showed a strong immune response after single dose JE-CV, with 95.0% seroprotection at D28.Citation11 A subset was then followed for long-term immunogenicity following JE-CV primary vaccination (study JEC05). Among those participants seroprotected at day 28, 68.6% (Kaplan-Meier analysis) were estimated to be still seroprotected at year 5.Citation16
Booster JE-CV vaccination elicits very strong immune responses in children previously vaccinated against JE, irrespective of whether primary vaccination was with inactivated JE vaccineCitation10 or JE-CV.Citation18 In JEC01, all children aged 2–5 years receiving a JE-CV booster dose 1 to 2 years after JE primary vaccination with MBDV were seroprotected 28 days after JE-CV booster vaccinationCitation10 and 97% were seroprotected at 5 years post-booster (sensitivity analysis).Citation15 No cases of symptomatic JE virus infection were recorded during the 5-year follow-up studyCitation15; however, given the endemicity of JE in Thailand, it is possible that the children came into contact with circulating JE virus during this time. It cannot be excluded that natural exposure to JE virus or another flavivirus may have caused a natural booster effect in some of these children, and thus may have impacted the findings from the current modeling exercise. In JEC15, among children who received a JE-CV booster dose 2 years after primary vaccination with a single dose of JE-CV, 100% (95% CI 98.9–100%) were seroprotected at D28 and nearly all (99.4% [95% CI 97.9–99.99%]) were seroprotected 1 year laterCitation18; 98.2% (95% CI, 96.2%–99.3%) of subjects remained seroprotected (Kaplan-Meier analysis) 5 years after booster with GMT of 161, 1/dil (95% CI 141–184).Citation19 A further subset of JE-CV primed children from the JEC02/JEC05 study received a JE-CV booster after a longer interval of 5 years following primary vaccination. A strong booster response was elicited, with 100% seroprotected at 28 days after booster; 92.8% had antibody titers ≥ 1,280 (1/dil).Citation20
The observation of strong booster responses very early after JE-CV booster vaccination in children primed with a single dose of JE-CV 2 years earlier suggests that immune memory is induced by the primary dose of JE-CV. Indeed, in JEC15, among JE-CV primed children whose neutralising antibody titers had fallen to below levels of protection (< 10 [1/dil]) before booster, 82.4% (95% CI 71.2–90.5%) were seroprotected at only 7 days after booster, when the primary response to vaccination is known to be low. This was compared to seroprotection levels of 15.4% (95% CI 5.9–30.5) in JE-vaccine naïve children 7 days after a JE-CV single dose primary immunization; GMTs (1/dil) were 44.3 and 6.41 for JE-vaccine primed and naïve children, respectively.Citation18 These findings suggest that primary JE-CV vaccination may provide additional protective potential for children who are re-exposed to the JE virus, potentially even for those whose neutralizing antibody titers have declined over time to below the threshold of protection. It is conceivable that any exposure to wild-type Japanese encephalitis virus in view of this memory will trigger a prompt and robust response. In such cases, immunological memory induced at primary vaccination may allow sufficient levels of antibody titers to neutralise the virus before undergoing replication cycles and crossing the blood-brain barrier.
The current study provides further insight into the potential long-term benefit of JE-CV booster vaccination in children. For children 2–5 years old, the piecewise linear model predicted long-term persistence of antibody titers following booster vaccination with JE-CV, with a median titer at 10 years (77 [41–144] 1/dil) that exceeds the threshold for protection.
In toddlers (12–24 months), our linear model starting from 6 months showed that the predicted level of antibody titers in toddlers decreased over 10 years to levels lower than for children who had been primed by JE vaccination 1 to 2 years before receiving a booster dose; the predicted median titers at 10 years for toddlers did remain just above the threshold for protection (10.8 [5.8–20.1] 1/dil).
The need for a booster dose of JE-CV in JE-naïve children was recommended as 1 to 2 years due to a continued risk of exposure in endemic countries; a booster vaccine at a later stage however still demonstrated a memory immune response. This is different to the recommendation in adults (age ≥ 18 years), both naïve and previously vaccinated, whereby only a single dose was recommended, and no booster doses up to five years after administration.Citation17 Adults had an initial stronger immune response with higher GMT levels and a lower rate of antibody loss over time.Citation14 Thus, a single dose was considered an effective long-term preventive intervention in adults.
The parameters of the model in the present study differed from the ones utilized to predict GMTs and seroprotection in adults up to 25 years after one dose of JE-CV. The intercept of the piecewise linear model was higher in children compared with adults (8.06 [7.74–8.38 for 2.5–97.5 percentiles] vs. 5.81 [5.36–6.58 for 5–95th percentiles], respectively).Citation14 These differences were reflected in the higher GMTs at D28 in children in the present study compared with adults in Desai et al. In addition, a more rapid decline in GMTs was predicted in children than adults by the piecewise linear model, with slope after S (b2 + b2i) of – 0.215 (–0.27, – 0.16) and – 0.109 (–0.172, – 0.034), respectively.
A number of limitations of this study must be considered. Two different models were used for the different age groups. The piecewise linear model, which provided a good fit with observed titers for children, did not give a good fit for the toddlers age group because of the limited number of time points between D28 and Y1 and a more rapid decrease in titers between D28 and M6. Therefore, a linear model using data from M6 was used for toddlers. Our predictions should be interpreted with caution given the fact that they are based on the assumption that the observed linear trend continues beyond five years. In particular, it should be highlighted that seroprotection estimates were derived from the predicted antibody titers; these derived seroprotection estimates appeared to be overestimated in the linear model used for toddlers (for Y2–Y5), and is not presented here.
Modeling approaches have been used to estimate the duration of protection for a number of other vaccine-preventable diseases. A recent modeling exercise, using piecewise and modified power law models based on real-life 5-year immunogenicity data following immunization with the human papillomavirus-16/18 AS04-adjuvanted vaccine predicted persistence of HPV antibodies above natural infection levels in girls (9–14 years) for over 20 yearsCitation21; mixed effects models have also been used to predict long-term seroprotection against hepatitis A for at least 20–30 years after vaccinationCitation22,Citation23; and to support the need for booster doses of 5-component acellular pertussis vaccine every 10 years.Citation24 Predictions of the duration of protection against such vaccine-preventable diseases are highly valuable for decision-making on future vaccination strategies. In the absence of real-life data beyond 5 years, the current modeling exercise supports growing evidence suggesting that primary immunization with a JE-CV single dose in toddlers may elicit a protective response that persists over 5 years despite a decrease in the Ab titers (and so justifying the booster dose to be administered 1 to 2 years after primary single dose immunization), and JE-CV booster vaccination in children confers protection without the need for further doses for at least 10 years.
Methods
Study data
Source data (observed individual JE antibody titers) were obtained from a phase 2 randomized controlled immunogenicity study (cross-over design) in Thailand, in which 100 children (2–5 years old) received 1 dose of JE-CV as a booster JE vaccination after primary immunization with the inactivated JE vaccine MBDV and 200 toddlers (12–24 months old) received a single dose of JE-CV as primary immunization.Citation10,Citation15 All participants received the control hepatitis A vaccine, administered either 28 days before or 28 days after the study vaccine JE-CV in a cross over design; both groups were treated with both products assuming no effect of hepatitis A vaccination on JE vaccination. Antibody titers were measured before JE-CV administration, then on D28, M6, and Y1, 2, 3, 4 and 5 after vaccination.
Sensitivity analyses were carried out to account for the discontinuation of subjects with low neutralizing antibody titers (i.e. below the threshold considered for protection), and who may have received another JE vaccination during the 5-year follow-up period, as previously described.Citation15 Briefly, for all follow-up visits between year 1 and year 5, missing values occurring after negative values were replaced by negative values; from M6 to Y5, positive values occurring after negative values were replaced by negative values. Titers increasing with a ratio ≥ 8 at Y4 or Y5 in comparison to previous values were deleted (other values were kept for those participants affected). Data from at least two time points had to be available for inclusion.
All data were anonymized such that no patient identifiers were present in the data files received for analysis.
Statistical models
Statistical models were developed to predict antibody titers over time for up to 10 years following JE-CV vaccination. It was also expected that the models could estimate the proportions of participants remaining seroprotected against JE from the predicted antibody titers (protective antibody titers ≥ 10 [1/dil]). Observed antibody titers from D28 to Y5 were used to construct models for children. For toddlers, as only limited data were available for the initial decay phase, models with data from D28 to Y5 didn’t fit the data, so models were constructed with data from M6 to year 5 only. Three mixed effect models were fitted to the data for each age group, to account for variation at the population level (fixed effects) and at the individual level (random effects).
Linear model
The first model (linear) estimated linear antibody decay and contained fixed and random effects for both slope and intercept parameters:
where Yij is the log of the neutralizing antibody titer for subject i observed at time tj .
a and b are the population-level (fixed) effects for the intercept and slope respectively and ai and bi are the individual-level (random) effects for the intercepts and the slope.
εij is the residual error.
Piecewise model
The second model was a 2-period piecewise linear model with fixed and random effects for the intercept (a,ai), 2 slope parameters (b,bi b2,b2i) and a change point Si, representing the point in time when the change in the rate of antibody decay occurs.
and
Exponential model
The third model was an Exponential model with fixed and random effects for the intercept (a,ai), the slope parameters (b,bi) and a Exponent (c,ci).
Model construction and validation
All models were constructed using a Bayesian Monte-Carlo Markov chain approach and were implemented with Open-Bugs V3.12.1.Citation25,Citation26 Models were constructed with three chains of 60,000 iterations, excluding the first 30,000 ‘burn-in’ estimates. Models were validated based on the convergence of various parameters: parameter iteration histories (the trace), the auto-correlation and the Gelman Rubin statistics. Models were compared using the Deviance Information Criterion (DIC).Citation27 The DIC is a generalization of the Akaike Information Criterion and is suitable for assessing mixed-effects models.
Estimation of seroprotection rates
Selected models were used to try to predict at each time point the proportion of subjects considered to be seroprotected (i.e. JE antibody titers ≥ 10 [1/dil]). Estimates were based on the distribution of the predicted antibody titers.
Disclosure of potential conflicts of interests
AB, FB and EF are employees of Sanofi Pasteur.
Author contributions
AB, FB and EF were involved in the design, analysis and interpretation of the analysis. All authors contributed to this publication and approved the final manuscript for submission. All authors had access to the study data and are responsible for the veracity and completeness of the data reported.
Supplemental Material
Download MS Word (98.3 KB)Acknowledgments
The authors thank and acknowledge the contribution of participation of the infants and parents in Bangkok, Thailand, as well as the investigational staffs at: Chulalongkorn Hospital, Bangkok, Thailand (Prof. U. Thisyakorn and Prof. C. Pancharoen), Siriraj Hospital, Bangkok, Thailand (Prof. K. Chokephaibulkit), Tropical Medicine Hospital, Bangkok, Thailand (Prof. A. Sabchareon), as well as Dr. Sutee Yoksan at the Centre for Vaccine Development, Mahidol University, Thailand, and the JE-CV Clinical Team in Sanofi Pasteur. Authors also thank Celine Monfredo (Sanofi Pasteur) for her contributions to designing the statistical methods and analyzing the data.
Editorial assistance with the preparation of the manuscript was provided by a professional medical writer, Juliette Gray of inScience Communications, Springer Healthcare, on behalf of Sanofi Pasteur.
Supplemental data
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Halstead SB, Jacobson J. Japanese encephalitis vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 5th ed. Philadelphia: Elsevier; 2008.
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–109. doi:10.1038/nm1144.
- Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, Marfin AA, Solomon T, Tsai TF, Tsu VD, et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ. 2011;89:766–74,74A-74E. doi:10.2471/BLT.10.085233.
- Arai S, Matsunaga Y, Takasaki T, Tanaka-Taya K, Taniguchi K, Okabe N, Kurane I. Japanese encephalitis: surveillance and elimination effort in Japan from 1982 to 2004. Jpn J Infect Dis. 2008;61:333–338.
- Wang H, Liang G. Epidemiology of Japanese encephalitis: past, present, and future prospects. Ther Clin Risk Manag. 2015;11:435–448. doi:10.2147/TCRM.S51168.
- Tsai TF. New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13-15 October 1998. Vaccine. 2000;18(Suppl 2):1–25.
- Halstead SB, Thomas SJ. Japanese encephalitis: new options for active immunization. Clin Infect Dis. 2010;50:1155–1164. doi:10.1086/651271.
- World Health Organization. Safety of SA 14-14-2 Japanese encephalitis (JE) vaccine. 2005. http://www.who.int/vaccine_safety/committee/topics/japanese_encephalitis/live_attenuated/June_2005/en/.
- Feroldi E, Pancharoen C, Kosalaraksa P, Chokephaibulkit K, Boaz M, Meric C, Hutagalung Y, Bouckenooghe A. Primary immunization of infants and toddlers in Thailand with Japanese encephalitis chimeric virus vaccine in comparison with SA14-14-2: a randomized study of immunogenicity and safety. Pediatr Infect Dis J. 2014;33:643–649. doi:10.1097/INF.0000000000000276.
- Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Sabchareon A, Pancharoen C, Bouckenooghe A, Gailhardou S, Boaz M, Feroldi E. Safety and immunogenicity of a single administration of live-attenuated Japanese encephalitis vaccine in previously primed 2- to 5-year-olds and naive 12- to 24-month-olds: multicenter randomized controlled trial. Pediatr Infect Dis J. 2010;29:1111–1117. doi:10.1097/INF.0b013e3181f68e9c.
- Feroldi E, Pancharoen C, Kosalaraksa P, Watanaveeradej V, Phirangkul K, Capeding MR, Boaz M, Gailhardou S, Bouckenooghe A. Single-dose, live-attenuated Japanese encephalitis vaccine in children aged 12-18 months: randomized, controlled phase 3 immunogenicity and safety trial. Hum Vaccin Immunother. 2012;8:929–937. doi:10.4161/hv.20071.
- Torresi J, McCarthy K, Feroldi E, Meric C. Immunogenicity, safety and tolerability in adults of a new single-dose, live-attenuated vaccine against Japanese encephalitis: randomised controlled phase 3 trials. Vaccine. 2010;28:7993–8000. doi:10.1016/j.vaccine.2010.09.035.
- Nasveld PE, Ebringer A, Elmes N, Bennett S, Yoksan S, Aaskov J, McCarthy K, Kanesa-Thasan N, Meric C, Reid M. Long term immunity to live attenuated Japanese encephalitis chimeric virus vaccine. Hum Vaccine. 2010;6:1038–1046. doi:10.4161/hv.6.12.13057.
- Desai K, Coudeville L, Bailleux F. Modelling the long-term persistence of neutralizing antibody in adults after one dose of live attenuated Japanese encephalitis chimeric virus vaccine. Vaccine. 2012;30:2510–2515. doi:10.1016/j.vaccine.2012.02.005.
- Chokephaibulkit K, Sirivichayakul C, Thisyakorn U, Pancharoen C, Boaz M, Bouckenooghe A, Feroldi E. Long-term follow-up of Japanese encephalitis chimeric virus vaccine: immune responses in children. Vaccine. 2016;34:5664–5669. doi:10.1016/j.vaccine.2016.09.018.
- Feroldi E, Yoksan S, Chokephaibulkit K, Sabchareon A, Thisyakorn U, Capeding MR., Boaz M, Bouckenooghe A. Long-term neutralizing antibody response after a JE-CV single dose primary immunization in JE-vaccine naïve toddlers. 5th Asian vaccine conference (ASVAC); 12–14 June; Hanoi, Vietnam 2015.
- Chotpitayasunondh T, Pruekprasert P, Puthanakit T, Pancharoen C, Tangsathapornpong A, Oberdorfer P, Kosalaraksa P, Prommalikit O, Tangkittithaworn S, Kerdpanich P, et al. Post-licensure, phase IV, safety study of a live attenuated Japanese encephalitis recombinant vaccine in children in Thailand. Vaccine. 2017;35:299–304. doi:10.1016/j.vaccine.2016.11.062.
- Feroldi E, Capeding MR, Boaz M, Gailhardou S, Meric C, Bouckenooghe A. Memory immune response and safety of a booster dose of Japanese encephalitis chimeric virus vaccine (JE-CV) in JE-CV-primed children. Hum Vaccin Immunother. 2013;9:889–897. doi:10.4161/hv.23087.
- Capeding MR, Alberto ER, Bouckenooghe A, Laot TM, Chansinghakul D, Monfredo C, Machabert T, Feroldi E. Five-year antibody persistence following a Japanese encephalitis chimeric virus vaccine (JE-CV) booster in JE-CV-primed children in the Philippines. J Infect Dis. 2018;217:567–571. doi:10.1093/infdis/jix601.
- Kosalaraksa P, Wiangnon S, Suphakunpinyo C, Kosuwon P, Hutagalung Y, Feroldi E., et al. Booster effect of Japanese encephalitis chimerix virus vaccine (JE-CV): 5 years after primary vaccination. 5th Asian vaccine conference (ASVAC); 12–14 June; Hanoi,Vietnam 2015.
- Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, Schulze K, Hillemanns P, Suryakiran P, Thomas F, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: five-year clinical data and modeling predictions from a randomized study. Hum Vaccine Immunother. 2016;12:20–29. doi:10.1080/21645515.2015.1065363.
- Hens N, Habteab Ghebretinsae A, Hardt K, Van Damme P, Van Herck K. Model based estimates of long-term persistence of inactivated hepatitis A vaccine-induced antibodies in adults. Vaccine. 2014;32:1507–1513. doi:10.1016/j.vaccine.2013.10.088.
- Lopez EL, Contrini MM, Mistchenko A, Kieffer A, Baggaley RF, Di Tanna GL, Desai K, Rasuli A, Armoni J. Modeling the long-term persistence of hepatitis A antibody after a two-dose vaccination schedule in Argentinean children. Pediatr Infect Dis J. 2015;34:417–425. doi:10.1097/INF.0000000000000605.
- Bailleux F, Coudeville L, Kolenc-Saban A, Bevilacqua J, Barreto L, Andre P. Predicted long-term persistence of pertussis antibodies in adolescents after an adolescent and adult formulation combined tetanus, diphtheria, and 5-component acellular pertussis vaccine, based on mathematical modeling and 5-year observed data. Vaccine. 2008;26:3903–3908. doi:10.1016/j.vaccine.2008.04.089.
- Bailleux F, Coudeville L, Monfredo C. A bayesian Monte-Carlo Markov chains approach (MCMC) to predict the long-term antibody persistence after Japanese encephalitis vaccination. 34th annual ISB conference; 2013; Munich, Germany.
- Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: evolution, critique and future directions. Stat Med. 2009;28:3049–3067. doi:10.1002/sim.3680.
- Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J Royal Stat Soc Series B (Stat Methodol). 2002;64:583–639. doi:10.1111/1467-9868.00353.